Abstract
Metacognition can be defined as knowing what one knows, and the question of whether nonhuman animals are metacognitive has driven an intense debate. We tested three language-trained chimpanzees in an information-seeking task in which the identity of a food item was the critical piece of information needed to obtain the food. In two experiments, the chimpanzees were significantly more likely to visit a container first on trials in which they could not know its contents but were more likely to just name the item without looking into the container on trials in which they had earlier seen the contents of that container. Thus, chimpanzees showed efficient information-seeking behavior that suggested they knew what they had or had not already seen when it was time to name a hidden item.
Keywords: metacognition, chimpanzees, Pan troglodytes, information-seeking
Metacognition can be defined as knowing what one knows (Flavell 1979; Koriat 1993; Nelson 1992; Schwartz 1994). Humans use metacognition frequently, as when we panic upon seeing an acquaintance whose name we cannot recall, or when we waver before answering the million dollar question in a game show and try to decide whether to “phone a friend” or trust our own knowledge. In essence, a metacognitive system supervises and surveys what necessary or relevant information a cognitive system has or does not have, and then generates responses accordingly. If one knows the answer to a question, or one can decide what to call something, an answer is generated. But if information is incomplete, or an answer is not clearly indicated, the metacognitive system generates responses to avoid responding, to seek additional information, or to request help.
Adult humans can show excellent metacognitive monitoring. Some nonhuman animal species have been reported to also show behavioral patterns that might reflect aspects of metacognition such as information-seeking behavior or uncertainty monitoring (Basile et al., 2009; Beran & Smith, 2011; Call & Carpenter, 2001; Foote & Crystal, 2012; Hampton, 2001; Kornell, Son, & Terrace, 2007; Smith et al., 1995, 1997, 1998, 2006), but the degree to which nonhuman animals’ performance reflects metacognition like that shown by humans is less clear and has driven an intense debate (see Carruthers, 2008, 2009; Crystal & Foote, 2009; Smith, 2009; Smith et al., 2008, 2009, 2012; Staddon et al., 2009).
One influential and useful paradigm in this area of inquiry is the information-seeking task. Typically, animals are exposed to two kinds of situations – one in which they are given the information they need to make an accurate response, and one in which more information is needed before they can respond accurately. For example, Call and Carpenter (2001) presented orangutans, chimpanzees and children with a test in which the goal was to reach into the container that held a prized reward. In some trials, the apes or children were shown exactly where the item was hidden. In other trials, that hiding event was done out of view, and so the apes or children could not know where the item was hidden without moving themselves into position to look into each of the possible hiding locations to find the item before reaching for it. The prediction was that, if children and apes knew when they knew the location, they would reach without looking. And, if they knew when they did not know where the item was, they should look first, and then reach after locating it. This is exactly the pattern that emerged in the responses for all three species. Subsequently, other species were tested, and some showed the potentially metacognitive pattern of responding (gorillas, orangutans, chimpanzees, bonobos: Call 2010; rhesus monkeys: Hampton et al. 2004) whereas others did not (capuchin monkeys: Basile et al. 2009; Paukner et al. 2006; dogs: Bräuer et al. 2004).
Additionally, concerns were raised about the test itself, and whether it required a metacognitive explanation. For example, Carruthers (2008) argued that animals could succeed—using only first-order mental states—in reaching immediately when they should and in looking further to collect information when they should. According to this argument, when an animal sees food in a tube, it believes food is in the tube. It also believes that if it selects that tube, food will be the result, and of course the animal desires food. Thus, it reaches immediately for the tube attached to these beliefs about getting food. When the baiting is not observed, the animal has no food beliefs attached to any of the tubes, and so the animal searches until such beliefs emerge because food is finally seen in one of the tubes. Put more simply, animals that lack information about the location of the food engage in search behavior until they locate the reward. Using associative terminology, the parallel argument could be made that the subjects learn a rule such as “If I see food, then don’t look; If I don’t see food, then do look,” based on the reinforcement histories of these different behavior chains in different contexts. Crystal and Foote (2011) also offered alternative explanations of the typical “metacognitive” results in these types of experiments by positing that two relatively simple principles could guide an animal’s behavior without need of metacognition: animals might have a “look before you go” response which supersedes random searches in space. The other principle assumes that spatially guided behavior should follow a rule of going to wherever something good is located. Together these two principles could potentially account for performance on information-seeking tasks without proposing a need for metacognition.
