Abstract
Developmental events in the life-cycle of the sleeping sickness parasite comprise integrated changes in cell morphology, metabolism, gene expression and signalling pathways. In each case these processes differ from the eukaryotic norm. In the past three years, understanding of these developmental processes has progressed from a description of the cytological events of differentiation to a discovery of its underlying molecular controls. With an expanding set of reagents for the identification of distinct parasite life-cycle stages in the tsetse, trypanosome differentiation is being studied from the molecular to the organismal and population level. Interestingly, the new molecular discoveries provide insights into the biology of the parasite in the field.
Introduction
The transmission of the trypanosome (Trypanosoma brucei), causative agent of African sleeping-sickness, occurs upon the bite of tsetse flies. In the tsetse, parasite development takes 20–30 days to reach maturation [1], during which the trypanosome undergoes enormous and multiple changes in its biology before regenerating a form that is infective to mammalian hosts. The reacquisition of infectivity exactly correlates with the expression on the parasite of a dense surface coat, composed of a homogenous layer of variant surface glycoprotein (VSG). In mammalian hosts, this can be periodically changed in an extreme form of antigen variation [2]. This is required because trypanosomes live extracellularly in the bloodstream and undergo antigenic variation to stay ahead of the lytic immunoglobulin response raised to successive antigenic types. Often the cyclical waves of parasitaemia characteristic of textbook descriptions of trypanosome infections are entirely ascribed to antigenic variation. However, it has been proposed for nearly 100 years that a parasite-derived factor is also important in modulation of the parasitaemia [3] and this has been supported recently at the biochemical level [4–6] and by mathematical modelling [7••]. Indeed, it was always apparent that the bloodstream parasite population was not uniform and that the proportion of different forms changed during the parasitaemia. This is described as pleomorphism, with its two extremes being the morphologically ‘slender’ and ‘stumpy’ forms [8]. Robertson (1912) [3] was clear that only stumpy forms were capable of transmission to the tsetse fly, but the situation has become more complicated since then. Part of the reason for this is that laboratory lines were selected for growth in the bloodstream (and antigenic stability), without a corresponding pressure for the maintenance of transmissibility through the tsetse fly. The result was the selection of monomorphic lines, which are able to proliferate rapidly and without control in mammalian hosts but which no longer produce stumpy forms. The analysis of these lines has caused controversy as to the relative importance of slender and stumpy forms in the developmental biology of the parasite since monomorphic cells, which resemble pleomorphic slender cells, remain able to differentiate to tsetse midgut (procyclic) forms in vitro.
Examining pleomorphism beyond morphology
The production of stumpy forms represents a form of quorum sensing. As parasite numbers increase through the proliferation of slender forms, a parasite-derived soluble factor accumulates which promotes cell-cycle arrest in G1/G0, mitochondrial elaboration and morphological transformation to the stumpy form [5,6] (Figure 1). This is accompanied by dysregulation of the monoallelic control of VSG gene expression sites [9]. The reason for this is unclear, though it may simply reflect the irreversibility of the slender–stumpy transition. Since stumpy cells are non-proliferative and are destined either for transmission or death, it may be of no consequence that the normal stringency of expression site control is lost in these forms.
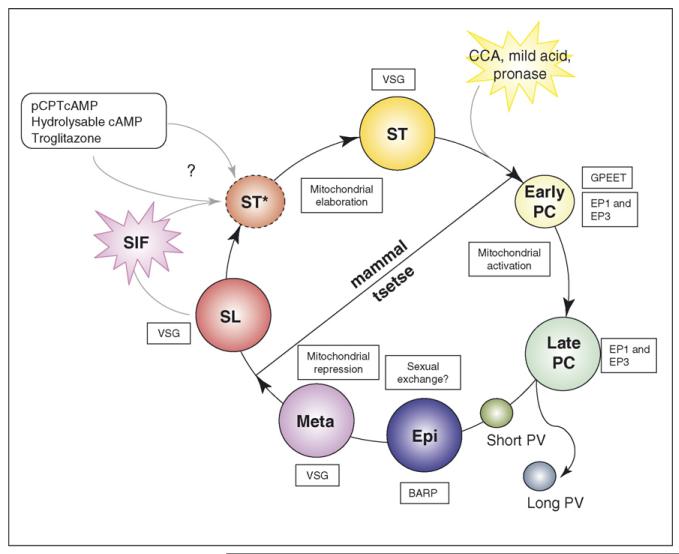
Figure 1. A schematic diagram of the life-cycle of Trypanosoma brucei.
Mammalian infection is initiated when metacyclic (‘Meta’) forms are inoculated by the bite of an infected tsetse fly. These develop to proliferative bloodstream slender (SL) forms that multiply in the blood to establish the infection. As numbers increase, a parasite-derived factor, stumpy induction factor (SIF), promotes division arrest and the generation of stumpy (ST) forms, commitment to this transition occurring while the parasite is still morphologically slender (this intermediate form has been termed Stumpy*; [25••]). The experimental induction of cells with stumpy characteristics can also be induced by various chemical treatments (8-pCPTcAMP, hydrolysable cAMP and troglitazone) although whether these act via the SIF signalling pathway is unclear. Once stumpy cells are up taken by tsetse flies they transform to procyclic (PC) forms, this being induced in vitro by exposure to citrate/cis-aconitate (CCA), mild acid or pronase treatment. Procyclic forms develop initially as early forms (which express GPEET procyclin), this being down regulated as they transform to late procyclic forms. Asymmetric division of proventricular (PV) forms generates a long and short form, the short form of which is believed to attach to the salivary gland and transform to proliferative epimastigote (‘Epi’) forms, which express BARP and are believed to be capable of sexual exchange. These eventually mature to detached metacyclic forms that express a VSG coat and are ready for transmission to a new mammalian host. Mitochondrial status is indicated inside the circle in boxes, surface antigen expression is indicated outside the circle.
