Abstract
We evaluated RS5444, a thiazolidinedione high affinity PPARγ agonist, for the ability to inhibit colon carcinogenesis in azoxymethane (AOM)-treated mice. In our initial experiment, mice were treated with RS5444 during AOM treatment, and the drug was withdrawn 12 weeks after the last injection of AOM. RS5444 significantly inhibited aberrant crypt focus formation under these circumstances. Furthermore, exposure to RS5444 during the course of AOM treatment effectively blocked colon tumor formation after withdrawal of the agonist. PPARγ expression and nuclear localization were reduced in adenomas. RS5444 did not inhibit DNA synthesis in tumor cells, suggesting that PPARγ activity was impaired in adenomas. To test this hypothesis, pre-existing adenomas were treated with RS5444 for 16 weeks. We observed a slight, albeit not statistically significant, reduction in tumor incidence in RS5444-treated mice. However, histological examination revealed that tumors from RS5444-treated mice were of significantly lower grade, as evaluated by the extent of dysplasia. Furthermore, carcinoma in situ was observed in about one-third of control tumors, but was never observed in RS5444-treated tumors. We conclude that RS5444 inhibits both initiation and progression of colon tumors in the AOM model of sporadic colon carcinogenesis.
Keywords: PPARγ, colon carcinogenesis, colon cancer chemoprevention, azoxymethane-induced colon tumors, nuclear receptors
Peroxisome proliferator-activated receptor-gamma (PPARγ) forms obligate heterodimers with the retinoid X receptors (RXR) and regulates gene expression through protein–DNA and protein–protein interactions. Although the endogenous ligands of PPARγ are unknown, a number of pharmacological agonists have been identified, including members of the thiazolidinedione class of insulin-sensitizing drugs.1–3 PPARγ is expressed at very high levels in the normal gastrointestinal epithelium4–7 and in human colon cancer cell lines.8–13 Gastrointestinal epithelial cell responses to activation of PPARγ include inhibition of proliferation and induction of markers of differentiation. Functional genomic analysis of PPARγ activation in human colon cancer cell lines indicates that thiazolidinediones regulate metabolism, proliferation and cell migration/motility functions,13 similar to those observed in non-transformed intestinal epithelial cells14 and normal colonic epithelial cells exposed to thiazolidinediones in vivo.7 Recent data from our laboratory indicate that these responses are, at least in part, attributable to induction of an endogenous calcineurin inhibitor, DSCR1, which antagonizes NFATc13 and/or NFκB15 signaling.
The data indicate that PPARγ promotes differentiation of some early stage colon cancer cells, suggesting that thiazolidinediones may have clinical efficacy in colon cancer treatment or prevention. A small phase II clinical trial was carried out using a weak PPARγ agonist, troglitazone, to treat patients with metastatic colon cancer who had failed available standard therapy.16 No objective response was observed in any patient, suggesting that the ability of PPARγ to affect differentiation or inhibit transformed growth is lost in very advanced disease. However, studies in rodents suggest that PPARγ may be effective in suppressing early stage colon cancer.
Three studies have been carried out to assess the effects of thiazolidinediones in the well-established azoxymethane (AOM) model of spontaneous colon cancer. The initial study, carried out in rats, indicated that the weak PPARγ agonist troglitazone inhibits the formation of preneoplastic aberrant crypt foci (ACF).17 This observation was confirmed in a second study, in which troglitazone was shown to inhibit both ACF and tumor formation in AOM-treated rats.18 A single study in wild type mice indicated that 2 higher affinity thiazolidinedione PPARγ agonists, rosiglitazone and pioglitazone, also inhibit both ACF and tumor formation in AOM-treated mice.19 In all of these experiments, the PPARγ agonists was given prior to the carcinogen, and taken together, these 3 studies indicate that activation of PPARγ inhibits some early stage in colon carcinogenesis in AOM-treated rodents.
In contrast, there are several reports that suggest that thiazolidinediones may have adverse effects on colon cancer in rodents. Long-term exposure to very high concentrations of thiazolidinediones induces caecal tumors in mice.20 The clinical significance of this observation is unclear, since caecal tumors are quite rare and since the concentration of thiazolidinediones used in these experiments was far greater than that which would be tolerated in humans. A potentially more disturbing observation was reported by 2 groups who independently observed that thiazolidinediones caused a slight increase in colon tumor size in APC+/Min mice.21,22 In contrast, Niho et al. did not observe any effect of pioglitazone on colon tumor formation in APC+/Min23 or APC+/Δ1309 mice.24 The conflicting results emphasize the need for additional studies to determine the effects of thiazolidinediones on growth and/or progression of established colon tumors.
We have undertaken to re-examine the effects of thiazoladinediones on colon carcinogenesis in AOM-treated wild type mice. In part, we were motivated by the availability of a new, third generation thiazolidinedione, RS5444, which is ~50 times more potent than rosiglitazone.14 We determined that this drug potently promotes differentiation and inhibits invasion of both normal and transformed gastrointestinal epithelial cells in culture,13,14,25 and a preliminary report indicated that RS5444 suppresses ACF formation in AOM-treated mice,7 and may therefore have chemopreventive efficacy. A major objective was to confirm the single report that thiazolidinediones inhibit colon carcinogenesis in mice,19 but we were also motivated to extent these studies and determine (1) if tumors appear in AOM-treated mice after withdrawal of the PPARγ agonist and (2) if thiazolidinediones have any effect on existing adenomas. More recently, it has been reported that patients treated with rosiglitazone (Avandia) are at slightly increased risk for cardiovascular disease.26 If this initial report is confirmed, then it is essential to begin to evaluate the tumor-suppressive potential of the newer, higher affinity PPARγ agonist, which has the potential to inhibit colon cancer initiation and/or progression at lower concentrations, and may therefore be less likely to produce undesirable side effects.
