Abstract
Objective
Accurate, efficient and cost-effective disposition of patients presenting to emergency departments (EDs) with symptoms suggestive of acute coronary syndromes (ACS) is a growing priority. Platelet activation is an early feature in the pathogenesis of ACS; thus, we sought to obtain an insight into whether point-of-care testing of platelet function: (1) may assist in the rule-out of ACS; (2) may provide additional predictive value in identifying patients with non-cardiac symptoms versus ACS-positive patients and (3) is logistically feasible in the ED.
Design
Prospective cohort feasibility study.
Setting
Two urban tertiary care sites, one located in the USA and the second in Argentina.
Participants
509 adult patients presenting with symptoms of ACS.
Main outcome measures
Platelet reactivity was quantified using the Platelet Function Analyzer-100, with closure time (seconds required for blood, aspirated under high shear, to occlude a 150 µm aperture) serving as the primary endpoint. Closure times were categorised as ‘normal’ or ‘prolonged’, defined objectively as the 90th centile of the distribution for all participants enrolled in the study. Diagnosis of ACS was made using the standard criteria. The use of antiplatelet agents was not an exclusion criterion.
Results
Closure times for the study population ranged from 47 to 300 s, with a 90th centile value of 138 s. The proportion of patients with closure times ≥138 s was significantly higher in patients with non-cardiac symptoms (41/330; 12.4%) versus the ACS-positive cohort (2/105 (1.9%); p=0.0006). The specificity of ‘prolonged’ closure times (≥138 s) for a diagnosis of non-cardiac symptoms was 98.1%, with a positive predictive value of 95.4%. Multivariate analysis revealed that the closure time provided incremental, independent predictive value in the rule-out of ACS.
Conclusions
Point-of-care assessment of platelet reactivity is feasible in the ED and may facilitate the rapid rule-out of ACS in patients with prolonged closure times.
Keywords: Acute coronary Syndromes, Platelet, Emergency Department:
Strengths and limitations of this study.
The study suggests that a technically straightforward and cost-effective test, with minimal patient risk, may serve as a useful adjunct to the current, standard emergency department practices for the rule-out of acute coronary syndromes. Importantly, the results are generalisable: the observation of a higher incidence of prolonged closure times in patients with non-cardiac symptoms was seen in participants from two distinct healthcare systems and populations.
Platelet Function Analyzer-100 testing will only contribute to the identification of a subset of patients with non-cardiac symptoms, with the size of the subset and the potential value of the test dependent on the threshold used to define the ‘prolonged’ closure times.
Limitations of this pilot study include the enrolment of patients via convenience sampling, the fact that patient outcomes were not monitored beyond hospital discharge and that a risk/benefit analysis was not included in the study design.
Accurate, efficient and cost-effective diagnosis of patients presenting to emergency departments (EDs) with symptoms suggestive of acute coronary syndromes (ACS)—and, in particular, the exclusion and early discharge of patients with non-cardiac chest pain—is a growing priority.1 2 In an effort to meet this challenge, interest has focused on the identification of new approaches to augment the standard ED procedures and facilitate the timely triage of patients with suspected ACS. For example, there is recent evidence that coronary CT angiography (CCTA) combined with routine clinical assessment may provide added prognostic value in the management of chest pain patients in the ED.3–8 Use of CCTA in low-to-intermediate risk patients is reportedly safe and reduces ED costs and hospital length of stay.3–5 7 8 However, these benefits are accompanied by exposure to radiation and associated with increases in diagnostic testing and subsequent invasive procedures.5 8
Assessment of platelet activation, an early feature in the pathogenesis of ACS,9–14 has also been investigated as a possible benign strategy to expedite the diagnosis of ACS.15 16 Application of flow cytometry, the ‘gold standard’ for the quantitation of molecular indices of platelet activation, is, however, impractical for routine use under emergent conditions.11 12 17 18 More importantly, classic molecular indices of platelet activation have not provided added benefit in the risk stratification of undifferentiated chest pain patients.15 16
We hypothesised that rapid assessment of platelet reactivity using a technically straightforward point-of-care device—specifically, the Platelet Function Analyzer (PFA)-100 (Siemens)—may represent a more feasible strategy to assist in the timely rule-out of ACS in the ED. Rather than quantifying molecular markers of platelet activation-aggregation, the output of the PFA-100 is the ‘closure time’: that is, the time required for whole blood, aspirated under high shear, to occlude a small, 150 μm aperture in a membrane coated with standard platelet agonists (collagen-ADP or collagen-epinephrine). Although the PFA-100 is typically utilised to investigate the responsiveness of patients to aspirin and other antiplatelet therapies and aid in the detection of platelet dysfunction,11 12 18–22, there is evidence to suggest that shortened closure times may be a marker of the acuity of coronary disease.10 Accordingly, our primary aims in this pilot study were to determine whether point-of-care testing of platelet function: (1) may assist in the rule-out of ACS; (2) may yield additional predictive value in identifying patients with non-cardiac symptoms versus ACS-positive patients and (3) is logistically feasible in an emergent setting. In addition, as a secondary analysis, we investigated whether the closure time may be of value in discriminating between ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI)/unstable angina (UA).
Methods
We conducted a prospective cohort feasibility study of patients presenting with potential ACS at two urban tertiary care sites: the ED at the University of Massachusetts (UMASS)-Memorial Medical Center, University Campus, Worcester, Massachusetts, USA, and the Cardiology Department, Instituto Modelo de Cardiologia Privado SRL, Cordoba, Argentina. The enrolment period was from January 2007 to December 2010. At each site, patients were entered on a convenience basis: that is, enrolments were not consecutive but, rather, coincided with the work schedules of the study investigators.