To date, the external signal promoting stumpy formation, termed stumpy-induction factor (SIF) [5], has eluded purification though intracellular signalling pathway components that stimulate stumpy formation when perturbed have been identified. These include a PX-FVYVE family zinc finger kinase, ZFK [10] and a MAP kinase family member, TbMAPK5 [11•]. In both cases, genetic deletion in pleomorphic lines resulted in accelerated development to stumpy forms and reduced growth in culture and in mice. Importantly, monomorphic lines, unable to generate stumpy forms, did not exhibit a phenotype when either gene was deleted, supporting a role for these genes in development rather than effects on virulence, for example. Although SIF remains unidentified, a number of chemical factors that mimic its action have been described which might give some clue as to how the factor operates or is signalled in the cell. Among these, compounds linked to the cAMP pathway induce convincing progression to stumpy forms [5,12••]. Importantly, this has not only been measured on the basis of cell morphology but also on the ability of treated cells to undergo efficient differentiation to tsetse midgut procyclic forms in vitro, the hallmark of stumpy-forms generated in vivo. Originally, a cell-permeant cAMP analogue, 8-pCPTcAMP, was observed to induce stumpy formation, invoking the cyclic nucleotide-signalling pathway [5]. More recently, however, hydrolysable cAMP analogues have been used to inhibit proliferation and promote the development of morphologically stumpy forms, though this was in monomorphic populations naturally resistant to SIF [12••]. If verified in SIF-responsive pleomorphic lines, this would suggest that the cyclic nucleotide-signalling pathway were not itself responsible for SIF signalling, but rather the breakdown products of these. Treatment with troglitazone, a thiazolidinedione that acts on the peroxisome proliferating-activated receptor gamma in higher eukaryotes, also promotes morphological transformation of pleomorphic lines and causes cell growth arrest in monomorphic cells (although not at a specific cell-cycle position) [13]. Cell-cycle arrest and development to a stumpy morphology has also been observed with several other compounds or genetic perturbations that inhibit cell growth in bloodstream forms [14,15]. However, the precise relationship between cells described as ‘stumpy-like’ and the stumpy cells that arise in response to SIF in vivo or in vitro is not clear. This reflects the absence of molecular markers for this cell type, these being necessary to recognise activation of a bona fide developmental pathway rather than growth arrest, mitochondrial activation and morphological changes that can accompany cells under certain non-physiological conditions.
Two specific cytological characteristics of stumpy forms have been defined for the first time by Engstler and Boshart [16••]. It has long been known that differentiation from bloodstream forms to tsetse midgut forms could be reproduced in vitro by a reduction in temperature and exposure to citrate/cis-aconitate (CCA) [17,18]. However, the required concentrations of these compounds appeared un-physiologically high for these molecules to be natural inducers of differentiation in the fly midgut [19]. Engstler and Boshart observed that when bloodstream forms were exposed to temperature reduction of >15 °C, then stumpy cells became hypersensitised to CCA, producing proliferative procyclic forms when treated with cis-aconitate in the low micromolar range. Although the relevance in flies is still to be validated, this is compatible with the reported concentration of CCA in the tsetse midgut (15 μM citrate; [20]); moreover the required temperature drop is consistent with tsetse feeding habits, where feeding at dusk exposes the ingested trypanosomes to cool night-time temperatures. Interestingly, it was also noted that bloodstream cells exposed to cold shock expressed EP procyclin (normally only detected on the surface of procyclic forms) but that this was trafficked to the cell surface in stumpy forms but not slender forms. Differential protein trafficking may be a key difference between these forms, which, through regulated surface access of a receptor, may explain the basis of stumpy form sensitivity to CCA (Figure 2).
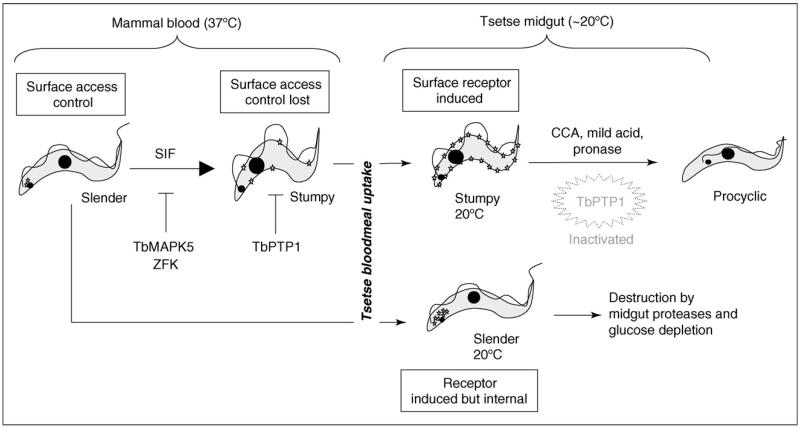
Figure 2. Cytological events leading from slender to procyclic forms.