Material and methods
AOM-mediated colon carcinogenesis
C57BL/6J mice were obtained from our local breeding colony, which is maintained as part of an American Association for Accreditation of Laboratory Animal Care facility. Animal experimentation was conducted in accordance with accepted standards of humane animal care, according to protocols approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee. Only female mice were used in these experiments, based upon our experience that there is less variance in tumorigenesis among females, and fewer animals are required to achieve statistical power as a result. At 6 weeks of age, mice received 4 intraperitoneal injections of AOM (Sigma) 10 mg/kg in 0.9% NaCl at weekly intervals, as previously described.27,28 ACF formation was assessed 12 weeks after the last injection of AOM. Mice were sacrificed by asphyxiation with CO2 followed by cervical dislocation. Colons were dissected, split lengthwise and fixed with 4% paraformaldehyde for 4 hr. Fixed colons were stained with 0.2% methylene blue, and ACF number was determined by examination under a low power dissecting microscope, as previously described.27,28 Tumor formation was assessed 40 weeks after the last injection of AOM. At this time, mice were sacrificed and their colons dissected, split, fixed and examined for tumor number, location and size, as previously described.27,28 Tumor volume was estimated as 0.5 (length × width).2 All tumor-bearing colons were embedded in paraffin and sectioned for histological examination of the tumors and adjacent uninvolved epithelium.
Thiazolidinedione treatment
RS5444 was obtained from Diaiichi Sankyo. This compound has been previously shown to activate PPARγ in vivo and in culture, and to regulate growth and differentiation of both normal and transformed gastrointestinal epithelial cells7,13,25 as well as transformed thyroid epithelial cells.29 In our initial experiments, mice received RS5444 in 0.5% carboxymethylcellulose (CMC) by oral gavage. A dose of 10 mg/kg/day was administered daily for 5 days a week starting 1 week prior to the first AOM injection. Forty-five mice were enrolled in the treatment (RS5444) or control (CMC) groups. Twenty-five mice of each group were sacrificed and examined for ACF formation 16 weeks after initiation of RS5444 and 12 weeks after the last injection of AOM, as described above. At that time, RS5444 treatment was terminated, and the remaining mice (~20/group) were allowed to proceed to tumor formation, assessed 40 weeks after the last injection of AOM, as described above. In all experiments, body weight of control and experimental mice was recorded weekly. We observed a slight but statistically significant increase in body weight among the RS5444-treated mice.
Alternatively, tumors were induced by AOM injection of untreated mice. Twenty-four weeks after the last injection of AOM, the mice were randomly assorted into 2 groups of 100 each. Half of these mice were fed a diet that contained RS5444 in AIN-76A chow. The RS5444-containing chow was formulated in pellets by the manufacturer, Research Diets, Inc. (New Brunswick, NJ), and control mice received standard AIN-76A chow. All mice were fed ad libidum. The dose was determined by measuring average weight and chow consumption of mice of the same age and sex, and the diet was then fortified with RS5444 sufficient to deliver an average dose of 10 mg/kg/day. Mice were sacrificed 40 weeks after the last injection of AOM, and colons were processed for tumor formation and histology, as described above. All mice were injected intraperitoneally with 100 mg/kg bromodeoxyuridine (BrdU) dissolved in PBS between 9 and 10 am and 1 hr prior to sacrifice, as previously described.27 As was the case when mice were given RS5444 by oral gavage, addition of RS5444 to the diet resulted in a significant weight gain of about 10%, compared to the animals on control diet. We did not examine any tissues other than the colon in these experiments.
Immunohistochemistry and histology
Fixed tissues were rehydrated, embedded in paraffin and cut into 5 µm thick sections. After deparaffinization and rehydration, nuclei were stained using the Oncogene Science BrdU antibody staining kit, as previously described.7 All stained sections were examined visually using a transmission light microscope. Only those longitudinally sectioned crypts that had an intact, complete and well-oriented structure, and that displayed lumen at the top and muscularis mucosa at the base were counted for BrdU labeling index. A minimum of 50 crypts was counted from each of 5 or more mice. A similar approach was used to determine BrdU labeling index of tumors, except that a grid was used to count multiple representative sections of the tumor. Ten or more sections were counted from each of a minimum of 5 tumors.
PPARγ immunohistochemistry was carried out as previously described.7 The PPARγ antibody was obtained from Cell Signaling (Danvers, MA). Antibody staining was detected using the Vectastain ABC Kit (Vector Laboratories, Burlinghame, CA). Quantification of nuclear staining intensity was carried out using the Aperio slide scanner and ImageScope v8.0.39.1059 employing the ‘IHC Nuclear’ algorithm.
Tumor histology was evaluated using H&E-stained sections prepared as described earlier. All sections were initially inspected using a transmission light microscope to assure uniform staining and appropriate sectioning through both tumor and normal adjacent epithelium. Multiple sections from each tumor were examined. Tumor sections were then scanned into digital images using the Aperio slide scanner. Digital images were evaluated blind for tumor grade using the following criteria: Grade 1, moderate nuclear atypia with evidence of differentiation; Grade 2, thickened tubular glands with no evidence of differentiation; Grade 3, highly branched glandular structures; Grade 4, multilayered glandular structures; Grade 5, carcinoma in situ as evidenced by complete loss of glandular structure and epithelial cell invasion into the adjacent stroma within the tumor, but no invasion of muscularis mucosa.
Statistical analyses
All statistical analyses were carried out using SigmaStat v3.5. Tumor incidence and incidence of carcinoma in situ were evaluated using the Fisher Exact test or Chi-squared analysis, as indicated in the figure legends. ACF formation, PPARγ nuclear staining, BrdU incorporation, tumor volume and tumor grade were evaluated using the Mann–Whitney rank sum test. PPARγ mRNA abundance was evaluated using the t-test.