Patient population
Patients ≥18 years of age with a potential diagnosis of ACS (at the discretion of the treating physician and reflected by orders for an ECG and cardiac biomarkers) were considered for enrolment in the study. The prospective exclusion criteria were pregnancy, renal insufficiency (defined as serum creatine levels ≥1.5 mg/dL), anaemia (haematocrit <30%), platelet count <100 000/µL, major bleeding, any gastrointestinal bleeding, trauma or the inability to provide informed consent for any reason. The use of antiplatelet agents was not an exclusion criterion.
Study enrolment and protocol
As soon as possible after evaluation by ED staff, potential participants were approached for enrolment into the study. If the patient was too ill or otherwise unable to provide written consent, then proxy consent was attempted. If consent was obtained by proxy and the patient later became cognisant, they were given the option to continue or withdraw their participation in the study. If consent was withdrawn, all collected data were discarded.
Blood used in the PFA-100 was drawn within <1 h after presentation, at the same time as cardiac biomarker testing, and the closure time was measured within 3 h of collection. All samples were obtained via peripheral venipuncture and drawn into tubes containing 3.2% sodium citrate. Immediately before analysis, each sample was gently inverted; a 900 μL aliquot of whole blood was then applied to a collagen-ADP test cartridge and the closure time was quantified. Maximum test duration for the PFA-100 is 5 min; if the aperture is not occluded within this period, a closure time of ‘>300 s’ is displayed.
On enrolment, patients were questioned regarding a history of bleeding or platelet function disorders and ingestion of known antiplatelet agents within the past 7 days (aspirin, clopidogrel, non-steroidal anti-inflammatory agents). In addition, the medical record was reviewed and the standard cardiac risk factors (diabetes, hypertension, hypercholesterolaemia, smoking, family history of heart disease) were recorded and used in the calculation of thrombolysis in myocardial infarction (TIMI) risk score.23
Patient diagnosis
The final diagnosis (non-cardiac symptoms vs ACS-positive) was established via a standardised chart review. All reviews were conducted by physicians in a blinded manner, without knowledge of the closure time data. For participants enrolled at the Instituto Modelo de Cardiologia Privado, patients were seen by a cardiologist at the time of hospital presentation, and all reviews were performed by a team of cardiologists (WQ-C, GFT, FRZ, ECC and JPS). At UMASS, each patient was first seen by an Emergency Medicine physician at the time of hospital presentation, and each chart was reviewed by an ED physician (CED). For patients with a definitive diagnosis of non-cardiac symptoms (ie, patients with no ischaemic ECG changes, no positive markers or provocative cardiovascular test results, and no history of a diagnosis of ACS of any type in the chart), no additional review was performed. However, for patients who: (1) had positive cardiac biomarkers during admission; (2) underwent cardiac catheterisation; (3) had a positive provocative cardiovascular test (eg, exercise stress test) or (4) had ischaemic ECG changes, the chart was reviewed by an ED physician and a cardiologist. In any case in which the diagnosis was uncertain, the decision was adjudicated by a cardiologist (CSS).
Patients considered to be ACS positive were categorised into one of the two groups, STEMI or NSTEMI/UA, in accordance with the standard guidelines.24–26 Patients who did not meet these criteria were classified as having a diagnosis of non-cardiac chest pain/symptoms.
Statistical analysis
Our target sample size in this exploratory study was empiric. We reasoned that, as an approximate order of magnitude, ∼100 patients would be required in the ACS-positive group in order to have the likelihood of discerning a potential difference in the closure times between patients with non-cardiac symptoms and the ACS-positive cohort. Assuming that 75% of patients enrolled in the study would have a diagnosis of non-cardiac chest pain (and thus 25% would be ACS positive), a total of approximately 400 patients would be required. To account for exclusions, target enrolment was set at ∼500 participants.
Univariate comparisons
Our primary analyses focused on the comparison of patients in the two main outcome groups: non-cardiac symptoms versus ACS positive. All categorical data were compared using the Fisher's exact test and are reported as percentages. For continuous data, the D'Agostino and Pearson test was applied to determine normality and the appropriate parametric (t test) or non-parametric alternative (Mann-Whitney) tests were utilised. Secondary analysis (comparison of closure times among non-cardiac chest pain, STEMI and NSTEMI/UA groups) was conducted using the Kruskal-Wallis test. Results are reported as mean±SD or medians with associated 10th and 90th centile ranges.
Sensitivity, specificity and multivariate analysis
We made the prospective and arbitrary decision to categorise the closure time into ‘normal’ and “prolonged” values based on the 90th centile of the distribution for all patients enrolled in the study. Utilising the normal and prolonged values of closure time, the sensitivity, specificity, negative and positive predictive values and likelihood ratio for identifying patients with non-cardiac symptoms were determined. Two reasons contributed to our choice of non-cardiac chest pain (rather than ACS positive) as the main outcome of interest: (1) this approach is consistent with current guidelines for the improvement of diagnostic accuracy1 8 and (2) we reasoned that prolonged closure times may be associated with the absence of ACS, whereas shorter closure times may be manifest in either group of patients. Sensitivity, specificity, predictive values and the likelihood ratio are reported with associated 95% CIs.