Slender cells proliferate in the mammalian bloodstream and produce SIF, triggering the density-dependent production of stumpy forms. This differentiation is inhibited by the action of signalling pathway(s) involving TbMAPK5 and ZFK. Once stumpy forms are generated, they are held poised for differentiation to procyclic forms by the action of TbPTP1 [25••]. These cells also express, at low level, a CCA receptor/transmitter for the signal to differentiate, which has access to the cell surface (shown as a ‘star’). Upon uptake by tsetse flies, Engstler and Boshart [16••] propose that a reduction in temperature of >15 °C promotes super-induction of the CCA receptor/transmitter that is trafficked to the stumpy cell surface, sensitising these cells to the inductive signal. Slender cells, in contrast, do not traffic the receptor/transmitter to the surface. Exposure to differentiation signals in the fly inactivates TbPTP1 and initiates transformation to procyclic forms, this being induced by CCA, proteolytic attack of the parasite surface or pH stress in the tsetse midgut and/or in vitro.
The division-arrest of stumpy forms is central to their role in the biology of trypanosomes in their mammalian host. Unless stumpy forms arose during a parasitaemia, the uncontrolled growth of slender forms would rapidly kill the host, thereby limiting the parasite’s transmission potential (reviewed in [21]). Cell-cycle arrest has also been proposed to be crucial in their transmission capacity [22]. This resulted from the observation that stumpy cells are uniformly arrested in G1/G0 [23] and initiate differentiation to procyclic forms in vitro synchronously and efficiently [5,22,24]. The molecular basis of the interrelationship between cell-cycle arrest and differentiation competence was investigated by analysis of a protein tyrosine phosphatase (TbPTP1) related to similar molecules with function in G0 in other systems [25••]. Transcript ablation of TbPTP1 by RNAi or pharmacological inhibition of the enzyme by a cell permeable PTP1B inhibitor induced spontaneous differentiation to procyclic forms, suggesting that this molecule normally inhibits this differentiation step [25••,26]. Although only a small proportion of cultured monomorphic bloodstream forms differentiated, uniformly arrested stumpy forms differentiated with nearly 100% efficiency when exposed to the inhibitor. This suggested a model in which cell-cycle arrest was required for differentiation and that in the bloodstream this was prevented by the activity of TbPTP1. Once in the fly, inactivation of this enzyme would allow differentiation of those cells competent to do so (Figure 2). Since differentiation can be stimulated by a number of alternative triggers including CCA, exposure of the parasite surface to limited protease treatment [19] or to reduced pH [27], it remains to be determined whether each pathway operates via the activity of TbPTP1 or whether multiple-independent pathways can act to initiate differentiation to procyclic forms.
Differentiation events in the tsetse fly: a model developmental system
Once stumpy forms initiate differentiation to procyclic forms they embark on a precisely regulated differentiation programme, the components of which occur in a consistent order and on a reproducible timescale in vitro. The major event is exchange of surface antigens between bloodstream and insect procyclic forms, that is, loss of the VSG and gain of procyclins. VSG is released from bloodstream forms by the combined action of a metalloendoprotease (MSP-B) and a glycophosphatidylinositol phospholipase C (GPI-PLC) activity [28]. MSP-B, which clips the VSG protein close to its C-terminus, is induced after the initiation of differentiation, and then remains expressed in procyclic forms. GPI-PLC, by contrast, is quickly downregulated during differentiation but gains access to the parasite surface (and hence its substrate) in the stumpy form. Other major events, each interlinked, fall broadly into three categories.
Gene expression
Gene expression changes are important in adaptation of trypanosomes from one life-cycle stage to the next, with microarray analysis suggesting that ~2% of genes show developmental regulation at the mRNA level [29]. This regulation is largely post-transcriptional because the trypanosome genome is organised into poly-cistronic transcription units where functionally unrelated genes are transcribed as a single unit and then processed into individual mRNAs. This means that protein levels are regulated through mRNA stability and degradation, coupled with translational efficiency and protein turnover. One common theme is that cycloheximide treatment increases the abundance of several stage-regulated transcripts, including EP procyclin [30], MSP-B [28], GPI-PLC [31] and several components of the cytochrome oxidase complex [32]. This effect does not appear to be mediated through signals in the 3′UTR [30,32], though these regions certainly do contain the dominant regulatory elements which govern the stage-specific expression of many genes [33].
A key ambition is the recognition of common motifs governing developmental gene expression [32]. One model for such analysis is the regulation of nuclear-encoded genes for components of the cytochrome oxidase (COX) complex. Trypanosomes are unusual in demonstrating developmental regulation of mitochondrial respiratory activity and require co-incident upregulation of COX subunits upon differentiation to procyclic forms. Reporter construct expression and bioinformatics analysis identified a dominant regulatory motif controlling COX V stage-regulated expression. This was similar to a ‘26mer’ element identified in procyclin mRNAs that operates to negatively regulate gene expression in bloodstream forms. Genome-wide analysis using an oligomer-counting algorithm suggested that its central region (UAUUUUUU) was highly enriched in the 3′UTR of procyclic-enriched transcripts, implicating this motif as a widespread regulator of developmental gene expression. A second element, as yet functionally uncharacterised, is over-represented in the mRNAs for nuclear-encoded mitochondrial proteins and may represent a general cis-acting signal regulating such transcripts. Expression analysis of the bloodstream-specific GPI-PLC mRNA used derepression in procyclic forms of an antibiotic resistance gene linked to the GPI-PLC 3′UTR to map regulatory regions [34]. Selected mutants spontaneously deleted the last 800 bases of the 2300 base long 3′UTR suggesting presence of a negative control element in this region that operates in procyclic forms.
