Abstract
OBJECTIVES
To follow-up the results of phase I testing by evaluating the clinical efficacy of the green tea extract Polyphenon E for patients with early stage chronic lymphocytic leukemia(CLL).
METHODS
Previously untreated patients with asymptomatic, Rai stage 0-II CLL and an absolute lymphocyte count(ALC) ≥10 ×109/L were eligible for this phase II trial. Polyphenon E with a standardized dose of epigallocatechin-3-gallate(2000 mg per dose) was administered twice daily.
RESULTS
Forty-two patients received Polyphenon E 2000 mg twice daily for up to 6 months. Among these, 29 (69%) had Rai stage I-II disease. Patients received a median of 6 cycles of treatment(range: 1-6). The most common grade 3 side effects were transaminitis (n=1), abdominal pain(n=1) and fatigue(n=1). Clinical activity was observed with 13(31%) patients experiencing a sustained ≥20% reduction in ALC and 20 of 29(69%) patients with palpable adenopathy experiencing at least a 50% reduction in the sum of the products of all nodal areas. EGCG plasma levels after 1 month of therapy correlated with reductions in lymphadenopathy (correlation 0.44; p=0.02). Overall, 29(69%) patients fulfilled the criteria for a biologic response with either a sustained ≥20% decline in ALC and/or a ≥30% reduction in the sum of the products of all nodal areas at some point during the 6 months of active treatment.
CONCLUSION
Daily oral EGCG in the Polyphenon E preparation was well tolerated by CLL patients in this phase II trial. Durable declines in ALC and/or lymphadenopathy were observed in the majority of patients.
INTRODUCTION
Green tea has long been promoted as a health promoting substance which reduces the risk of cancer.1, 2 After 3 case-control studies demonstrated that green tea intake was associated with a reduced risk of leukemia3, 4 and non-Hodgkin lymphoma5, a population based cohort study of ~42,000 individuals prospectively followed for 9 years was conducted.6 Green tea consumption was inversely associated with the risk of lymphoid malignancies even after adjusting for 16 other personal characteristics including age, sex, smoking history, level of education, occupation, consumption of other dietary products, and family history of leukemia.6
Tea polyphenols (catechins) exert multi-targeted effects on malignant cells.7-10 Epigallocatechin gallate(EGCG), the major catechin in tea induces apoptotic cell death in animals models of human non-Hodgkin lymphoma,11 B-cell lymphoma cell lines,12, 13 and primary CLL B-cells.14 Subsequent case reports in patients with low-grade B-cell malignancies suggested these preclinical findings may have clinical relevance.15
Based on this series of observations and the favorable toxicity profile of green tea extracts in human testing,16, 17 we conducted a phase I/II trial of daily, oral Polyphenon E(a standardized, pharmaceutical grade catechin preparation) for patients with asymptomatic, Rai stage 0-II CLL.18 As previously reported, daily oral EGCG was well tolerated at the maximal dose tested(2000 mg twice per day) in the phase I component of the trial and declines in the absolute lymphocyte count(ALC) and/or lymphadenopathy were observed in the majority of the 36 treated patients treated.18 Coincident with our CLL trial, a randomized, placebo control trial of green tea catechins in patients with high grade prostate intraepithelial neoplasia found that tea catechins reduced the risk of progression to prostate cancer(progression to prostate cancer at 1 and 2 years: tea catechins 3% and 11%; placebo 30% and 53%; p<0.01).19, 20 Similar studies have also suggested EGCG may result in clinical benefit in patients with high risk premalignant oral lesions.21 These studies extend the extensive pre-clinical evidence and suggest green tea catechins may have clinical benefits in patients with premalignant and indolent malignant conditions. Here we report the results of the phase II component of the CLL trial exploring the clinical benefits of Polyphenon E.
MATERIALS AND METHODS
Patient Eligibility
We instituted a phase I/II trial of Polyphenon E in patients with previously untreated patients with asymptomatic, Rai stage 0-II CLL who did not meet National Cancer Institute Working Group(NCI-WG)22 criteria to initiate chemotherapy. Patients were required to have a confirmed diagnosis of CLL by standard criteria.22 Mantle cell lymphoma was excluded in all patients by fluorescent in situ hybridization(FISH) assessing for a t(11;14). The eligibility criteria for the phase II portion of the trial were identical to those reported for the phase I portion of the study18 with two exceptions. First, although patients who had used over the counter green tea or green tea extracts with medicinal intent were eligible for the phase I portion of the trial provided they had not used such medications within 8 weeks of registration; such individuals were excluded from the phase II portion of the trial so that it better reflected the experience of patients naïve to treatment with green tea extracts. Second, the phase I portion of the trial required patients have an ALC of at least 5 × 109/L while the phase II portion of the trial required patients have an ALC of at least 10 × 109/L to allow more accurate characterization of changes in absolute lymphocyte count (ALC). The protocol was reviewed and approved by the Mayo Clinic Institutional Review Board and registered with the National Institute of Health(clinicaltrials.gov). All patients provided written informed consent prior to study enrollment in accordance with the Declaration of Helsinki.