Results
RS5444 inhibits early stage colon carcinogenesis in AOM-treated mice
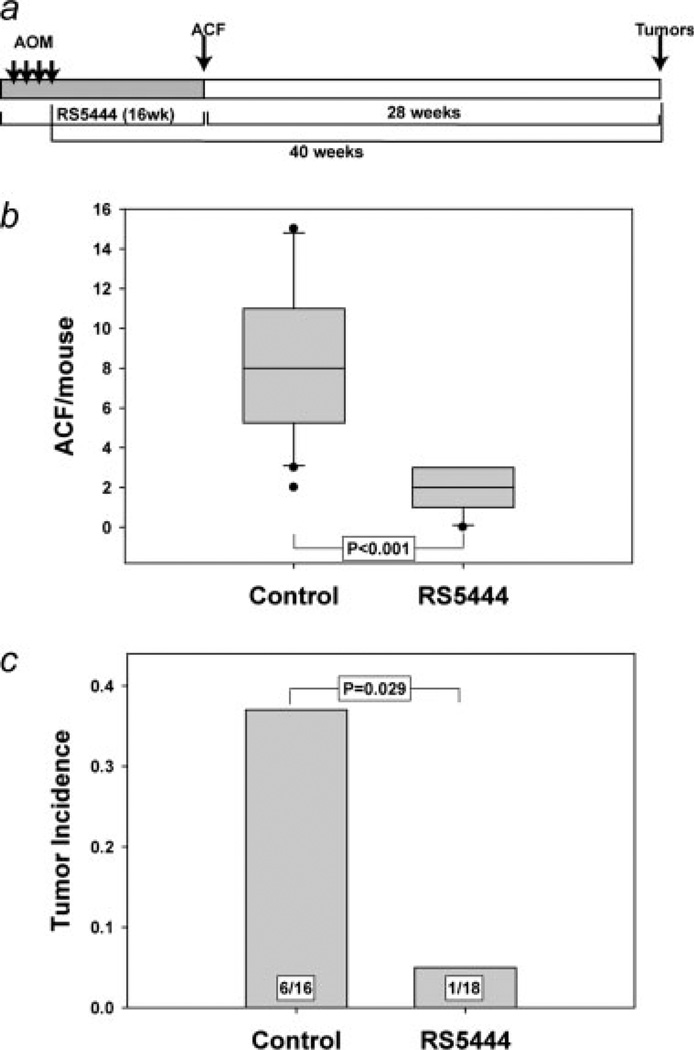
Our initial experiment was designed to determine if RS5444 inhibited the formation of preneoplastic lesions and colon tumors in the AOM model of rodent colon carcinogenesis. To this end, mice were treated with 10 mg/kg/day of RS5444 or CMC by oral gavage beginning 1 week prior to the first injection of AOM, as illustrated in Figure 1a. One group of control and another of RS5444-treated mice were sacrificed 12 weeks after the last injection of AOM, and colons were examined for formation of preneoplastic ACF. As shown in Figure 1b, the control mice contained a median of 8 crypts per mouse, whereas the median number of ACF in RS5444-treated mice was 2. The observation that RS5444 significantly inhibited ACF formation is consistent with published data with other thiazolidinediones17–19 and confirms our initial observation that RS5444 is a potent inhibitor of early events in colon carcinogenesis in AOM-treated mice.7
Figure 1.
RS5444 inhibits ACF formation and blocks tumor incidence in AOM-treated mice. Mice were given 10 mg/kg/day RS5444 or CMC by oral gavage for 1 week prior to the initial injection of AOM, as shown in Panel a. ACF were counted in colons from control mice and mice that had received RS5444 starting 1 week prior to the first injection of AOM (Panel b). Statistical analysis used the Mann–Whitney rank sum test. Oral gavage of the remaining mice (16 controls and 18 RS5444-treated) was halted 12 weeks after the last injection of AOM and tumor incidence was determined at 40 weeks after the last injection of AOM (Panel c). Statistical analysis was carried out using the Fisher Exact test.
ACF number is a well known risk factor for colon tumor formation in both rodents and humans.30–32 The observation that RS5444 inhibited ACF formation suggests that this drug may also inhibit colon tumor formation. However, it appears that only a small subset of ACF are capable of progression to adenomas,33 and it is formally possible that RS5444 might have no effect on that hypothetical subpopulation of ACF that are capable of progression. It is also possible that RS5444 might restrain the progression of preneoplastic lesions, rather than block their initiation. In such a case, RS5444 might block tumor formation only as long as the drug is present, and tumors might develop after the drug is withdrawn. To evaluate these possibilities, RS5444 was withdrawn 12 weeks after the last injection of AOM (as illustrated in Fig. 1a), and tumor formation was assessed 28 weeks later (40 weeks after the last injection of AOM). As shown in Figure 1c, about one-third of the control AOM-treated mice formed tumors, which were exclusively in the distal colon. However, RS5444 significantly inhibited tumor formation in AOM-treated mice, even when the drug was withdrawn after AOM injection. These data indicate that RS5444 blocked initiation of transformation, and validate the prophylactic efficacy of RS5444 in the AOM model of colon carcinogenesis.
RS5444 inhibits colon tumor progression with little or no effect on growth of adenomas
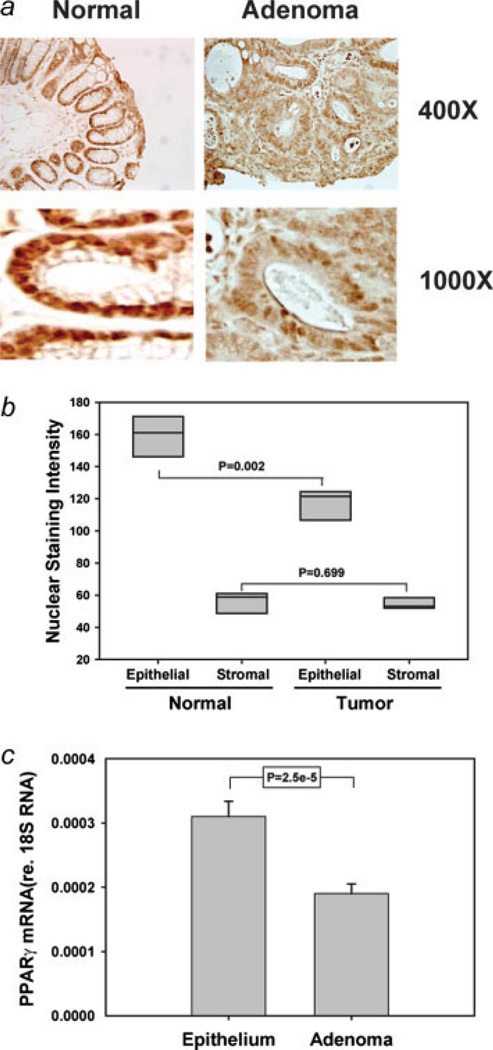
Our data indicate that RS5444 blocks initiation of colon carcinogenesis. However, the effects of thiazolidinediones on growth and progression of established AOM-induced colonic adenomas have never been examined. We initially evaluated PPARγ expression and cellular distribution in adenomas and in adjacent uninvolved distal epithelial, using tumors from control animals in the experiment described above. As we have previously reported,7 PPARγ staining in the distal epithelium is more or less uniform throughout the crypts and is primarily localized to the nuclei of distal epithelial cells (Fig. 2a). However, PPARγ staining in the adenomatous epithelial cells was distinctly different from that observed in adjacent uninvolved epithelia (Fig. 2a). Nuclear staining was less intense in adenomatous epithelial cells, compared to normal adjacent epithelial cells (Fig. 2b). As a control, we measured nuclear staining of stromal cells in tumors and normal epithelia. No significant difference in stromal PPARγ staining was observed (Fig. 2b). The decreased nuclear staining intensity suggested that PPARγ expression was reduced in adenomatous epithelial cells. Furthermore, the abundance of PPARγ mRNA was significantly lower in adenomas than in isolated epithelial cells (Fig. 2c). Similar observations have been reported for PPARγ mRNA abundance in human colonic polyps.34
Figure 2.