As further cis-acting sequence elements are identified within the genome there is a need to identify the trans-acting regulatory proteins recognising these signals. The T. brucei genome encodes an abundance of RNA-binding proteins (RBPs) [35–37] and linking these to their target sequence element will help to decipher the mechanisms involved in developmental gene regulation. Candidate molecules include small RNA-binding proteins presumed to act through modular interaction with other effector proteins. These include the T. cruzi UBP proteins [38], their T. brucei equivalents [39] and a group of CCCH zinc finger proteins, TbZFPs, one of which is required for kinetoplast repositioning during differentiation to procyclic forms [40]. Further members of this protein family associate with the translational apparatus in a stage-specific manner and enhance differentiation [41]. Interestingly, immunoprecipitation of one protein (TbZFP3) co-selects EP procyclin mRNA, suggesting that it may be positively regulating this transcript at the level of stability or translation.
In addition to trans-acting RNA-binding proteins other mechanisms may also regulate developmental gene expression. One mechanism, recently characterised in mammalian cells, is the action of the RNAi pathway on transcripts containing AU-rich elements [42]. However, at present there is no evidence for this in trypanosomes, with Argonaut mutants lacking a functional RNAi pathway and still exhibiting normal life-cycle progression and an absence of detectable changes in stage-regulated mRNAs [43]. Histone modification is another potential regulatory mechanism for gene expression, with deletion of the DOT1B gene, responsible for trimethylation of H3K76, apparently causing defective differentiation to procyclic forms [44•]. Finally, gene expression may correlate with nuclear architecture. The monoallelleic expression characteristic of VSG expression site control is mediated through an expression site body proposed to associate with the active telomere at the nuclear periphery [45]. Upon differentiation to procyclic forms this subnuclear association appears to be lost, with telomere redistribution in the nucleus perhaps contributing to VSG gene silencing [46].
Signalling pathways
Accompanying changes in gene expression, various signalling components have been identified that regulate differentiation to procyclic forms. It can be difficult to distinguish signalling molecules that act on the differentiation process itself from those that simply regulate outgrowth and viability of the differentiated procyclic form population. Nonetheless, several protein kinases that act in a stage-specific manner have been identified, as have cell-cycle regulatory kinases that elicit developmental stage-specific effects [47–52]. Study of the complete complement of protein kinases encoded in the trypanosomatid genomes [53•] revealed an absence of tyrosine-specific kinases, though tyrosine phosphorylation patterns clearly change during life-cycle development [54], presumably through the action of dual specificity protein kinases. Analysis of the full complement of phosphatases encoded in the trypanosomatid genomes also reveals very few predicted tyrosine-specific phosphatases, with a corresponding increase in serine/threonine phosphatases (R Brenchley, H Tariq, H McElhinney, B Szoor, R Stevens, K Matthews, L Tabernero, unpublished data). Interestingly, a classic tyrosine phosphatase encoded in Leishmania major and T. cruzi is missing in T. brucei. Genetic ablation experiments in L. major [55] implicate this molecule as being important in the virulence of intracellular amastigote forms, perhaps explaining the absence of an orthologous gene in the exclusively extracellular T. brucei.
Metabolism and surface antigen regulation
During differentiation in the fly, trypanosomes exhibit complex changes in metabolism. The major differences between forms is that in bloodstream stages glycolysis is responsible for ATP synthesis, with mitochondrial activity being repressed except the activity of a plant-like alternative oxidase which maintains the redox balance of glycosomes [56]. In procyclic forms, by contrast, substrate level phosphorylation is responsible for ATP production in the presence of glucose, whereas in its absence, amino acids become the major carbon source, with ATP being generated via oxidative phosphorylation. These changes in mitochondrial activity are not only important in the metabolic activity of the cell but also in regulating the surface antigen expression. Thus, GPEET procyclin shows alternative expression depending on the activity of mitochondrial enzymes, with downregulation of the acetate, succinate-CoA transferase/succinyl-CoA synthetase (ASCT) pathway leading to expression of GPEET in late procyclic forms, this being reversible by drug inhibition of the alternative oxidase [57•].
Differentiation events in the tsetse fly
Although several components of the trypanosome life-cycle can be reproduced in vitro, analysis of events in the fly is also revealing. The best characterised is the expression profile of different procyclin isoforms. In culture, EP and GPEET procyclin (phosphorylated by a GPEET kinase restricted to the flagellar pocket; [58]) are expressed early, with GPEET procyclin being downregulated upon development of late procyclic forms. However, EP procyclin is also regulated. There are three isoforms of this molecule, EP1, EP2 and EP3. Analysis of their expression in the fly has been monitored by mass spectrometry for protein expression [59,60] and by RTPCR analysis of mRNA expression using primers directed to regions of the 3′UTR that distinguish each type [61]. This revealed that EP1 and EP3 replace GPEET procyclin in late procyclic forms, and that EP2, which unlike EP1 and EP3 is not glycosylated, is not detectable at the protein level. After multiplication in the midgut/proventriculus, procyclic forms migrate toward the salivary glands, undergoing an asymmetric cell division generating a long epimastigote form and a short epimastigote form [62]. Only short forms are believed to be destined for attachment and proliferation in the fly salivary gland, where EP procyclin mRNA continues to be expressed although procyclin protein is not detectable on most parasites — this repression being due to sequences present in the procyclin coding region [61]. Instead of procyclins, a further surface coat protein, brucei alanine rich protein (BARP), is expressed on epimastigotes though its function is unclear [63•]. Epimastigotes are believed to be the site of sexual exchange between trypanosome lines, with hybrids being detectable in the salivary gland after parasite lines tagged with distinct fluorescent reporters are transmitted through flies [64]. Finally, the parasites mature to metacyclic forms and reacquire the VSG coat [65]. Upon detachment from the salivary gland wall, these metacyclic forms are infectious for mammalian hosts, this being initiated when they are expelled during a tsetse blood meal.