Protocol Treatment
Polyphenon E capsules containing ~200 mg of EGCG were supplied by the NCI or directly by Polyphenon E International(PEI). All patients in the Phase II portion of the trial received Polyphenon E at a dose of 1000 mg orally twice per day for the first 7 days of cycle 1 at which point they increased the dose to 2000 mg orally twice per day. Polyphenon E was administered with a light meal/snack. All study subjects were provided a medication diary to indicate the time and quantity of medication usage which was reviewed at each follow-up visit. Patients remained on active treatment for up to 6 months and were evaluated once every 4 weeks by physical examination and laboratory testing. Treatment was discontinued in the event of excessive toxicity or progressive disease(PD) as defined by the NCI-WG criteria.8 At the completion of 6 months of active treatment, patients entered observation. With the approval of the treating hematologist, patients who had not experienced disease progression and who desired to remain on treatment were provided with Polyphenon E containing capsules at their assigned dose level for up to 12 additional months.
Toxicity was graded using NCI Common Terminology Criteria for Adverse Events version 3.0. Because there is a low tolerance for toxicity in the treatment of CLL patients not meeting standard criteria for progressive disease, dose modifications were required for adverse events >grade 1 that were attributed to Polyphenon E and that did not respond to supportive care. In general, for grade 2 adverse events attributed to Polyphenon E, therapy was held until symptoms resolved to ≤grade 1 and then re-initiated at the original dose along with supportive care measures. For grade 3-4 adverse events attributed to study treatment or recurrent grade 2 events, Polyphenon E was held until symptoms resolved to ≤grade 1 and then re-initiated at the next lower dose level (reduction of 200 mg per dose) along with supportive care measures. Due to a FDA mandate, the notable exception to this approach was the response to any elevation in transaminases(AST or ALT) in which case Polyphenon E was held for grade 1 adverse events regardless of attribution until these values returned to normal when Polyphenon E was re-initiated at the same dose level. If grade 1 transaminitis recurred, Polyphenon E was held and re-initiated at the next lower dose(reduction of 200 mg BID) once AST or ALT values had returned to normal. Regardless of attribution, patients with ≥grade 2 transaminitis were required by the FDA to permanently discontinue study treatment.
Risk-stratification Parameters
All patients underwent a comprehensive CLL prognostic evaluation including assessment of CD38 and ZAP-70 expression, FISH detectable cytogenetic defects, and IGHV gene mutation testing as previously described.23, 24
Criteria for Response
Best response during the 6 months of active therapy was evaluated using the NCI-WG criteria.22 Given the favorable toxicity profile of Polyphenon E in healthy adults16 and the intention to evaluate the efficacy of this agent to delay or prevent disease progression in CLL, we also evaluated an additional response category termed “biologic response” among patients that did not meet standard NCI-WG criteria for complete or partial remission. The criteria for Biologic response was prospectively defined in the study protocol after discussion and approval of this endpoint by the NCI because of recognition that anti-cancer botanicals such as Polyphenon E may work through unique, non-cytotoxic mechanisms and these criteria were evaluated and published for the patients participating in the phase 1 trial18 before the phase 2 trial was initiated. Biologic response was defined as a reduction in the ALC of >20% from the pre-treatment level that was sustained for at least 2 months or a ≥30% reduction in all palpable lymphadenopathy. Biologic response evaluation was included as a primary endpoint in the study protocol with NCI approval to ensure that the study assessed the potential ability of EGCG to prevent CLL progression in patients that did not achieve a conventional response.
Plasma Polyphenol Levels
Trough(~12 hours after last dose) plasma EGCG levels were measured at the end of the first month of therapy in the laboratory of C.S. Yang using an established HPLC procedure with a coulochem electrode array system.25
Statistical Analysis
The primary outcomes for this phase II trial were assessment of tolerability and clinical response. Confirmed clinical responses(NCI-WG complete or partial remission) on 2 consecutive evaluations (e.g. over 2 month interval) and “biologic responses” as defined above were used as measures of clinical response and were summarized by simple descriptive statistics. Differences in response by key patient characteristics(i.e. ZAP-70 status) were compared using the Fisher exact test. Correlations between plasma EGCG level and dose/response were evaluated with the Spearman rank coefficient and the Wilcoxon rank sum test where appropriate. Treatment-free survival was defined as the time from the date of registration to the date of treatment for progressive CLL or death. Patients who are alive and treatment-free were censored on the date of last follow-up.