There are counter-arguments to these criticisms (e.g., Call, 2010), but there are also methods that can get around this problem if one changes the task. For example, one can ensure that a subject always has the belief that there is a valuable item in a container, but sometimes the subject cannot know what that item is. This approach was used in Experiment 1 with language trained apes capable of communicating the identity of items. Rather than requiring chimpanzees to determine where an item was hidden, we required them to name what was in a container, so that there was always a belief that food was in the container, and there was always a desire to get the food that was in the container. But, there was the restriction that items could only be obtained if they were properly named. Thus, if a chimpanzee knew what was in the container, it could name the item forthwith. But, if it did not, it would have to approach the container, a behavior that was more costly in terms of time and effort, determine what was inside, and then name the item. Thus, the missing information was not about where the food reward was, and not about its value (all food rewards were valued), but about what the identity of the item was so that it could be named – a piece of information that had to be communicated to an experimenter. This method eliminates the concerns about spatial gradients and default responses of looking before one chooses a container (Crystal & Foote, 2011) because the goal is not to find the item but to name it.
Even success in this new variation of the task could be attributed to a multi-component rule such as chimpanzees learning to approach the container when they had not seen any food but to name immediately if they saw food at the start of the trial. To control for this possibility, Experiment 2 involved always showing food in one container, and never showing the food in a second container, and then choosing one of the containers for placement in the enclosure where naming took place. Now, chimpanzees could not simply name whatever they saw in the first part of the trial, because sometimes that item stayed in the container that was not moved to the naming area, and they could not use a rule such as “look when no food has been seen, and name when any food has been seen” because food was seen on every trial. Instead, the chimpanzees had to attend to whether the container that was moved was the one holding the item they had seen, and if so, they could name that food item immediately. But, if the container that was moved had not been opened to show its contents, the chimpanzees would have to instead move to the container, look inside, and then name the item. High performance from the outset would discount these alternate explanations of success in Experiment 1, and strengthen the conclusion that chimpanzees used flexible information-seeking behaviors to accompany their knowledge states, in a manner that would reflect metacognitive monitoring.
Experiment 1
Method
Participants
Three chimpanzees, Lana (female, 41 years old), Sherman (male, 38 years old), and Panzee (female, 25 years old) were tested. All three chimpanzees had been reared from an early age to use lexigram symbols to request and label objects, actions, locations, and individuals as well as to respond to requests by humans using those symbols (Brakke & Savage-Rumbaugh, 1995; Rumbaugh, 1977; Rumbaugh & Washburn, 2003; Savage-Rumbaugh, 1986). All three chimpanzees had participated in a large number of previous experiments, some of which made use of lexigram symbols to assess various aspects of cognition (e.g., Beran, Savage-Rumbaugh, Pate, & Rumbaugh, 1999; Heimbauer, Beran & Owren, 2011; Menzel, 1999).
The chimpanzees were tested in their home-cage area, which included indoor enclosures and outdoor yards (see Figure 1). Each chimpanzee was tested while singly housed, and tests occurred two to four times per week between 10:30 h and 13:00 h.