Conclusions
There are still fundamental barriers to be overcome in the study of the trypanosome life-cycle. Among the most important is the identification of molecular markers for distinct life-cycle stages, particularly stumpy forms. The ambiguity of morphological descriptors of this life-cycle stage has, firstly, created controversy regarding the relative capacity of slender and stumpy form for differentiation to procyclic forms; secondly, hindered the development of effective bioassays for the generation of stumpy forms, an important component in systematic approaches to identify SIF biochemically and, thirdly, restricted accurate modelling of the dynamics of trypanosome profiles in the chronic infections characteristic of the disease in the field. A mathematical model was developed recently that effectively predicted the trypanosome parasitaemia in relation to the relative probabilities of antigen gene activation [7••]. However, such models can only be as good as the data upon which key assumptions are based. In consequence the contribution of the slender–stumpy transition to the developed models was, through necessity, based on data generated using relatively subjective morphological markers. Unambiguous markers are clearly needed to allow these transitions to be modelled quantitatively and, thereby, the balance between maintenance of the parasitaemia (as slender forms) and transmissibility of the parasite (as stumpy forms) to be understood.
Notwithstanding these issues, understanding the cell biology of differentiation in trypanosomes is moving from a descriptive era to one where molecular controls underlying several of the key transitions are becoming known. The identification of these regulators offers exciting prospects for analysing the pathways involved in differentiation control with the potential to identify routes to either block them or stimulate them inappropriately. Clearly, breaking the life-cycle offers new hope in blocking the cycle of infection for these important parasites.
Acknowledgement
Work in Keith Matthews’ Laboratory is funded by a Programme grant from the Wellcome Trust (073358).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Aksoy S, Gibson WC, Lehane MJ. Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Adv Parasitol. 2003;53:1–83. doi: 10.1016/s0065-308x(03)53002-0. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JE, Rudenko G. Switching trypanosome coats: what’s in the wardrobe? Trends Genet. 2006;22:614–620. doi: 10.1016/j.tig.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Robertson M. Notes on the polymorphism of Trypanosoma gambiense in the blood and its relation to the exogenous cycle in Glossina palpalis. Proc Biol Sci. 1912;85:241–539. [Google Scholar]
- 4.Hamm B, Schindler A, Mecke D, Duszenko M. Differentiation of Trypanosoma brucei bloodstream trypomastigotes from long slender to short stumpy-like forms in axenic culture. Mol Biochem Parasitol. 1990;40:13–22. doi: 10.1016/0166-6851(90)90075-w. [DOI] [PubMed] [Google Scholar]
- 5.Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- 6.Reuner B, Vassella E, Yutzy B, Boshart M. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol Biochem Parasitol. 1997;90:269–280. doi: 10.1016/s0166-6851(97)00160-6. [DOI] [PubMed] [Google Scholar]
- 7••.Lythgoe KA, Morrison LJ, Read AF, Barry JD. Parasite-intrinsic factors can explain ordered progression of trypanosome antigenic variation. Proc Natl Acad Sci U S A. 2007;104:8095–8100. doi: 10.1073/pnas.0606206104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on the differential activation frequency of surface antigen genes coupled with the balance of slender and stumpy forms, this paper accurately models trypanosome parasitaemias in chronic infections via mathematical prediction.
- 8.Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- 9.Amiguet-Vercher A, Perez-Morga D, Pays A, Poelvoorde P, Van Xong H, Tebabi P, Vanhamme L, Pays E. Loss of the mono-allelic control of the VSG expression sites during the development of Trypanosoma brucei in the bloodstream. Mol Microbiol. 2004;51:1577–1588. doi: 10.1111/j.1365-2958.2003.03937.x. [DOI] [PubMed] [Google Scholar]
- 10.Vassella E, Kramer R, Turner CM, Wankell M, Modes C, van den Bogaard M, Boshart M. Deletion of a novel protein kinase with PX and FYVE-related domains increases the rate of differentiation of Trypanosoma brucei. Mol Microbiol. 2001;41:33–46. doi: 10.1046/j.1365-2958.2001.02471.x. [DOI] [PubMed] [Google Scholar]
- 11•.Domenicali Pfister D, Burkard G, Morand S, Renggli CK, Roditi I, Vassella E. A mitogen-activated protein kinase controls differentiation of bloodstream forms of Trypanosoma brucei. Eukaryot Cell. 2006;5:1126–1135. doi: 10.1128/EC.00094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a mitogen-activated kinase whose deletion in pleomorphic parasites accelerates the production of stumpy forms by inducing this transition at lower parasitaemia. In consequence this molecule is implicated in the SIF-sensing pathway
- 12••.Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci U S A. 2006;103:19194–19199. doi: 10.1073/pnas.0608971103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hydrolysis products of cAMP analogues induce the production of stumpy forms in monomorphic parasites. This suggests that the breakdown products of cyclic nucleotides may be involved in the SIF-signalling pathway rather than an involvement of the PKA pathway.