RESULTS
Patient Demographics
Thirty-seven patients were accrued to the phase II portion of the study between September 2007 and October 2010. One patient was deemed ineligible for evaluation because study drug was not administered correctly during cycle 1. Per protocol, the remaining 36 patients along with the 6 patients in the phase I portion of the trial treated at the phase II dose level were evaluated to determine tolerability and efficacy of the phase II dose level(n=42). The clinical characteristics of the 42 eligible patients are presented in Table 1. A majority of patients (n=29; 69%) had Rai stage I-II disease. Most patients had favorable prognostic profiles on FISH, ZAP-70, CD38, and IGHV gene mutation analysis at study entry consistent with the eligibility requirements that patients be asymptomatic and have earlier stage disease.
Table 1.
Patient Characteristics
| N=42 | ||
|---|---|---|
| Median Age | 60(41-78) | |
| Median time from diagnosis to registration | 16 months(0.7 – 106) | |
| Male | 30(71%) | |
| Median ALC (×109/L) | 33(10–258) | |
| Rai Stage | 0 | 13(31%) |
| I | 24(57%) | |
| II | 5(12%) | |
| ZAP-70 | ≥20% | 12(29%) |
| CD38 | ≥30% | 7(17%) |
| IGHV1 | Unmutated | 6(18%) |
| FISH | (del)13q14.2 | 27(64%) |
| normal | 10(24%) | |
| Trisomy 12 | 4(10%) | |
| (del)11q22 | 1(2%) | |
| (del)17p13 | 0(0%) | |
Although IGHV mutation analysis was performed in all patients, it was non-informative in 9.
Toxicity and Tolerability
Median overall compliance with the prescribed dose as assessed using pill diaries was 96%(range 51%-104%). During the 6 months of active treatment 13/42 (31%) of patients required a dose reduction. Side effects at least possibly attributed to therapy during the 6 months of active treatment were generally mild with 18(43%) patients having a maximum of a grade 2 event and 3(7%) patients having a grade 3 event (Table 2). Thirty patients completed 6 cycles of active therapy. Twelve patients discontinued therapy early: 9 experienced an adverse event and 3 progressed. Per FDA requirement in all human trials of Polyphenon E, 6 patients were forced to discontinue treatment after experiencing ≥grade 2 transaminitis(5 during the first 6 cycles of treatment and 1 patient during continuation). Changes in ALC in 6 patients forced to discontinue treatment after experiencing to ≥grade 2 transaminitis are shown in Figure 1.
Table 2.
Side Effects(at least possibly related)
| CTCAE Classification | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Nausea | 23(55%) | 2(5%) | - |
| Abdominal pain | 9(21%) | 3(7%) | 1(2%) |
| Transaminitis | 13(31%) | 6(14%) | 1(2%) |
| Anorexia | 12(29%) | 1(2%) | - |
| Diarrhea | 19(45%) | 4(10%) | - |
| Dyspepsia | 11(26%) | 1(2%) | - |
| Flatulence | 13(31%) | 2(5%) | - |
| Fatigue | 11(26%) | 3(7%) | 1(2%) |
| Hyperglycemia | 1(2%) | - | - |
Figure 1.