To determine whether the PFA closure time provides an additional predictive value to the standard clinical diagnostic information, a logistic regression was developed to assess its independent association with the main outcome of non-cardiac symptoms. Independent predictor variables considered to be mandatory in the model were the closure time (utilising continuous rather than the categorised values; the main predictor of interest) and the TIMI risk score (representing standard clinical information to predict ACS outcomes). To account for potential differences between UMASS and Cordoba, the study site was also added to the regression model. Other non-mandatory variables considered for inclusion were sex, a medical history of diabetes, hypertension, hypercholesterolaemia, smoking and clopidogrel use. As age and aspirin use are individually incorporated in the TIMI risk score, these variables were not considered for separate inclusion in the model. Non-mandatory variables were left in the model only if they yielded an increase in predictive value as determined by receiver operating characteristic (ROC) analysis or were of additional importance based on the 2 log-likelihood ratio test.
The reported estimate and adjusted OR for closure time were calculated for an increase in platelet closure time of 10 s (rather than 1 s), a choice based on the premise that a 1 s increase would be of limited clinical usefulness. The final model was evaluated by c-statistic (area under the ROC curve) and the Hosmer and Lemeshow fit test. As a sensitivity test, the results were calculated using the general estimating equation (PROC GENMOD) with study site evaluated as a subject factor (cluster) rather than a term in the model. The Net Reclassification Index (NRI) and Integrated Discrimination Improvement (IDI) statistic were not included in the analyses, given the scepticism and criticisms that have been raised concerning their value in predicting the potential, incremental prognostic impact of novel biomarkers.27 28
Analyses were performed using GraphPad Prism V.5.04 (San Diego, California, USA) and SAS V.9.3 (Carey, North Carolina, USA).
Results
Enrolment and exclusions
A combined total of 509 patients were enrolled at the two study sites (figure 1). Sixty-one participants were excluded because the closure time data were not available. The reasons included technical errors (n=14), closure times >300 s (possibly due to mild thrombocytopenia, anaemia or inadequate mixing of the blood sample prior to testing; n=36) and failure to measure the closure time despite obtaining consent (n=11). An additional five participants were removed from analysis because exclusion criteria were identified after consent was obtained (n=4), or consent was revoked after the blood sample was collected (n=1). For the remaining 443 patients in whom the closure time was quantified, 8 were diagnosed with thrombotic events that were non-cardiac in origin and thus were excluded from further analysis. Accordingly, results are reported for 435 participants (324 enrolled at UMASS and 111 enrolled in Cordoba): 105 were diagnosed with ACS and 330 had non-cardiac symptoms.
Figure 1.
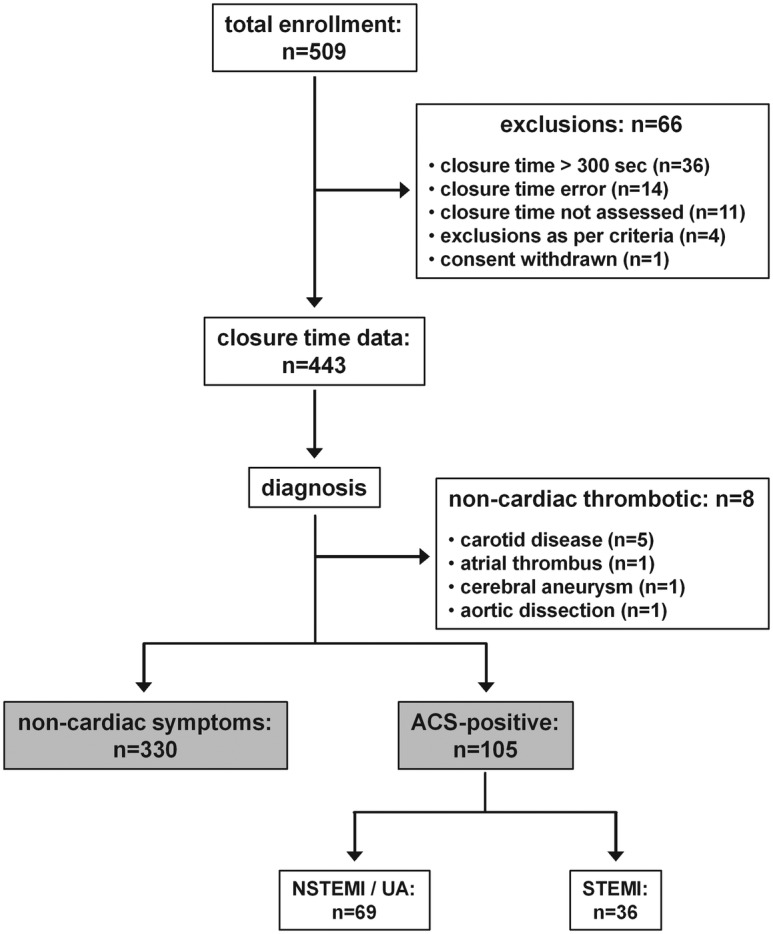
Inclusion flow chart.
Demographics
As expected,29 the ACS-positive group had higher TIMI scores, was older, and had a higher proportion of male participants versus patients with non-cardiac symptoms (table 1). In addition, the incidence of hypercholesterolaemia and the use of clopidogrel were significantly higher in the ACS group. There were, however, no differences in the use of aspirin, incidence of hypertension or diabetes, proportion of smokers or a reported family history of heart disease between the two groups.
Table 1.