- 13.Denninger V, Figarella K, Schonfeld C, Brems S, Busold C, Lang F, Hoheisel J, Duszenko M. Troglitazone induces differentiation in Trypanosoma brucei. Exp Cell Res. 2007;313:1805–1819. doi: 10.1016/j.yexcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Penketh PG, Divo AA, Shyam K, Patton CL, Sartorelli AC. The effects of the methylating agent 1,2-bis(methylsulfonyl)-1-methylhydrazine on morphology, DNA content and mitochondrial function of Trypanosoma brucei subspecies. J Protozool. 1991;38:172–177. doi: 10.1111/j.1550-7408.1991.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 15.Scory S, Stierhof YD, Caffrey CR, Steverding D. The cysteine proteinase inhibitor Z-Phe-Ala-CHN2 alters cell morphology and cell division activity of Trypanosoma brucei bloodstream forms in vivo. Kinetoplastid Biol Dis. 2007;6:2. doi: 10.1186/1475-9292-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Engstler M, Boshart M. Cold shock and regulation of surface protein trafficking convey sensitization to inducers of stage differentiation in Trypanosoma brucei. Genes Dev. 2004;18:2798–2811. doi: 10.1101/gad.323404. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that a reduction of >15 °C stimulates the expression of EP procyclin, with stumpy forms trafficking this to the cell surface. Furthermore, at low temperature stumpy forms exhibit greatly enhanced sensitivity to cis aconitate with respect to their initiation of differentiation to procyclic forms. A new model is presented which relates ambient temperature to the potential for differentiation from stumpy forms to procyclic forms in the tsetse fly.
- 17.Czichos J, Nonnengaesser C, Overath P. Trypanosoma brucei: cis-aconitate and temperature reduction as triggers of synchronous transformation of bloodstream to procyclic trypomastigotes in vitro. Exp Parasitol. 1986;62:283–291. doi: 10.1016/0014-4894(86)90033-0. [DOI] [PubMed] [Google Scholar]
- 18.Brun R, Schönenberger M. Stimulating effect of citrate and cis-Aconitate on the transformation of Trypanosoma brucei blood-stream forms to procyclic forms in vitro. Zeitschrift Fur Parasitenkunde-Parasitol Res. 1981;66:17–24. doi: 10.1007/BF00941941. [DOI] [PubMed] [Google Scholar]
- 19.Sbicego S, Vassella E, Kurath U, Blum BIR. The use of transgenic Trypanosoma brucei to identify compounds inducing the differentiation of bloodstream forms to procyclic forms. Mol Biochem Parasitol. 1999;104:311–322. doi: 10.1016/s0166-6851(99)00157-7. [DOI] [PubMed] [Google Scholar]
- 20.Hunt M, Brun R, Kohler P. Studies on compounds promoting the in vitro transformation of Trypanosoma brucei from bloodstream to procyclic forms. Parasitol Res. 1994;80:600–606. doi: 10.1007/BF00933009. [DOI] [PubMed] [Google Scholar]
- 21.Matthews KR, Gull K. Cycles within cycles: the interplay between differentiation and cell division in Trypanosoma brucei. Parasitol Today. 1994;10:473–476. doi: 10.1016/0169-4758(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 22.Matthews KR, Gull K. Evidence for an interplay between cell cycle progression and the initiation of differentiation between life cycle forms of African trypanosomes. J Cell Biol. 1994;125:1147–1156. doi: 10.1083/jcb.125.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro SZ, Naessens J, Liesegang B, Moloo SK, Magondu J. Analysis by flow cytometry of DNA synthesis during the life cycle of African trypanosomes. Acta Trop. 1984;41:313–323. [PubMed] [Google Scholar]
- 24.Ziegelbauer K, Quinten M, Schwarz H, Pearson TW, Overath P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. Eur J Biochem. 1990;192:373–378. doi: 10.1111/j.1432-1033.1990.tb19237.x. [DOI] [PubMed] [Google Scholar]
- 25••.Szoor B, Wilson J, McElhinney H, Tabernero L, Matthews KR. Protein tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in trypanosomes. J Cell Biol. 2006;175:293–303. doi: 10.1083/jcb.200605090. [DOI] [PMC free article] [PubMed] [Google Scholar]; A classic tyrosine phosphatase is identified whose inactivation leads to the spontaneous differentiation from stumpy forms to procyclic forms in the absence of other triggers for this process. This implicates TbPTP1 as a ‘brake’ inhibiting differentiation in the bloodstream until the appropriate differentiation signal is received upon uptake of parasites into the tsetse midgut.