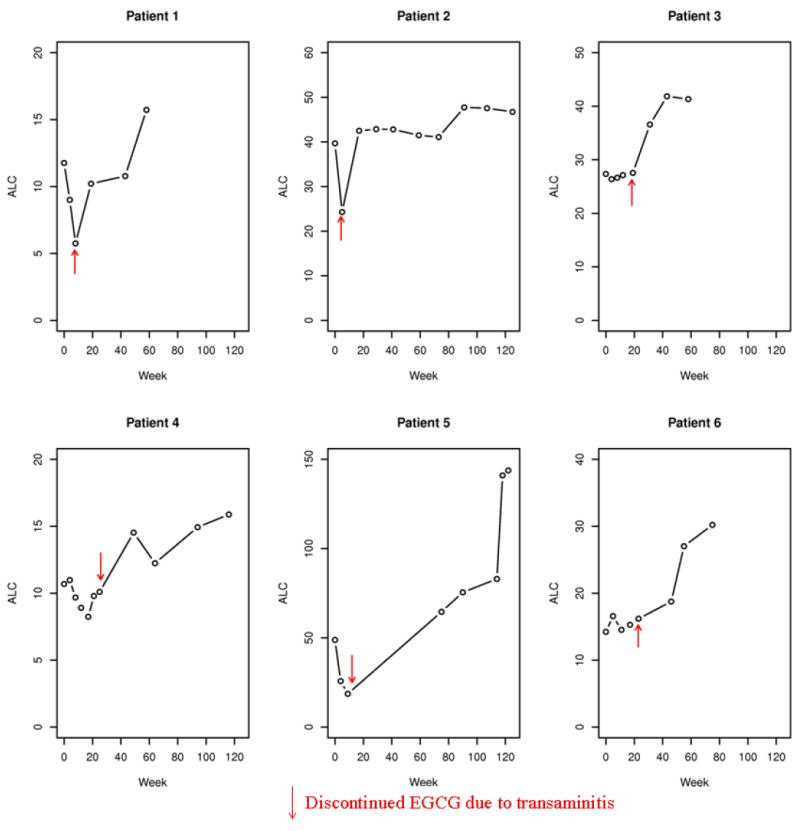
Absolute lymphocyte counts (ALC) in Patients Discontinuing Therapy because of Increased Serum Transaminase Levels.
Time 0 indicates the ALC prior to initiating EGCG therapy. Red arrows indicate the ALC at the time EGCG was discontinued due to the increase in serum transaminase levels.
Response to Therapy
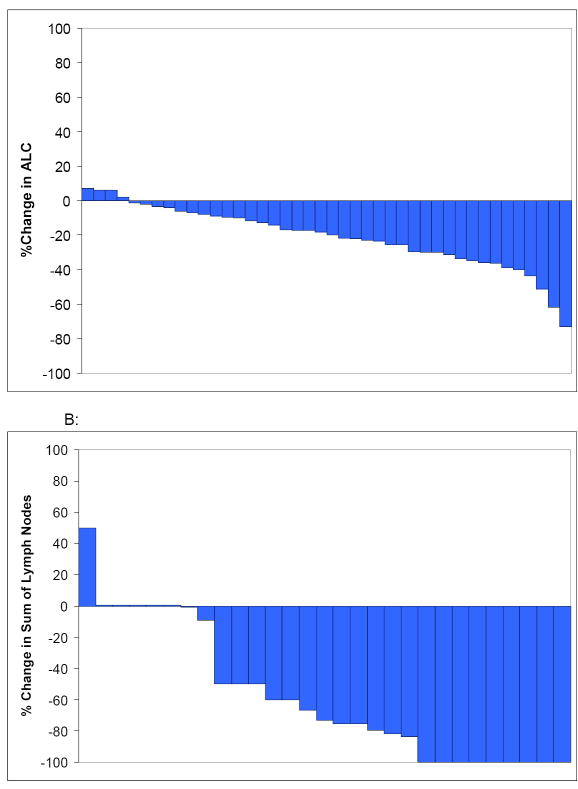
One patient treated at the phase II dose level of the phase I trial achieved a partial remission according to the NCI working group criteria. Most (67%) patients had a reduction in ALC(Table 3; Figure 2A) which was transient in some patients while others had a steady, sustained stepwise reduction throughout the 6 months of active therapy. Among the 22(52%) patients who had a ≥20% reduction in ALC, 13(31% overall study population) had a sustained decrease ≥20% persisting for at least 2 months and thus fulfilled the criteria for biologic response (see methods). Among patients who completed 6 cycles of therapy, the ALC at the completion of therapy was below the baseline ALC in 15(47%) patients. Among the 29 patients with palpable adenopathy at enrollment, 20(69%) experienced at least a 50% reduction in the sum of the products of all palpable nodal areas at some point during treatment. Collectively, 12 of the 29 (41%) patients with Rai stage I/II disease were down staged during treatment including 8/24 (33%) stage 1 patients who had resolution of adenopathy and would be down staged to Rai 0 and 4 (100%) stage 2 patients (1 down staged to Rai 0 and 3 down staged to staged to Rai 1; Figure 2B). Among patients with lymphadenopathy at enrollment who completed 6 cycles of therapy, the sum of the nodal products at the completion of therapy was below baseline for 13 (65%) patients. Overall 29(69%) patients fulfilled the criteria for a biologic response.
Table 3.