Demographics: all patients
| Non-cardiac symptoms (total n=330) | ACS positive (total n=105) | p Value | |
|---|---|---|---|
| Age (years): mean±SD | 57±14 (n=328) | 61±13 (n=104) | 0.034 |
| Male | 65% (n=330) | 80% (n=105) | 0.004 |
| TIMI score: mean±SD | 1.9±1.4 (n=320) | 3.1±1.4 (n=104) | <0.0001 |
| Aspirin | 71% (n=321) | 64% (n=104) | 0.222 (ns) |
| Clopidogrel | 11% (n=325) | 20% (n=104) | 0.031 |
| Smoker | 29% (n=322) | 28% (n=105) | 0.901(ns) |
| Hypertension | 57% (n=322) | 62% (n=105) | 0.425 (ns) |
| Hypercholesterolaemia | 57% (n=322) | 69% (n=105) | 0.030 |
| Diabetes | 24% (n=322) | 27% (n=105) | 0.601 (ns) |
| Family history | 40% (n=319) | 41% (n=105) | 0.909 (ns) |
ACS, acute coronary syndromes; TIMI, thrombolysis in myocardial infarction.
Closure time
There was a modest but significant difference in the median closure times in patients with non-cardiac symptoms versus the ACS-positive group: 91 vs 87 s, respectively (p=0.0061; figure 2A). When the ACS-positive group was divided into STEMI and combined NSTEMI/UA cohorts, the modest differences in the closure time in the secondary three-group analysis remained significant (p=0.0001; figure 2C).
Figure 2.
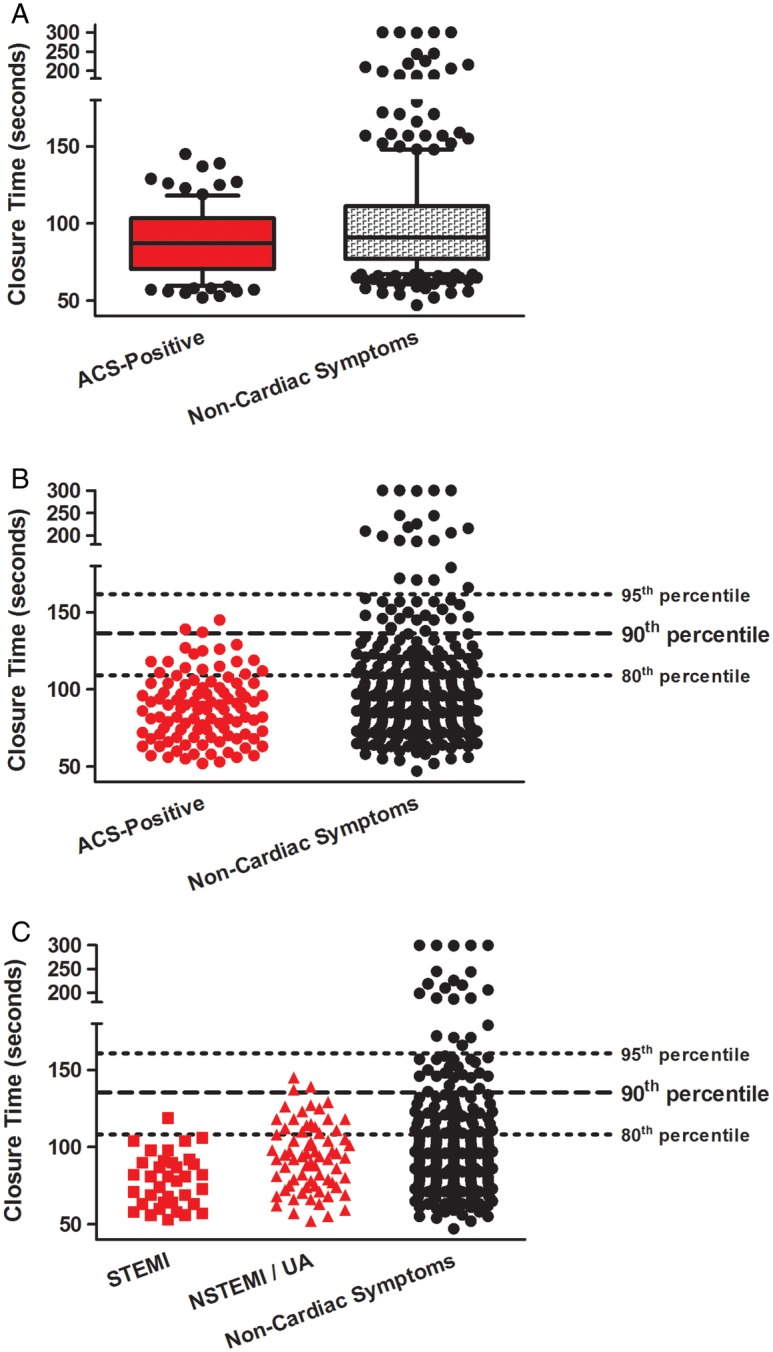
Platelet Function Analyzer-100 closure time (s). (A) Median values with 10th, 25th, 75th and 90th centiles: acute coronary syndromes (ACS)-positive patients and patients with non-cardiac symptoms. (B) Individual data points for all participants: ACS-positive patients and patients with non-cardiac symptoms. Lines denote the 80th, 90th and 95th centiles of closure times for all patients enrolled in the study. (C) Individual data points for all participants: ST-elevation myocardial infarction, non-ST-elevation myocardial infarction/unstable angina cohorts and patients with non-cardiac symptoms. Lines denote the 80th, 90th and 95th centiles of closure times for all patients enrolled in the study.