PPARγ expression is reduced in AOM-induced adenomas. Sections from colons from tumor-bearing control mice (from the experiment described in Fig. 1) were stained for PPARγ, and representative photomicrographs were obtained from adenomas and adjacent normal epithelia from the same stained section (Panel a). Nuclear staining intensity in epithelial and stromal cells was quantified using the Aperio Slide Scan algorithm (Panel b). Three distal adenomas from AOM-treated mice were dissected, pooled and total RNA was extracted. In parallel, RNA was extracted from purified normal distal epithelial cells. The abundance of PPARγ mRNA was measured by quantitative real time PCR and was normalized to the abundance of 18S rRNA (Panel c). Statistical significance of nuclear staining was assessed by Mann–Whitney, whereas the t-test was used to compare mRNA abundance in normal epithelial cells and tumors. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
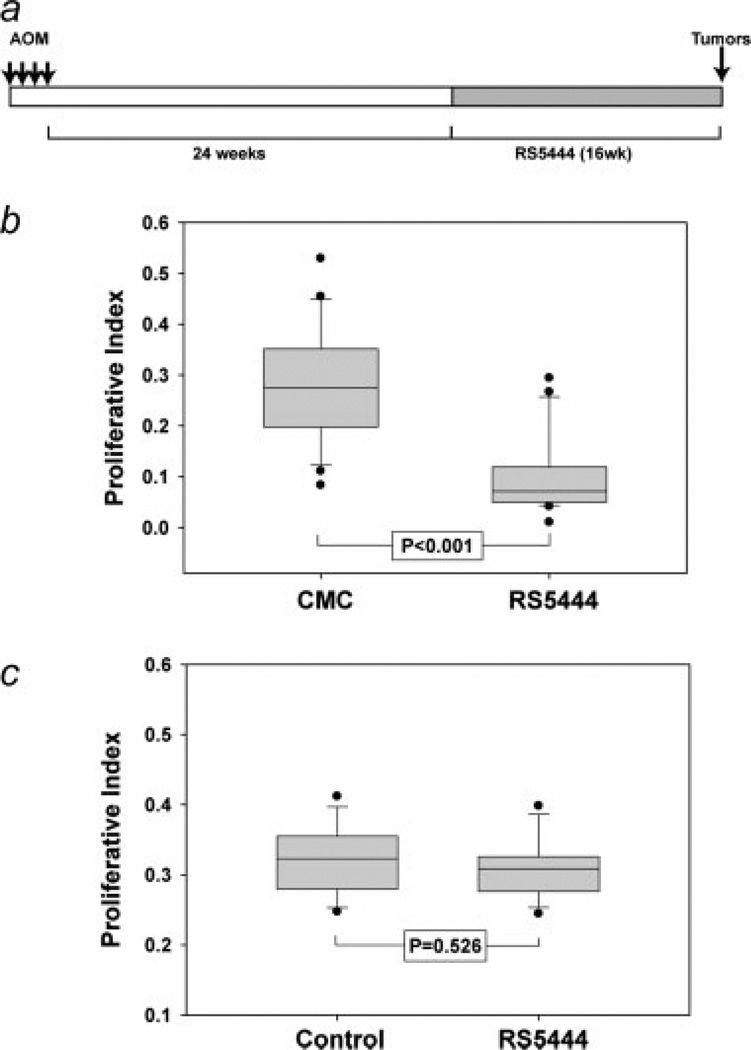
Immunohistochemical analysis of PPARγ expression and nuclear localization suggested that the function of this receptor may be impaired in adenomatous epithelial cells. If this is the case, thiazolidinedione PPARγ agonists such as RS5444 may have little or no effect on growth of adenomas. To test this hypothesis, we examined the effects of RS5444 in preformed adenomas in the colons of AOM-treated mice. As illustrated in Figure 3a, AOM-treated mice were allowed to progress for 24 weeks after the last injection of AOM. At this time, we anticipated from historical controls that some of the mice should have adenomas (N. Murray, unpublished observations). Beginning at 24 weeks after the last injection of AOM, mice received RS5444 in a diet that was calculated to deliver ~10 mg/kg/day, based upon average mouse weight and chow consumption rate (N. Murray, data not shown). Mice were harvested 40 weeks after the last injection of AOM. All mice were injected with BrdU immediately prior to sacrifice. Tumors and adjacent uninvolved epithelial were sectioned, stained with BrdU antibodies and nuclear labeling indices were determined for adenomatous epithelial cells and adjacent uninvolved epithelia. As shown in Figure 3b, RS5444 potently inhibited BrdU incorporation in normal epithelial cells, confirming our initial report that PPARγ inhibits proliferation of normal colonic epithelial cells.7 However, RS5444 had no statistically significant effect on BrdU labeling of adenomatous epithelial cells (Fig. 3c), suggesting that the ability of PPARγ to inhibit colonic epithelial cell proliferation is lost during early stage carcinogenesis.
Figure 3.