Best Response in ALC and Nodes
| Best reduction ALC | N (%) | ||
|---|---|---|---|
| At least 10% decline | 28/42(67%) | ||
| At least 20% decline | 22/42(52%) | ||
| At least 30% decline | 12/42(29%) | ||
| At least 40% decline | 4/42(10%) | ||
| At least 50% decline | 3/42(7%) | ||
| Best reduction Lymphadenopathy | |||
| At least 50% reduction sum products | 20/29*(69%) | ||
| PR or Biologic Response | 29/42(69%) | ||
| PR or Biologic Response by Prognostic Parameter | Proportion with at Least Biologic Response | P value | |
| ZAP-70 | Negative(<20%) | 21/30(70%) | 1.0 |
| Positive(≥20%) | 8/12(67%) | ||
| CD38 | Negative(<30%) | 25/35(71%) | 0.66 |
| Positive(≥30%) | 4/7(57%) | ||
| IGHV | Mutated | 19/27(70%) | 1.0 |
| Unmutated | 5/6(83%) | ||
| FISH | (del)13q14.2 | 19/27(70%) | 0.84 |
| normal | 7 /10(70%) | ||
| Trisomy 12 | 2/4(50%) | ||
29 patients had palpable lymphadenopathy at study entry
Figure 2.
Reductions in ALC and Lymphadenopathy
A: Waterfall plot of best ALC declines during treatment
B: Waterfall plot of best reduction in sum of lymph nodes
The proportion of patients with a PR or biologic response by each prognostic parameter is shown in Table 3. No differences in response were observed based on IGHV, ZAP-70, or CD38, or on FISH testing although sample size for some comparisons was small.
After median follow-up of 32 (range 21-51) months from registration and median 56 months from diagnosis, 12(29%) patients have progressed to require treatment for CLL(Figure 3A). The 24-month treatment free survival(TFS) from registration was 79%(95%CI: 62-92) and appeared similar in ZAP-70+ and ZAP-70 negative patients(Figure 3B).
Figure 3.
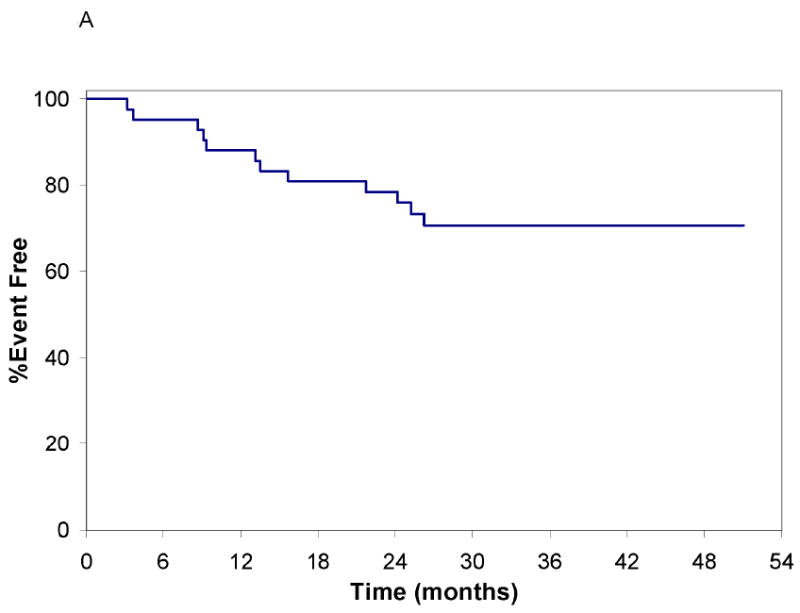
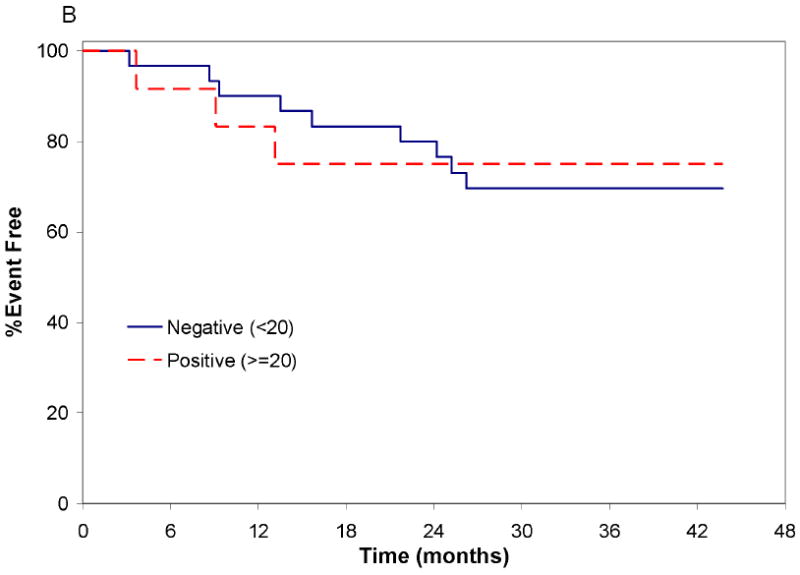
Treatment Free Survival from Date of Registration
A: Treatment Free Survival All Patients from the Date of Registration: Time in months is shown on the x axis. The event free survival is shown on the y axis (initiation of CLL treatment or death were considered events). No deaths have been observed to date; 8 patients required treatment for progressive CLL.