Of potentially greater importance, patients with non-cardiac symptoms versus ACS-positive patients were distinguished by differences in the proportion of the prolonged closure times. We found that 41/330 (12.4%) of patients with non-cardiac symptoms had closure times ≥138 s (ie, the 90th centile of the distribution for all patients enrolled in the study) while, in contrast, 2/105 (1.9%) of patients in the ACS-positive group had closure times ≥ this value (p=0.0006; figure 2B and table 2). The specificity and sensitivity of ‘prolonged’ closure times (≥138 s) for a diagnosis of non-cardiac symptoms were 98.1% and 12.4%, respectively, with a positive predictive value of 95.4%, a negative predictive value of 26.3% and a likelihood ratio of 6.52 (table 3).
Table 2.
Incidence of prolonged closure time (≥138 s, defined as the 90th centile of the distribution of the study population)
| Non-cardiac symptoms | ACS positive | Total | |
|---|---|---|---|
| Prolonged closure time | |||
| Yes | 41 | 2 | 43 |
| No | 289 | 103 | 392 |
| Total | 330 | 105 | 435 |
ACS, acute coronary syndromes.
Table 3.
Sensitivity, specificity, positive and negative predictive values and likelihood ratio of prolonged closure time for a diagnosis of non-cardiac symptoms
| 95% CI | ||
|---|---|---|
| Sensitivity (%) | 12.4 | 9.1 to 16.5 |
| Specificity (%) | 98.1 | 93.3 to 99.8 |
| Positive predictive value (%) | 95.4 | 84.2 to 99.4 |
| Negative predictive value (%) | 26.3 | 22.0 to 30.9 |
| Likelihood ratio | 6.52 | 1.61 to 26.51 |
Site differences: UMASS versus Cordoba
Patients at both sites displayed the expected differences in TIMI score, age and sex between non-cardiac chest pain and ACS-positive groups (data not shown). However, the participants enrolled in Cordoba were significantly younger, with a higher proportion of males and smokers but lower proportion of patients with diabetes, when compared with UMASS (table 4). In addition, and as expected,30 31 aspirin use was significantly lower among the cohort in Cordoba versus the UMASS population (40% vs 80%, p<0.0001, table 4). Despite these demographic differences, a higher incidence of prolonged closure times in patients with non-cardiac symptoms was observed at both sites: that is, the proportion of participants with closure times ≥138 s was 13% vs 10% in patients with non-cardiac chest pain and 1.9% vs 2% in ACS-positive patients at UMASS versus Cordoba, respectively.
Table 4.
Demographics: UMASS vs Cordoba
| UMASS all (total n=324) | Cordoba all (total n=111) | p Value | |
|---|---|---|---|
| Age (years): mean±SD | 59±14 (n=324) | 56±12 (n=108) | 0.036 |
| Male | 65% (n=324) | 78% (n=111) | 0.009 |
| TIMI score: mean±SD | 2.3±1.5 (n=313) | 2.0±1.4 (n=111) | 0.142 (ns) |
| Aspirin | 80% (n=314) | 40% (n=104) | <0.0001 |
| Clopidogrel | 15% (n=319) | 9% (n=111) | 0.146 (ns) |
| Smoker | 26% (n=316) | 37% (n=111) | 0.038 |
| Hypertension | 61% (n=316) | 51% (n=111) | 0.093 (ns) |
| Hypercholesterolaemia | 62% (n=316) | 53% (n=111) | 0.115 (ns) |
| Diabetes | 28% (n=313) | 14% (n=111) | 0.005 |
| Family History | 44% (n=313) | 31% (n=51) | 0.014 |
TIMI, thrombolysis in myocardial infarction.
Closure time as an independent predictor of diagnosis
Logistic regression analysis was first performed with mandatory variables (closure time, TIMI risk score) and non-mandatory variables (study site, sex, diabetes, hypertension, hypercholesterolaemia, smoking, clopidogrel use) included in the regression model (data not shown). ROC analysis revealed that, among the non-mandatory variables, only study site (UMASS vs Cordoba) contributed to an increase to the predictive value of the model for a diagnosis of non-cardiac symptoms. Comparison of ROC curves obtained by including the closure time, TIMI score and site in the model versus TIMI score and site alone showed a significant, incremental increase in area under the curve with the addition of closure time (0.818 vs 0.795; p=0.009; figure 3). Accordingly, the final multivariate logistic regression model incorporated these three variables (table 5).
Figure 3.
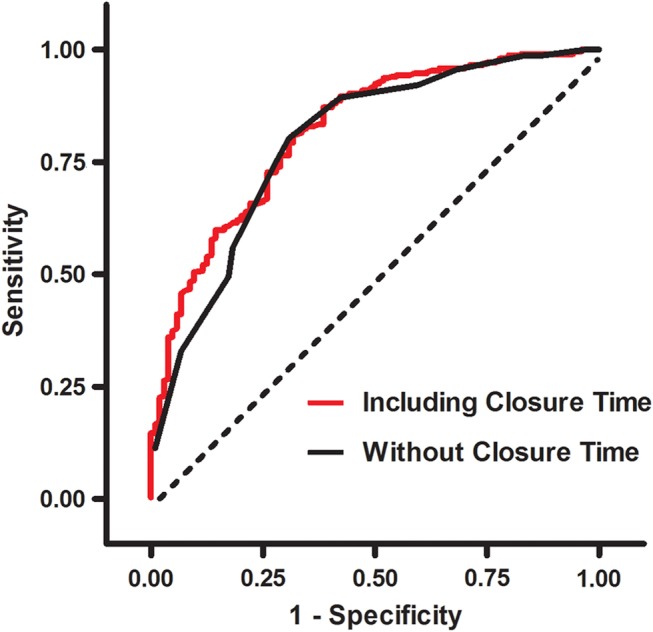
Receiver operating characteristic (ROC) analysis. Comparison of ROC curves obtained by including the closure time, thrombolysis in myocardial infarction (TIMI) score and study site in the regression model versus the TIMI score and site alone showing a significant, incremental increase in area under the curve with the addition of closure time (0.818 vs 0.795; p=0.009).