RS5444 does not inhibit DNA synthesis in adenomas. Adenomas were induced by AOM injection as illustrated in Panel a. Proliferative index was determined by counting BrdU labeled and unlabeled cells in adjacent normal epithelial crypts (Panel b) or adenomas (Panel c). Five control and 5 RS5444-treated tumors were analyzed and statistical analysis was carried out using the Mann–Whitney rank sum test.
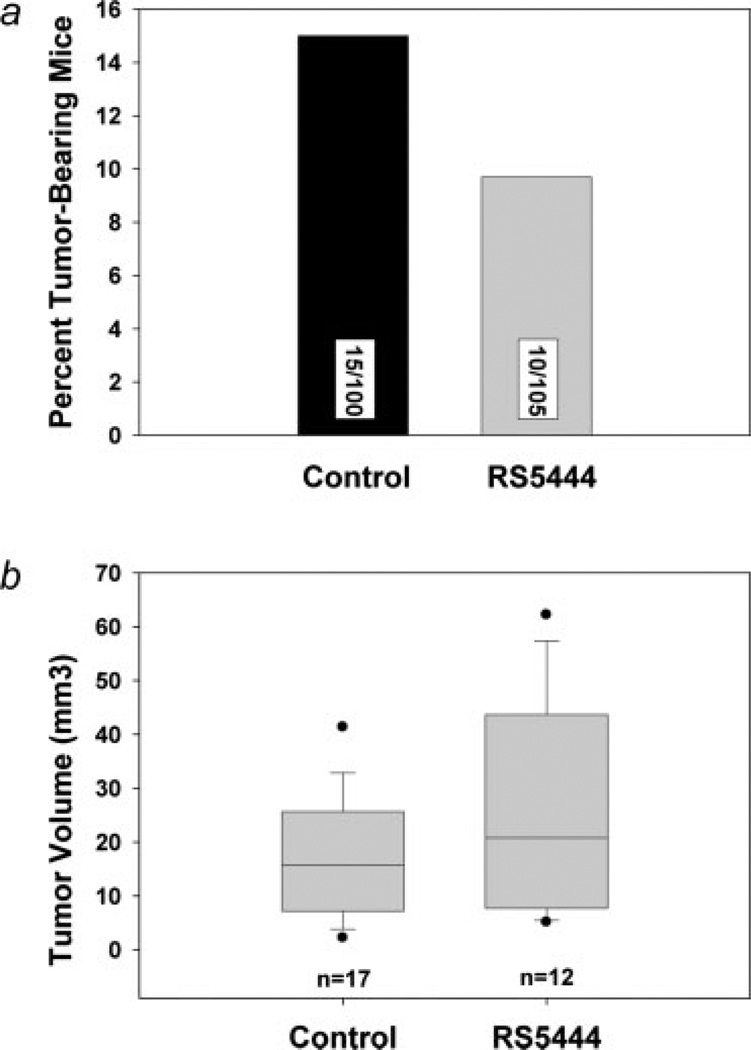
Tumor number and size were measured in control and RS5444-treated mice. As shown in Figure 4a, we observed a decrease of about 30% in tumor incidence in the RS5444-treated mice. However, the experiment lacked statistical power to detect a significant change in tumor incidence of this magnitude. Tumor volume was highly variable. The median tumors volumes for control and RS5444-treated mice were 16 and 21 mm,3 respectively, and no significant effect on tumor size was observed (Fig. 4b).
Figure 4.
RS5444 has no significant effect on incidence or growth of pre-existing tumors. Tumors were induced and treated with RS5444 as indicated in Figure 3a. Two groups of 100 mice each were sacrificed, colons were dissected and tumor number and size measured. Chi-squared analysis was used to determine the significance of tumor incidence (Panel a), whereas the Mann–Whitney rank sum test was used to analyze tumor volume (Panel b).
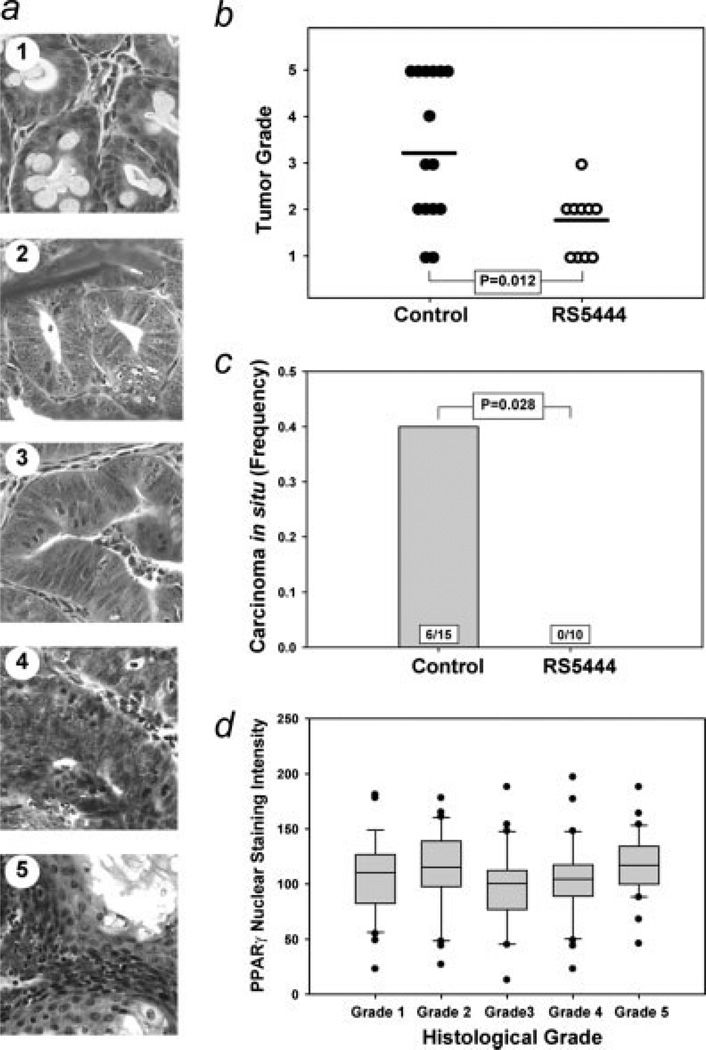
Since PPARγ is known to promote differentiation of colon cancer cells in vitro,35,36 we asked if RS5444 had any effect on the extent of dysplasia exhibited by AOM-induced adenomas. Tumors from control and RS5444-treated animals were sectioned, stained with H&E and scanned images were evaluated for tumor histology. Adenomas were scored for dysplasia on a basis of 1–5, as described in the Material and Methods and illustrated in Figure 5a. Significantly higher tumor grade was observed in the control tumors (Fig. 5b), indicating more advanced dysplasia in control tumors compared to RS5444-treated tumors. The median tumor grade for control animals was 3, with a significant number of tumors with Grades 4 and 5 dysplasia observed in this group. These results are consistent with our experience with dysplasia of AOM-induced tumors in this mouse strain.37 In contrast, the median grade for RS5444-treated tumors was 2; only 1 Grade 3 tumor and no higher grade tumors were observed in this group.