B.Treatment Free Survival from Date of Registration by ZAP-70 Status: The event free survival of ZAP-70 positive (n=12) and ZAP-70 negative (n=30) patients are shown (Log rank p-value: 0.53).
Plasma Polyphenol Levels
Median trough total plasma EGCG level after 1 month of therapy was 188.6 ng/mL with a wide range of 5.2-4,342 ng/mL(0.001 - 9.56 uM). There was a moderate correlation between plasma EGCG levels and reductions in lymphadenopathy(sum of nodal products; correlation 0.44; p=0.02) at one month but not in the reductions in ALC(correlation 0.18; p=0.28).
DISCUSSION
Although a majority of patients with CLL have Rai stage 0-I disease at diagnosis, ~70% eventually progress to require treatment and a majority will ultimately die from CLL or CLL related complications.26-30 Accordingly, patients with asymptomatic early to intermediate stage CLL represent an appropriate patient population in which to test the ability of nutraceutical agents with a favorable toxicity profile to prevent or delay disease progression. This approach is conceptually different than early administration of conventional chemotherapy28, 31 since it does not prematurely expend/exhaust an agent to be used as a treatment later in the course of the disease, is less likely to cause major toxicity (e.g. DNA damage) and should not induce chemotherapy resistance. In this phase II trial, EGCG in the Polyphenon E preparation was well tolerated at a dose of 2000 mg orally twice per day for 6 months in patients with asymptomatic, Rai stage 0-II CLL. The most severe toxicity was ≤grade 2 for 93% of patients. Although eligibility for this phase II trial required a higher baseline ALC than our previous phase I trial(10 ×109/L rather than 5 ×109/L), the clinical activity observed was nearly identical to the Phase I study with ~30% of patients experiencing a sustained decline in the ALC of ≥20% and ~70% of those with lymphadenopathy at study entry experiencing a ≥50% reduction in the sum of the nodal products during treatment. Overall, ~70% of patients had a biologic response, a protocol specified endpoint developed after discussion and approval by the NCI prior to the previously published phase I study.18 No difference in the proportion of patients achieving a biologic response was observed based on ZAP-70, CD38, or IGHV gene mutation status. This rate of biologic responses exceeded the protocol specified decision rule that a biologic response rate ≥50% would suggest EGCG was worthy of further clinical testing.
A higher proportion of patients achieved a biologic response in the phase II trial than the phase I study (~70% vs. 55%). This occurred even though the phase II trial accrued a higher proportion of Rai stage I-II CLL patients (69% vs. 45%). The higher biologic response rate could have occurred because the phase II trial enrolled exclusively EGCG naïve patients. Alternatively this finding could suggest that higher doses of EGCG are more effective. This possibility is supported by the higher rate of biologic responses in patients taking doses ≥1200 mg BID in the phase I study compared to those taking <1200 mg BID(biologic response 76% vs. 17%) and the observation that EGCG plasma levels correlated with reductions in lymphadenopathy in the phase II trial.
While spontaneous regressions occasionally occur in patients with CLL, such remissions are rare(~1% in most series32-34). While fluctuations in ALC and lymphadenopathy can be expected in a subset of patients, the expected pattern for most patients is that of a rising ALC and progressive lymphadenopathy. Among the patients completing 6 months of EGCG the ALC at the completion of therapy was below baseline in ~50% and the sum of the nodal products at the completion of therapy was below baseline for ~65%. The rapid decline in ALC and/or lymphadenopathy observed in a majority of patients after starting EGCG also strongly suggests that this was a treatment effect. This conclusion is supported by the effect of cessation of EGCG therapy in patients who developed grade 2 transaminitis. Three of these 6 patients(patients 1, 2, 5 of Figure 1) had a rapid and substantial(~50%) decline in their ALC within the first 2 months of starting EGCG followed by an immediate return to near pre-treatment levels after discontinuing EGCG.