- 26.Wiesmann C, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, Fahr BJ, Zhong M, Taylor L, Randal M, et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11:730–737. doi: 10.1038/nsmb803. [DOI] [PubMed] [Google Scholar]
- 27.Rolin S, Hancocq-Quertier J, Paturiaux-Hanocq F, Nolan DP, Pays E. Mild acid stress as a differentiation trigger in Trypanosoma brucei. Mol Biochem Parasitol. 1998;93:251–262. doi: 10.1016/s0166-6851(98)00046-2. [DOI] [PubMed] [Google Scholar]
- 28.Gruszynski AE, van Deursen FJ, Albareda MC, Best A, Chaudhary K, Cliffe LJ, del Rio L, Dunn JD, Ellis L, Evans KJ, et al. Regulation of surface coat exchange by differentiating African trypanosomes. Mol Biochem Parasitol. 2006;147:211–223. doi: 10.1016/j.molbiopara.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Brems S, Guilbride DL, Gundlesdodjir-Planck D, Busold C, Luu VD, Schanne M, Hoheisel J, Clayton C. The transcriptomes of Trypanosoma brucei Lister 427 and TREU927 bloodstream and procyclic trypomastigotes. Mol Biochem Parasitol. 2005;139:163–172. doi: 10.1016/j.molbiopara.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Schürch N, Furger A, Kurath U, Roditi I. Contributions of the procyclin 3′ untranslated region and coding region to the regulation of expression in bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1997;89:109–121. doi: 10.1016/s0166-6851(97)00107-2. [DOI] [PubMed] [Google Scholar]
- 31.Webb H, Burns R, Ellis L, Kimblin N, Carrington M. Developmentally regulated instability of the GPI-PLC mRNA is dependent on a short-lived protein factor. Nucleic Acids Res. 2005;33:1503–1512. doi: 10.1093/nar/gki298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayho M, Fenn K, Craddy P, Crosthwaite S, Matthews K. Post-transcriptional control of nuclear-encoded cytochrome oxidase subunits in Trypanosoma brucei: evidence for genome-wide conservation of life-cycle stage-specific regulatory elements. Nucleic Acids Res. 2006 doi: 10.1093/nar/gkl598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clayton CE. Life without transcriptional control? From fly to man and back again. EMBO J. 2002;21:1881–1888. doi: 10.1093/emboj/21.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb H, Burns R, Kimblin N, Ellis L, Carrington M. A novel strategy to identify the location of necessary and sufficient cis-acting regulatory mRNA elements in trypanosomes. RNA. 2005;11:1108–1116. doi: 10.1261/rna.2510505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 37.De Gaudenzi J, Frasch AC, Clayton C. RNA-binding domain proteins in kinetoplastids: a comparative analysis. Eukaryot Cell. 2005;4:2106–2114. doi: 10.1128/EC.4.12.2106-2114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jager AV, De Gaudenzi JG, Cassola A, D’Orso I, Frasch AC. mRNA maturation by two-step trans-splicing/polyadenylation processing in trypanosomes. Proc Natl Acad Sci U S A. 2007;104:2035–2042. doi: 10.1073/pnas.0611125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartmann C, Benz C, Brems S, Ellis L, Luu VD, Stewart M, D’Orso I, Busold C, Fellenberg K, Frasch AC, et al. The small trypanosome RNA-binding proteins TbUBP1 and TbUBP2 influence expression of F-box protein mRNAs in bloodstream trypanosomes. Eukaryot Cell. 2007 Sep 14; doi: 10.1128/EC.00279-07. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendriks EF, Matthews KR. Disruption of the developmental programme of Trypanosoma brucei by genetic ablation of TbZFP1, a differentiation-enriched CCCH protein. Mol Microbiol. 2005;57:706–716. doi: 10.1111/j.1365-2958.2005.04679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterou A, Walrad P, Craddy P, Fenn K, Matthews K. Identification and stage-specific association with the translational apparatus of TbZFP3, a CCCH protein that promotes trypanosome life cycle development. J Biol Chem. 2006;281(51):39002–39013. doi: 10.1074/jbc.M604280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 43.Janzen CJ, van Deursen F, Shi H, Cross GA, Matthews KR, Ullu E. Expression site silencing and life-cycle progression appear normal in Argonaute1-deficient Trypanosoma brucei. Mol Biochem Parasitol. 2006;149:102–107. doi: 10.1016/j.molbiopara.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Janzen CJ, Hake SB, Lowell JE, Cross GA. Selective di- or trimethylation of histone H3 lysine 76 by two DOT1 homologs is important for cell cycle regulation in Trypanosoma brucei. Mol Cell. 2006;23:497–507. doi: 10.1016/j.molcel.2006.06.027. [DOI] [PubMed] [Google Scholar]; Identification and biochemical characterisation of the enzymes involved in histone modification in Trypanosoma brucei. As well as involvement in cell-cycle control, one enzyme, DOT1B, may have a role in life cycle development between bloodstream and procyclic forms.