Table 5.
Multivariable logistic regression model (outcome modeled: non-cardiac symptoms)
| Predictor | Adjusted OR | 95% CI |
|---|---|---|
| Closure time | 1.17 | 1.06 to 1.29 |
| TIMI risk score | 0.48 | 0.40 to 0.59 |
| Study site | 7.21 | 4.05 to 12.86 |
(UMASS vs Cordoba).
TIMI, thrombolysis in myocardial infarction.
For every 10 s increase in the closure time, the adjusted odds of a diagnosis of non-cardiac symptoms was 1.17 (95% CI 1.06 to 1.29; table 5). That is, when controlling for TIMI risk score and study site: for every 10 s increase, the prolonged closure time was associated with a 17% increase in the patient having non-cardiac chest pain. These results demonstrate that, irrespective of TIMI score and site, the closure time was associated with the diagnosis of non-cardiac symptoms, with closure time providing additional, incremental predictive value beyond that obtained by TIMI score. The model demonstrated good predictive characteristics (c=0.82) and model fit (χ2=5.24, df=8; p=0.73). Finally, the sensitivity analysis that utilised a general estimating equation accounting for potential correlations among sites did not result in any important changes in the direction of the effects (adjusted odds for every 10 s increase in closure time: 1.15 with 95% CI 1.10 to 1.23) or conclusions.
Discussion
In this study, we demonstrate that point-of-care testing of platelet reactivity using the PFA-100 is feasible in the environment of the ED. The results of our primary analysis revealed differences in closure times between patients with non-cardiac symptoms versus ACS-positive cohorts, with the most notable, discriminating feature being the higher incidence of prolonged closure times in the group with non-cardiac chest pain. Finally, the outcome of our multivariate analysis is consistent with the concept that the assessment of closure time provides incremental, independent prognostic value beyond that obtained using the standard clinical predictor of TIMI score.
Assessment of platelet reactivity as a diagnostic tool for ACS in emergent settings
It is well-established that heightened platelet activity occurs in the setting of ACS.9–11 13 14 32 Indeed, measurement of platelet reactivity has been utilised in an effort to discern the stability of coronary disease,10 predict the future incidence and outcomes of major adverse cardiovascular events,11 33–37 and, although results have been disappointing, guide the dosing of antiplatelet agents with the goal of improving outcomes.32 38 39 Our current study differs from these previous reports, in that it focused on the assessment of platelet reactivity as an adjunct strategy to risk stratify patients with potential ACS in emergent conditions. Accordingly, the novelty of our study lies in our expanded analysis of the closure time, and the identification of a more practical and feasible application of these data in the prognosis of ACS.
Our observation of a modest but significant reduction in the median/mean closure times in patients with ACS is consistent with the outcomes of two previous, small ED studies.40 41 However, this ∼4 s difference is of a limited practical significance given the broad and overlapping distributions of closure times for patients with non-cardiac symptoms and the ACS-positive cohort (figure 2). Rather, we propose that the clinical utility of this test lies in the identification of patients with a prolonged closure time, a threshold that we objectively defined as the 90th centile of the distribution of the study population. In this regard, we found that the prolonged closure time (≥138 s) had a high specificity, positive predictive value and likelihood ratio for a diagnosis of non-cardiac chest pain.
Strengths and weaknesses
The results of our pilot study are consistent with the hypothesis that point-of-care testing of platelet reactivity may assist in the timely rule-out of ACS in the ED. Strengths of this approach include the fact that the assessment of closure time is technically straightforward and cost effective, with minimal patient risk (ie, does not involve exposure to radiation or additional invasive testing). In addition, our data suggest that the concept is generalisable. We observed significant differences in demographics (age and gender), the prevalence of specific risk factors (diabetes, smoking) and the use of antiplatelet therapy (aspirin: table 4) between UMASS and Cordoba—findings that are in agreement with the published reports focused on Argentine cohorts,30 31 42 and may underlie the robust contribution of study site as a predictor of outcome in our logistic regression model. Nonetheless, our results, obtained from two distinct healthcare systems and populations, consistently revealed a higher proportion of prolonged closure times in patients with non-cardiac symptoms.
There is, however, an important caveat to this strategy. PFA testing will only contribute to the identification of a subset of patients with non-cardiac symptoms, with the size of the subset and the potential value of the test dependent on the threshold used to define ‘prolonged’ closure times (figure 2 and table 6). For example, the prospective and arbitrary criterion used in our analysis (the 90th centile of closure times for all patients enrolled in the study) discerned an arguably modest 12.4% of patients with a diagnosis of non-cardiac symptoms. However, expediting the discharge of even a small proportion of patients with a non-cardiac diagnosis would limit the costs, potential risks and patient stress associated with unneeded advanced diagnostic testing and possibly invasive procedures.1 2 Reducing the threshold (ie, to the 80th centile of the distribution) would increase the size of the subset identified as ACS negative, with an accompanying (and increasingly unacceptable) loss in specificity because of the growing proportion of false positives (ACS-positive patients with prolonged closure times: figure 2 and table 6). In contrast, increasing the threshold (ie, to the 95th centile) would identify a diminishing proportion of patients with non-cardiac symptoms with an increasing specificity (figure 2 and table 6). The appropriate definition of ‘prolonged’ closure time will therefore require refinement based on risk/benefit analysis.