Although ~70% of the patients in this study had Rai stage I-II CLL, it should be emphasized that the patients enrolled were asymptomatic, and did not meet criteria to initiate conventional chemotherapy treatment.22 It is unknown whether the modest clinical effects observed translate into a delay in disease progression or need for subsequent chemotherapy. It should also be emphasized that EGCG can in no way be considered a substitute for traditional chemotherapy and/or monoclonal antibody based treatment once need for treatment develops.22 The EGCG containing preparation used in the present study was a pharmaceutical grade product with a standardized and verified EGCG dose/content confirmed by the NCI and/or the pharmaceutical manufacturer. Accordingly it is unknown how the clinical effects reported here translate to use of over-the-counter, food supplement grade EGCG containing products that are not subject to stringent quality control. Further, some animal studies suggest that a mixture of polyphenols is needed for maximal anti-tumor effect35-37 and it is unknown whether the effects observed were related to polyphenols present in Polyphenon E other than EGCG or the specific composition of this EGCG-containing preparation. Although correlative in vitro studies in the present trial did not suggest EGCG induced resistance to fludarabine or chlorambucil, it is unknown if these are an accurate approximation of in vivo effects or whether long term use of EGCG containing products may influence future sensitivity to conventional chemotherapeutic agents.
There remains great interest on the part of both CLL patients and their physicians in identifying low toxicity interventions with the potential to delay disease progression, particularly for those patients whose molecular prognostic markers(e.g. ZAP, IGHV mutation status) predict a higher risk of disease progression. An effective disease stabilizing agent must be both efficacious and safe enough for extended use since short-term administration would not be expected to substantially affect the risk of progression over the longer term. EGCG appears to fulfill many of these criteria. Oral EGCG preparations with improved bioavailability are also being developed38-40 and could be more effective. Ultimately, the ability of EGCG or other nutraceutical compounds to delay disease progression will need to be determined in a randomized trial.
Acknowledgments
We grateful to the CLL patients who participated in this trial and made this research possible.
Research Support: Support through grants from the National Cancer Institute (NCI CA 113408, CA6912, CA133021), Gabrielle’s Angel Research Foundation, CLL Global Research Foundation, CLL Topics, Commonwealth Foundation for Cancer Research, Polyphenon E International, and the Mayo Clinic Cancer Center are gratefully acknowledged.
References
- 1.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81(7):519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CS, Wang ZY. Tea and cancer. Journal of the National Cancer Institute. 1993;85(13):1038–49. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Zhao X, Zhang X, Holman CD. Possible protective effect of green tea intake on risk of adult leukaemia. Br J Cancer. 2008;98(1):168–70. doi: 10.1038/sj.bjc.6604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frankenfeld CL, Cerhan JR, Cozen W, et al. Dietary flavonoid intake and non-Hodgkin lymphoma risk. Am J Clin Nutr. 2008;87(5):1439–45. doi: 10.1093/ajcn/87.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo YC, Yu CL, Liu CY, et al. A population-based, case-control study of green tea consumption and leukemia risk in southwestern Taiwan. Cancer Causes Control. 2009;20(1):57–65. doi: 10.1007/s10552-008-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naganuma T, Kuriyama S, Kakizaki M, et al. Green tea consumption and hematologic malignancies in Japan: the Ohsaki study. Am J Epidemiol. 2009;170(6):730–8. doi: 10.1093/aje/kwp187. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389(6647):134–5. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269(2):269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature reviews Cancer. 2009;9(6):429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63(23):8118–21. [PubMed] [Google Scholar]
- 11.Bertolini F, Fusetti L, Rabascio C, Cinieri S, Martinelli G, Pruneri G. Inhibition of angiogenesis and induction of endothelial and tumor cell apoptosis by green tea in animal models of human high-grade non-Hodgkin’s lymphoma. Leukemia. 2000;14(8):1477–82. doi: 10.1038/sj.leu.2401854. [DOI] [PubMed] [Google Scholar]
- 12.Nakazato T, Ito K, Ikeda Y, Kizaki M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res. 2005;11(16):6040–9. doi: 10.1158/1078-0432.CCR-04-2273. [DOI] [PubMed] [Google Scholar]
- 13.Shanafelt T, Lee YK, Geyer SM, et al. The Green Tea Extract Epigallocatechin Induces In VItro Cell Death in Primary Human Lymphoma Cells through an ROS Dependent Mechanism. Blood. 2006;108 Abstract 234. [Google Scholar]