Figure 5.
RS5444 inhibits progression of pre-existing tumors. Tumors were induced and treated with RS5444 as indicated in Figure 3a. Tumors were fixed and stained with H&E and tumor histology was evaluated for degree of dysplasia (Panel a). Dysplasia scores for control and RS5444-treated tumors were tabulated and analyzed by the Mann–Whitney rank sum test (Panel b). Histological examination revealed that control tumors exhibited carcinoma in situ (Panel c). The frequency of carcinoma in situ (#CIS/total tumors) was calculated and statistical significance was evaluated using the Fisher Exact test. (Panel d) Sections from tumors were immunostained using a PPARγ antibody. The slides were scanned and nuclear staining was analyzed using the Aperio scanner as described for the data shown in Figure 2b. A minimum of 30 areas conforming to each of the individual histological grades ware analyzed and subjected to statistical evaluation using the Mann–Whitney test. No statistically significant differences were observed.
Invasion into the submucosa was not observed in any AOM-induced tumor, consistent with our experience with this model. However, carcinoma in situ, characterized by loss of glandular structure and invasion of epithelial cells into the adjacent stroma (Fig. 5c), was observed in 6 tumors from control animals. Carcinoma in situ was not observed in the RS5444-treated tumors indicating that this drug had a statistically significant effect on progression of preformed colonic adenomas (Fig. 5c).
We also measured nuclear PPARγ staining in tumors of different degrees of dysplasia. As shown in Figure 5d, we did not observe any progression-related change in PPARγ expression. We also stained for nuclear β-catenin in tumor sections from both control and RS5444-treated mice. All tumor cells exhibited pronounced nuclear β-catenin staining, consistent with reports that almost all AOM-induced tumors harbor β-catenin mutations.38 Adjacent normal epithelial cells never exhibited significant nuclear β-catenin staining (data not shown). We detected no significant progression-related difference in nuclear β-catenin staining of tumor nuclei, neither did we observe any difference in nuclear β-catenin staining in tumors from control and RS5444-treated mice.
Discussion
RS5444 is about 50 times more potent than rosiglitazone in culture.14 The increased potency of RS5444 in vitro suggests that this compound may be efficacious as a chemopreventive agent in vivo at lower concentrations than those previously reported for other thiazolidinediones (generally in the range of 50–200 mg/kg/day in mouse studies). The use of lower concentrations of thiazolidinediones is potentially desirable, since such drugs are known to have side effects that are not mediated by PPARγ.39–42 Furthermore, recent concerns about potential cardiovascular complications associated with rosiglitatone (Avandia)26 warrant evaluation of other thiazolidinediones as potential chemopreventive agents. We report here that RS5444 inhibits ACF formation and blocks tumor formation. These data are consistent with those reported by Osawa et al.,19 who observed inhibition of tumor formation in the colons of AOM-treated mice receiving pioglitazone or rosiglitazone.
In previous studies, thiazolidinedione treatment was initiated prior to AOM treatment and was continued throughout the experiment. This protocol makes it difficult to discriminate between early and late effects of such drugs. For example, the tumor-suppressive effects of thiazolidinediones could result from blocking transformation at some early stage or from inhibiting the development of transformed cells into preneoplastic lesions. In the latter case, transformed cells might persist in a latent state and tumors might develop after withdrawal of the drug. However, we did not observe significant tumor formation after withdrawal of RS5444 from AOM-treated mice, suggesting that thiazolidinediones block some early event in transformation.
We have recently shown that RS5444 causes early stage human colonic adenocarcinoma cells to withdraw irreversibly from the cell cycle.13 We and others have also shown that PPARγ agonists promote differentiation of both normal and transformed gastrointestinal epithelial cells.13,14,35,36 The data are consistent with the hypothesis that the ability of PPARγ agonists to inhibit early stage transformation in the colon is inherent in the ability of such drugs to promote differentiation of minimally transformed colonic epithelial cells. Alternatively, the ability to block initiation of transformation may be due to inhibition of proliferation of normal colonic epithelial cells, resulting in an effective decrease in the ‘target size’ for the carcinogen. Finally, we have observed that RS5444 induces several drug metabolic pathways in the colonic epithelium,7 and it is possible that PPARγ may inhibit adduct formation in AOM-treated mice, thereby reducing the number of mutations that are induced by this carcinogen. Although the mechanism that accounts for the tumor-suppressive effects of thiazolidinediones is unknown, it is clear that transient activation of PPARγ by treatment with RS5444 very effectively blocks colon tumor formation in AOM-treated mice.
A significant objective of our experiments was to determine the effect of thiazolidinediones on growth of established tumors. We were motivated in this regard by conflicting reports on the effects of thiazolidinediones on colon tumor growth in APC+/Min mice. It has previously been reported that virtually all AOM-induced tumors harbor activating β-catenin mutations,38 and our observation that all such tumors exhibit a high level of nuclear β-catenin staining is consistent with the conclusion that AOM-induced tumors have undergone mutational activation of the Wnt/APC/β-catenin signaling pathway. It was therefore important to determine if PPARγ agonists promoted growth or progression of such tumors. We observed a slight, albeit not statistically significant, decrease in tumor incidence when RS5444 was administered to tumor-bearing mice, with no significant effect on tumor size. Our results are consistent with those of Niho et al.,23,24 as well as more recent genetic studies, which indicate that PPARγ knockout promotes, rather than inhibits, tumor growth in APC+/Min mice.43 We conclude, in contrast to the initial reports, that PPARγ does not promote growth of colon tumors.