- 45.Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414:759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- 46.Landeira D, Navarro M. Nuclear repositioning of the VSG promoter during developmental silencing in Trypanosoma brucei. J Cell Biol. 2007;176:133–139. doi: 10.1083/jcb.200607174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller IB, Domenicali-Pfister D, Roditi I, Vassella E. Stage-specific requirement of a mitogen-activated protein kinase by Trypanosoma brucei. Mol Biol Cell. 2002;13:3787–3799. doi: 10.1091/mbc.E02-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammarton TC, Clark J, Douglas F, Boshart M, Mottram JC. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J Biol Chem. 2003;278:22877–22886. doi: 10.1074/jbc.M300813200. [DOI] [PubMed] [Google Scholar]
- 49.Ellis J, Sarkar M, Hendriks E, Matthews K. A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal extension. Mol Microbiol. 2004;53:1487–1499. doi: 10.1111/j.1365-2958.2004.04218.x. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Salcedo JA, Nolan DP, Gijon P, Gomez-Rodriguez J, Pays E. A protein kinase specifically associated with proliferative forms of Trypanosoma brucei is functionally related to a yeast kinase involved in the co-ordination of cell shape and division. Mol Microbiol. 2002;45:307–319. doi: 10.1046/j.1365-2958.2002.03019.x. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Wang CC. Changing roles of aurora-B kinase in two life cycle stages of Trypanosoma brucei. Eukaryot Cell. 2006;5:1026–1035. doi: 10.1128/EC.00129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hua SB, Wang CC. Differential accumulation of a protein kinase homolog in Trypanosoma brucei. J Cell Biochem. 1994;54:20–31. doi: 10.1002/jcb.240540104. [DOI] [PubMed] [Google Scholar]
- 53•.Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]; Complete analysis of the kinases encoded within the genomes of kinetoplastid parasites. These organisms completely lack tyrosine-specific kinase activity, this role being replaced by dual specificity kinases. A complementary analysis of the phosphatase complement of the kinetoplastids reveals a corresponding paucity of classic tyrosine phosphatases (R Brenchley, H Tariq, H McElhinney, B Szoor, R Stevens, K Matthews, L Tabernero, unpublished data).
- 54.Parsons M, Valentine M, Deans J, Schieven GL, Ledbetter JA. Distinct patterns of tyrosine phosphorylation during the life cycle of Trypanosoma brucei. Mol Biochem Parasitol. 1991;45:241–248. doi: 10.1016/0166-6851(91)90091-j. [DOI] [PubMed] [Google Scholar]
- 55.Nascimento M, Zhang WW, Ghosh A, Houston DR, Berghuis AM, Olivier M, Matlashewski G. Identification and characterization of a protein tyrosine phosphatase in Leishmania; involvement in virulence. J Biol Chem. 2006 doi: 10.1074/jbc.M606256200. [DOI] [PubMed] [Google Scholar]
- 56.Bringaud F, Riviere L, Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. 2006;149:1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 57•.Vassella E, Probst M, Schneider A, Studer E, Renggli CK, Roditi I. Expression of a major surface protein of Trypanosoma brucei insect forms is controlled by the activity of mitochondrial enzymes. Mol Biol Cell. 2004;15:3986–3993. doi: 10.1091/mbc.E04-04-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]; The expression of GPEET procyclin is shown to be regulated by mitochondrial activity, with repression of the succinate-CoA transferase/succinyl-CoA synthetase cycle causing re-expression of GPEET in late procyclic forms. This extends previous work in which GPEET expression was found to be regulated in culture by glycerol via a sequence in the 3′UTR of the GPEET mRNA, the so-called ‘glycerol-response element’.
- 58.Schlaeppi AC, Malherbe T, Butikofer P. Coordinate expression of GPEET procyclin and its membrane-associated kinase in Trypanosoma brucei procyclic forms. J Biol Chem. 2003;278:49980–49987. doi: 10.1074/jbc.M309548200. [DOI] [PubMed] [Google Scholar]
- 59.Vassella E, Acosta-Serrano A, Studer E, Lee SH, Englund PT, Roditi I. Multiple procyclin isoforms are expressed differentially during the development of insect forms of Trypanosoma brucei. J Mol Biol. 2001;312:597–607. doi: 10.1006/jmbi.2001.5004. [DOI] [PubMed] [Google Scholar]
- 60.Acosta-Serrano A, Vassella E, Liniger M, Kunz Renggli C, Brun R, Roditi I, Englund PT. The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc Natl Acad Sci U S A. 2001;98:1513–1518. doi: 10.1073/pnas.041611698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urwyler S, Vassella E, Van Den Abbeele J, Renggli CK, Blundell P, Barry JD, Roditi I. Expression of procyclin mRNAs during cyclical transmission of Trypanosoma brucei. PLoS Pathog. 2005;1:e22. doi: 10.1371/journal.ppat.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Den Abbeele J, Claes Y, van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118(Pt 5):469–478. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- 63•.Urwyler S, Studer E, Renggli CK, Roditi I. A family of stage-specific alanine-rich proteins on the surface of epimastigote forms of Trypanosoma brucei. Mol Microbiol. 2007;63:218–228. doi: 10.1111/j.1365-2958.2006.05492.x. [DOI] [PubMed] [Google Scholar]; The BARP protein is demonstrated to be a surface glycoprotein specific to epimastigote forms. Although earlier analyses implicated BARP expression in bloodstream forms, this paper demonstrates their specificity for tsetse salivary gland epimastigote forms.
- 64.Gibson W, Peacock L, Ferris V, Williams K, Bailey M. Analysis of a cross between green and red fluorescent trypanosomes. Biochem Soc Trans. 2006;34:557–559. doi: 10.1042/BST0340557. [DOI] [PubMed] [Google Scholar]
- 65.Tetley L, Turner CM, Barry JD, Crowe JS, Vickerman K. Onset of expression of the variant surface glycoproteins of Trypanosoma brucei in the tsetse fly studied using immunoelectron microscopy. J Cell Sci. 1987;87(Pt 2):363–372. doi: 10.1242/jcs.87.2.363. [DOI] [PubMed] [Google Scholar]