- 14.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104(3):788–94. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 15.Shanafelt TD, Lee YK, Call TG, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–12. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Chow H-HS, Cai Y, Hakim IA, et al. Pharmacokinetics and Safety of Green Tea Polyphenols after Multiple-Dose Administration of Epigallocatechin Gallate and Polyphenon E in Healthy Individuals. Clin Cancer Res. 2003;9:3312–19. [PubMed] [Google Scholar]
- 17.Pisters KM, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19(6):1830–8. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 18.Shanafelt T, Call TG, Zent CS, et al. Phase 1 Trial of Daily, Oral Polyphenon E in Patients with Asymptomatic, Rai Stage 0-II Chronic Lymphocytic Leukemia. J Clin Oncol. 2009;27:3808–14. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer research. 2006;66(2):1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 20.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. European urology. 2008;54(2):472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 21.Tsao AS, Liu D, Martin J, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer prevention research. 2009;2(11):931–41. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group Guidelines fo Chronic Lymphocytic Leukemia: Reised Guidelines for Diagnosis and Treatment. Blood. 1996;87:4990–97. [PubMed] [Google Scholar]
- 23.Shanafelt TD, Geyer SM, Bone ND, et al. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. Br J Haematol. 2008;140(5):537–46. doi: 10.1111/j.1365-2141.2007.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewald G, Brockman S, Paternoster S, et al. Chromosome anomalies detected by interphase fluorscence in hybridization: correlation with significant biological features of chronic lymphocytic leukemia. British Journal of Haematology. 2003;121:287–95. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279(2):164–9. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- 26.Call T, Phyilky R, Noel P, et al. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clinic Proceedings. 1994;69:323–28. doi: 10.1016/s0025-6196(12)62215-0. [DOI] [PubMed] [Google Scholar]
- 27.Cavotsky D, Fooks J, Richards S. Prognostic factors in chronic lymphocytic leukaemia: the importance of age, sex and response to treatment in survival. Br J Haematol. 1989;72:141–49. doi: 10.1111/j.1365-2141.1989.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 28.Dighiero G, Maloum K, Desablens B, et al. Cholorambucil in indolent chronic lymphocytic leukemia. Frech Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med. 1998;338:1506–14. doi: 10.1056/NEJM199805213382104. [DOI] [PubMed] [Google Scholar]
- 29.Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer. 1999;86(12):2684–92. [PubMed] [Google Scholar]
- 30.Molica S, Levato D. What is changing in the natural history of chronic lymphocytic leukemia. Haematologica. 2001;86:8–12. [PubMed] [Google Scholar]
- 31.Chemotherapeutic Options in Chronic Lymphocytic Leukemia: a Meta-analysis of the Randomized Trials. CLL Trialists’ Collaborative Group. Journal of the National Cancer Institute. 1999;91:861–68. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 32.Thomas R, Ribeiro I, Shepherd P, et al. Spontaneous clinical regression in chronic lymphocytic leukaemia. British journal of haematology. 2002;116(2):341–5. [PubMed] [Google Scholar]
- 33.Ribera JM, Vinolas N, Urbano-Ispizua A, Gallart T, Montserrat E, Rozman C. “Spontaneous” complete remissions in chronic lymphocytic leukemia: report of three cases and review of the literature. Blood cells. 1987;12(2):471–83. [PubMed] [Google Scholar]
- 34.Del Giudice I, Chiaretti S, Tavolaro S, et al. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood. 2009;114(3):638–46. doi: 10.1182/blood-2008-12-196568. [DOI] [PubMed] [Google Scholar]
- 35.Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer prevention research. 2009;2(6):514–7. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu H, He J, Mei F, et al. Lung cancer inhibitory effect of epigallocatechin-3-gallate is dependent on its presence in a complex mixture (polyphenon E) Cancer prevention research. 2009;2(6):531–7. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y, Cook J, McQuillan J, et al. Chemopreventive effect of aerosolized polyphenon E on lung tumorigenesis in A/J mice. Neoplasia. 2007;9(5):401–5. doi: 10.1593/neo.07160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janle EM, Morre DM, Morre DJ, Zhou Q, Zhu Y. Pharmacokinetics of Green Tea Catechins in Extract and Sustained-Release Preparations. Journal of dietary supplements. 2008;5(3):248–63. doi: 10.1080/19390210802414279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui IA, Adhami VM, Bharali DJ, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer research. 2009;69(5):1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landis-Piwowar KR, Huo C, Chen D, et al. A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer research. 2007;67(9):4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]