Although RS5444 did not inhibit growth of established tumors, we observed that control tumors were significantly more dysplastic than the tumors from RS5444-treated mice. Carcinoma in situ was observed in about one-third of the control tumors, but invasion of epithelial cells into the adjacent stroma was never observed in RS5444-treated tumors. We and others have reported that PPARγ inhibits invasion by early stage colon cancer cell lines, as measured by the ability to penetrate Matrigel-coated transwell filters in vitro.13,44 Our data demonstrate that thiazolidinediones also inhibit invasion by transformed colonic epithelial cells in vivo.
In conclusion, we have shown that the high affinity PPARγ agonist RS5444 blocks early events in AOM-mediated carcinogenesis and inhibits progression of pre-existing AOM-induced adenomas. These data suggest that high affinity thiazolidinedione PPARγ agonists may have clinical efficacy as a chemopreventive agents for the treatment of patients who are at increased risk for colon cancer. However, recent concerns over potential cardiovascular side effects of Avandia (rosiglitazone)26 must be resolved, and as is invariably the case with pharmaceutical agents, the potential liability due to long term side effects must be assessed relative to the risk of development of colon cancer. On the other hand, it is clear that ACF represent a significant risk factor for human colon cancer,45–47 and it is possible that high affinity PPARγ agonists such as RS5444 may have clinical efficacy in short term prophylactic treatment of individuals with large numbers of such lesions. The ability to reduce the number of ACF in such patients would likely translate into a significant reduction in colon cancer risk.
Acknowledgements
We would like to acknowledge the expert assistance of Ms. Pam Kreinest and the Mayo Clinic Tumor Histology facility.
Grant sponsor: Daiichi Sankyo, Inc.; National Cancer Center, National Institutes of Health, United States Public Health Service; Grant numbers: CA121349, CA94122, CA81436.
Abbreviations
- ACF
aberrant crypt foci
- AOM
azoxymethane
- APC
adenomatous polyposis coli
- BrdU
bromodexoyuridine
- Min
multiple intestinal neoplasia
- NFATc
cytoplasmic component of nuclear factor of activated T cells
- NFκB
nuclear factor kappa light chain of B cells
- PPARγ
peroxisome proliferator-activated receptor-gamma
- RXR
retinoid X receptor
References
- 1.Fujiwara T, Horikoshi H. Troglitazone and related compounds: therapeutic potential beyond diabetes. 2000;67:2405–2416. doi: 10.1016/s0024-3205(00)00829-8. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara T, Wada M, Fukuda K, Fukami M, Yoshioka S, Yoshioka T, Horikoshi H. Characterization of CS-045, a new oral antidiabetic agent. II. Effects on glycemic control and pancreatic islet structure at a late stage of the diabetic syndrome in C57BL/KsJ-db/db mice. Metabolism. 1991;40:1213–1218. doi: 10.1016/0026-0495(91)90218-l. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor [IMAGE] (PPARIMAGE]) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 4.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee R, Jow L, Croston GE, Paterniti JR., Jr Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem. 1997;272:8071–8076. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre M, Paulweber B, Fajas L, Woods J, McCrary C, Colombel JF, Najib J, Fruchart JC, Datz C, Vidal H, Desreumaux P, Auwerx J. Peroxisome proliferator-activated receptor gamma is induced during differentiation of colon epithelium cells. J Endocrinol. 1999;162:331–340. doi: 10.1677/joe.0.1620331. [DOI] [PubMed] [Google Scholar]
- 7.Su W, Bush CR, Necela BM, Calcagno SR, Murray NR, Fields AP, Thompson EA. Differential expression, distribution, and function of PPARγ in the proximal and distal colon. Physiol Genomics. 2007;30:342–353. doi: 10.1152/physiolgenomics.00042.2007. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizumi T, Ohta T, Ninomiya I, Terada I, Fushida S, Fujimura T, Nishimura G, Shimizu K, Yi S, Miwa K. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int J Oncol. 2004;25:631–639. [PubMed] [Google Scholar]
- 9.Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H. Induction of differentiation and peroxisome proliferator-activated receptor gamma expression in colon cancer cell lines by troglitazone. J Cancer Res Clin Oncol. 2004;130:73–79. doi: 10.1007/s00432-003-0510-2. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RA, Sarraf P, Mueller E, Brockman JA, Prusakiewicz JJ, Eng C, Willson TM, DuBois RN. Peroxisome proliferator-activated receptor gamma-mediated differentiation: a mutation in colon cancer cells reveals divergent and cell type-specific mechanisms. J Biol Chem. 2003;278:22669–22677. doi: 10.1074/jbc.M300637200. [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor gamma (PPARgamma) ligand induced apoptosis in colon cancer cells. Gut. 2002;50:658–664. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 13.Bush CR, Havens JM, Necela BM, Su W, Chen L, Yanagisawa M, Anastasiadis PZ, Guerra R, Luxon BA, Thompson EA. Functional genomic analysis reveals cross-talk between peroxisome proliferator-activated receptor gamma and calcium signaling in human colorectal cancer cells. J Biol Chem. 2007;282:23387–23401. doi: 10.1074/jbc.M702708200. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Bush CR, Necela BM, Su W, Yanagisawa M, Anastasiadis PZ, Fields AP, Thompson EA. RS5444, a novel PPARgamma agonist, regulates aspects of the differentiated phenotype in nontransformed intestinal epithelial cells. Mol Cell Endocrinol. 2006;251:17–32. doi: 10.1016/j.mce.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Cho KO, Lee HJ, Kim SY, Sato Y, Cho YJ. Down syndrome candidate region 1 increases the stability of the IkappaBalpha protein: implications for its anti-inflammatory effects. J Biol Chem. 2006;281:39051–39061. doi: 10.1074/jbc.M604659200. [DOI] [PubMed] [Google Scholar]