Table 6.
Effect of definition of ‘Prolonged’ closure time on specificity and positive predictive value for a diagnosis of non-cardiac symptoms
| Threshold | Specificity, 95% CI (%) | Positive predictive value, 95% CI (%) | Patients with non-cardiac symptoms identified (%) |
|---|---|---|---|
| 95th centile (≥160 s) | 100 (96.6 to 100) | 100 (83.9 to 100) | 6.4 (21/330) |
| 90th centile (≥138 s) | 98.1 (93.3 to 99.8) | 95.4 (84.2 to 99.4) | 12.4 (41/330) |
| 80th centile (≥117 s) | 88.6 (80.9 to 94.0) | 86.2 (77.2 to 92.7) | 22.7 (75/330) |
Finally, we emphasise that point-of-case assessment of platelet reactivity and identification of patients with ‘prolonged’ closure times clearly cannot function as a stand-alone test for the rule-out of ACS. Rather, measurement of PFA closure times may serve as an adjunct to the current, standard ED practices. In support of this concept, our multivariate logistic regression model revealed that, irrespective of the TIMI score and study site (and, thus, irrespective of differences in demographics and aspirin use between sites), closure time was an independent predictor of the diagnosis of non-cardiac symptoms.
Summary, limitations and future directions
We report that measurement of the closure time using the PFA-100 provides additional and independent, incremental predictive value in the rule-out of ACS. Limitations of this pilot feasibility study include the enrolment of patients via convenience sampling, and differences in the logistics of the chart review process between the two sites. In addition, neither monitoring of patient outcomes beyond hospital discharge (raising the possibility of potential misclassification of some patients) nor risk/benefit analysis of PFA testing was incorporated into the study design. We emphasise that point-of-care assessment of platelet reactivity cannot serve as a stand-alone test to either discern patients with non-cardiac symptoms versus ACS-positive patients or distinguish between STEMI versus NSTEMI/UA in the emergent setting—limitations that in all likelihood reflect the complex and multifactorial pathophysiology of acute myocardial ischaemia and infarction. Rather, our results suggest that the assessment of closure times may provide benefit by augmenting the standard ED diagnostic practices, a concept that warrants further large-scale multicentre investigation.
Supplementary Material
Footnotes
Contributors: KP, CED and ADM participated in conception and design of the study; all coauthors participated in analysis and interpretation of the data and final approval of the manuscript; KP, CED, RDW, PW, JASM, CSS and ADM participated in drafting of the manuscript or revising it critically for important intellectual content; all authors have had full access to all data and take responsibility for the integrity of the data and the accuracy of the analysis. KP is the guarantor.
Funding: Siemens Inc provided the Emergency Department at the University of Massachusetts Medical School and the Cardiology Department, Instituto Modelo de Cardiologia Privado SRL with PFA-100 devices on a loan basis, as well as partial support for the purchase of test cartridges used in this study. CED was supported in part by a Research Training Grant from the Society for Academic Emergency Medicine (SAEM), Des Plaines, Illinois, USA.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Institutional Review Boards from the two study sites, University of Massachusetts (UMASS)-Memorial Medical Center, University Campus, Worcester, Massachusetts, USA, and the Cardiology Department, Instituto Modelo de Cardiologia Privado SRL, Cordoba, Argentina.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010;122:1756–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darling CE, Michelson AD, Volturo GA, et al. Platelet reactivity and the identification of acute coronary syndromes in the emergency department. J Thromb Thrombolysis 2009;28:31–7 [DOI] [PubMed] [Google Scholar]
- 3.Hollander JE, Chang AM, Shofer FS, et al. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295–304 [DOI] [PubMed] [Google Scholar]
- 4.Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–403 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann U, Truong QA, Fleg JL, et al. Design of the rule out myocardial ischemia/infarction using computer assisted tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am Heart J 2012;163:330–8, 8 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer AJ, Domingo A, Thode HC, Jr,et al. Utilization of coronary computed tomography angiography for exclusion of coronary artery disease in ED patients with low- to-intermediate-risk chest pain: a 1-year experience. Am J Emerg Med 2012;30:1706–11 [DOI] [PubMed] [Google Scholar]
- 8.Hulten E, Pickett C, Bittencourt MS, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol 2013;61:880–92 [DOI] [PubMed] [Google Scholar]
- 9.Ault KA, Cannon CP, Mitchell J, et al. Platelet activation in patients after an acute coronary syndrome: results from the TIMI-12 trial. Thrombolysis in myocardial infarction. J Am Coll Cardiol 1999;33:634–9 [DOI] [PubMed] [Google Scholar]
- 10.Linden MD, Furman MI, Frelinger AL, III,et al. Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost 2007;5:761–5 [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Becker RC, Mann KG, et al. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol 2007;50:1822–34 [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Montalescot G. Platelet function testing and implications for clinical practice. J Cardiovasc Pharmacol Ther 2009;14:157–69 [DOI] [PubMed] [Google Scholar]