- 16.Kulke MH, Demetri GD, Sharpless NE, Ryan DP, Shivdasani R, Clark JS, Spiegelman BM, Kim H, Mayer RJ, Fuchs CS. A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J. 2002;8:395–399. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kohno H, Yoshitani S, Takashima S, Okumura A, Hosokawa M, Yamaguchi N, Tanaka T. Troglitazone, a ligand for peroxisome proliferator-activated receptor gamma, inhibits chemically-induced aberrant crypt foci in rats. Jpn J Cancer Res. 2001;92:396–403. doi: 10.1111/j.1349-7006.2001.tb01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Kohno H, Yoshitani S, Takashima S, Okumura A, Murakami A, Hosokawa M. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–2428. [PubMed] [Google Scholar]
- 19.Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Fan KH, Lamprecht SA, Edelmann W, Kopelovich L, Kucherlapati R, Lipkin M. Peroxisome proliferator-activated receptor gamma agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in Apc1638 N/+ Mlh1+/− double mutant mice. Int J Cancer. 2005;116:495–499. doi: 10.1002/ijc.21018. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 22.Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 23.Niho N, Takahashi M, Shoji Y, Takeuchi Y, Matsubara S, Sugimura T, Wakabayashi K. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR gamma ligand. Cancer Sci. 2003;94:960–964. doi: 10.1111/j.1349-7006.2003.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niho N, Takahashi M, Kitamura T, Shoji Y, Itoh M, Noda T, Sugimura T, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–6095. [PubMed] [Google Scholar]
- 25.Chen L, Necela BM, Su W, Yanagisawa M, Anastasiadis PZ, Fields AP, Thompson EA. Peroxisome proliferator-activated receptor gamma promotes epithelial to mesenchymal transformation by Rho GTPase-dependent activation of ERK1/2. J Biol Chem. 2006;281:24575–24587. doi: 10.1074/jbc.M604147200. [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 27.Murray NR, Davidson LA, Chapkin RS, Clay Gustafson W, Schattenberg DG, Fields AP. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, Anastasiadis P, Gatalica Z, Thompson EA, Fields AP. Protein kinase Cι is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copland JA, Marlow LA, Kurakata S, Fujiwara K, Wong AK, Kreinest PA, Williams SF, Haugen BR, Klopper JP, Smallridge RC. Novel high-affinity PPARgamma agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25:2304–2317. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 30.McLellan EA, Medline A, Bird RP. Dose response and proliferative characteristics of aberrant crypt foci: putative preneoplastic lesions in rat colon. Carcinogenesis. 1991;12:2093–2098. doi: 10.1093/carcin/12.11.2093. [DOI] [PubMed] [Google Scholar]
- 31.McLellan EA, Medline A, Bird RP. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: putative preneoplastic lesions. Cancer Res. 1991;51:5270–5274. [PubMed] [Google Scholar]
- 32.Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol. 1991;22:287–294. doi: 10.1016/0046-8177(91)90163-j. [DOI] [PubMed] [Google Scholar]
- 33.Shpitz B, Hay K, Medline A, Bruce WR, Bull SB, Gallinger S, Stern H. Natural history of aberrant crypt foci. A surgical approach. Dis Colon Rectum. 1996;39:763–767. doi: 10.1007/BF02054441. [DOI] [PubMed] [Google Scholar]
- 34.Matthiessen MW, Pedersen G, Albrektsen T, Adamsen S, Fleckner J, Brynskov J. Peroxisome proliferator-activated receptor expression and activation in normal human colonic epithelial cells and tubular adenomas. Scand J Gastroenterol. 2005;40:198–205. doi: 10.1080/00365520410009573. [DOI] [PubMed] [Google Scholar]
- 35.Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn J Cancer Res. 1999;90:75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray NR, Weems C, Chen L, Leon J, Yu W, Davidson LA, Jamieson L, Chapkin RS, Thompson EA, Fields AP. Protein kinase C betaII and TGFbetaRII in omega-3 fatty acid-mediated inhibition of colon carcinogenesis. J Cell Biol. 2002;157:915–920. doi: 10.1083/jcb.200201127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120. [PubMed] [Google Scholar]
- 39.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPARgamma) ligand selectively induces the early growth response-1 gene independently of PPARgamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 40.Brunmair B, Gras F, Neschen S, Roden M, Wagner L, Waldhausl W, Furnsinn C. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-γ-mediated changes in gene expression. Diabetes. 2001;50:2309–2315. doi: 10.2337/diabetes.50.10.2309. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Fu M, D’Amico M, Albanese C, Zhou J-N, Brownlee M, Lisanti MP, Chatterjee VKK, Lazar MA, Pestell RG. Inhibition of cellular proliferation through IκB kinase-independent and peroxisome proliferator-activated receptor γ-dependent repression of cyclin D1. Mol Cell Biol. 2001;21:3057–3070. doi: 10.1128/MCB.21.9.3057-3070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X, Lin Y, Zhang J, Fu M, Mao Z, Chen YE. Thiazolidinediones, a class of anti-diabetic drugs, inhibit Id2 expression through a PPARgamma-independent pathway in human aortic smooth muscle cells. Cell Mol Life Sci. 2003;60:212–218. doi: 10.1007/s000180300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer. 2006;119:2339–2346. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 44.Batlle E, Verdu J, Dominguez D, del Mont Llosas M, Diaz V, Loukili N, Paciucci R, Alameda F, de Herreros AG. Protein kinase C-alpha activity inversely modulates invasion and growth of intestinal cells. J Biol Chem. 1998;273:15091–15098. doi: 10.1074/jbc.273.24.15091. [DOI] [PubMed] [Google Scholar]
- 45.Yokota T, Sugano K, Kondo H, Saito D, Sugihara K, Fukayama N, Ohkura H, Ochiai A, Yoshida S. Detection of aberrant crypt foci by magnifying colonoscopy. Gastrointest Endosc. 1997;46:61–65. doi: 10.1016/s0016-5107(97)70212-8. [DOI] [PubMed] [Google Scholar]
- 46.Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niitsu Y. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 47.Adler DG, Gostout CJ, Sorbi D, Burgart LJ, Wang L, Harmsen WS. Endoscopic identification and quantification of aberrant crypt foci in the human colon. Gastrointest Endosc. 2002;56:657–662. doi: 10.1067/mge.2002.128540. [DOI] [PubMed] [Google Scholar]