- 13.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov 2010;9:154–69 [DOI] [PubMed] [Google Scholar]
- 14.Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol 2013;61:1–11 [DOI] [PubMed] [Google Scholar]
- 15.Hollander JE, Muttreja MR, Dalesandro MR, et al. Risk stratification of emergency department patients with acute coronary syndromes using P-selectin. J Am Coll Cardiol 1999;34:95–105 [DOI] [PubMed] [Google Scholar]
- 16.Furman MI, Benoit SE, Barnard MR, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol 1998;31:352–8 [DOI] [PubMed] [Google Scholar]
- 17.Michelson AD, Barnard MR, Krueger LA, et al. Evaluation of platelet function by flow cytometry. Methods 2000;21:259–70 [DOI] [PubMed] [Google Scholar]
- 18.Michelson AD. Methods for the measurement of platelet function. Am J Cardiol 2009;103:20A–6A [DOI] [PubMed] [Google Scholar]
- 19.Crescente M, Mezzasoma AM, Del Pinto M, et al. Incomplete inhibition of platelet function as assessed by the platelet function analyzer (PFA-100) identifies a subset of cardiovascular patients with high residual platelet response while on aspirin. Platelets 2011;22:179–87 [DOI] [PubMed] [Google Scholar]
- 20.Linnemann B, Schwonberg J, Rechner AR, et al. Assessment of clopidogrel non-response by the PFA-100 system using the new test cartridge INNOVANCE PFA P2Y. Ann Hematol 2010;89:597–605 [DOI] [PubMed] [Google Scholar]
- 21.Poulsen TS, Jorgensen B, Korsholm L, et al. Prevalence of aspirin resistance in patients with an evolving acute myocardial infarction. Thromb Res 2007;119:555–62 [DOI] [PubMed] [Google Scholar]
- 22.Poulsen TS, Mickley H, Korsholm L, et al. Using the Platelet Function Analyzer-100 for monitoring aspirin therapy. Thromb Res 2007;120:161–72 [DOI] [PubMed] [Google Scholar]
- 23.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42 [DOI] [PubMed] [Google Scholar]
- 24.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–55 [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–35 [DOI] [PubMed] [Google Scholar]
- 26.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e663–828 [DOI] [PubMed] [Google Scholar]
- 27.Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Stat Med 2013:Epub 2 April 2013 [DOI] [PubMed] [Google Scholar]
- 28.Kerr KF, Wang Z, Janes H, et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology 2014;25:114–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly AM. What is the incidence of major adverse cardiac events in emergency department chest pain patients with a normal ECG, thrombolysis in myocardial infarction score of zero and initial troponin <=99th centile: an observational study? Emerg Med J 2013;30:15–18 [DOI] [PubMed] [Google Scholar]
- 30.Bernardi V, Szarfer J, Summay G, et al. Long-term versus short-term clopidogrel therapy in patients undergoing coronary stenting (from the Randomized Argentine Clopidogrel Stent (RACS) trial). Am J Cardiol 2007;99:349–52 [DOI] [PubMed] [Google Scholar]
- 31.Bernztein RG, Drake I. Use of aspirin in the public primary care level: experience of the Remediar Program, Argentina. Rev Argent Cardiol 2010;78:330–8 [Google Scholar]
- 32.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100–9 [DOI] [PubMed] [Google Scholar]
- 33.Christie DJ, Kottke-Marchant K, Gorman RT. Hypersensitivity of platelets to adenosine diphosphate in patients with stable cardiovascular disease predicts major adverse events despite antiplatelet therapy. Platelets 2008;19:104–10 [DOI] [PubMed] [Google Scholar]
- 34.Atiemo AD, Ng'Alla LS, Vaidya D, et al. Abnormal PFA-100 closure time is associated with increased platelet aggregation in patients presenting with chest pain. J Thromb Thrombolysis 2008;25:173–8 [DOI] [PubMed] [Google Scholar]
- 35.Bevilacqua S, Alkodami AA, Volpi E, et al. Risk stratification after coronary artery bypass surgery by a point-of-care test of platelet function. Ann Thorac Surg 2009;87:496–502 [DOI] [PubMed] [Google Scholar]
- 36.Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919–33 [DOI] [PubMed] [Google Scholar]
- 37.Breet NJ, van Werkum JW, Bouman HJ, et al. The relationship between platelet reactivity and infarct-related artery patency in patients presenting with a ST-elevation myocardial infarction. Thromb Haemost 2011;106:331–6 [DOI] [PubMed] [Google Scholar]
- 38.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097–105 [DOI] [PubMed] [Google Scholar]
- 39.Ang L, Thani KB, Ilapakurti M, et al. Elevated plasma fibrinogen rather than residual platelet reactivity after clopidogrel pre-treatment is associated with an increased ischemic risk during elective percutaneous coronary intervention. J Am Coll Cardiol 2013;61:23–34 [DOI] [PubMed] [Google Scholar]
- 40.Frossard M, Fuchs I, Leitner JM, et al. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation 2004;110:1392–7 [DOI] [PubMed] [Google Scholar]
- 41.Harrison P, Mackie I, Mathur A, et al. Platelet hyper-function in acute coronary syndromes. Blood Coagul Fibrinolysis 2005;16:557–62 [DOI] [PubMed] [Google Scholar]
- 42.Moran A, Degennaro V, Ferrante D, et al. Coronary heart disease and stroke attributable to major risk factors is similar in Argentina and the United States: the Coronary Heart Disease Policy Model. Int J Cardiol 2011;150:332–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