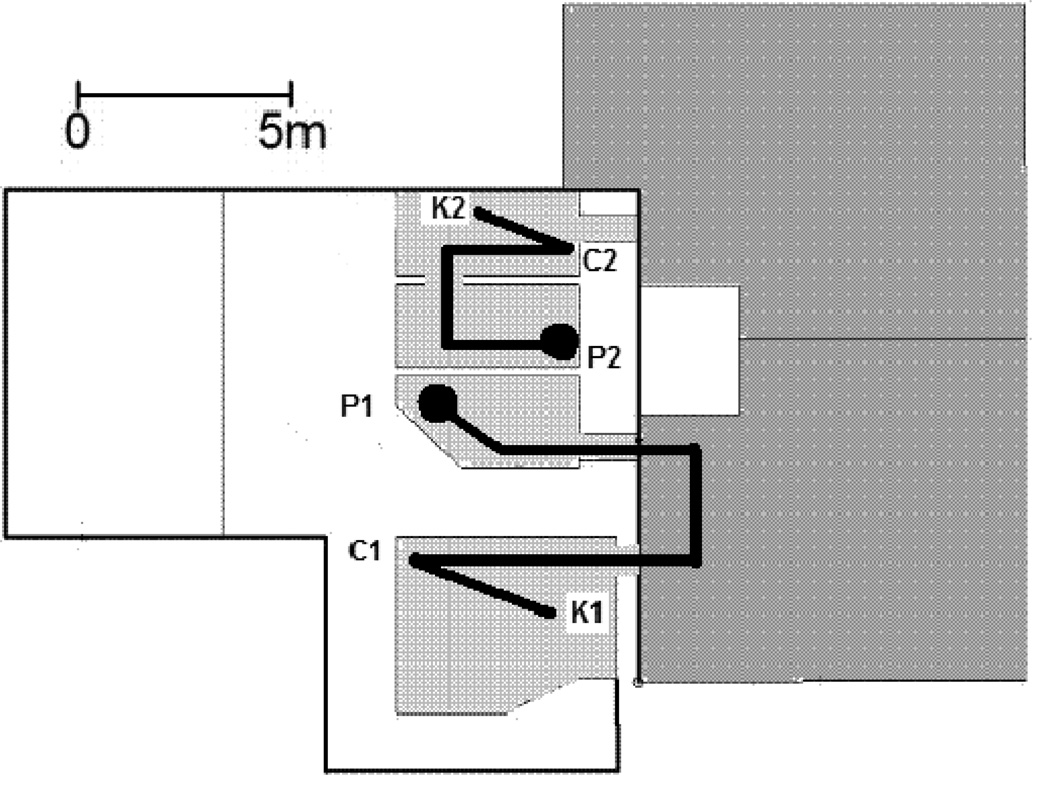
Figure 1.
A schematic of the test area. The areas show in light grey color are indoor housing areas for the chimpanzees. The dark grey areas are outdoor yards for the chimpanzees. P1 (Sherman and Panzee) and P2 (Lana) refer to the locations where the experimenter either showed or did not show the contents of the container initially before moving the container to location C1 (Sherman and Panzee) or C2 (Lana). K1 was the board used by Sherman and Panzee, and K 2 was the keyboard used by Lana to indicate the contents of the container. The dark circles show the starting locations for the chimpanzees, and the dark lines show the routes the chimpanzees had to take if they chose to first view the contents of the container after it was moved before they went to the keyboard to name the item in the container.
Design and Procedure
At the outset of each trial, Experimenter 1 moved out of view of the chimpanzee and placed a single food item into an opaque container. This item was always a preferred food item for which that chimpanzee could reliably name the item with its corresponding lexigram. Experimenter 1 then moved within view of the chimpanzee, removed the lid from the container, and made one of two motions – either tilting the container toward the chimpanzee so that it could see the contents of the container, or tilting the container away from the chimpanzee so that the contents remained unknown. Experimenter 1 then moved to a location adjacent to the area the chimpanzee was currently in, and placed the container on a chair so that its contents could only be viewed from a particular location in that other area (Figure 1). Experimenter 1 then left the test area for the remainder of the trial. The chimpanzee then had to leave its original area, and move through space such that it lost visual contact with the container for a period of time before arriving in the second location. Once there, the chimpanzee could take one of two routes – move directly to the lexigram keyboard and indicate the contents of the container to the second experimenter or move instead to the container and a position from which it could view the contents. Critically, the chimpanzee could not see what was in the container from the keyboard location. Thus, moving to the container was costly, and on average added approximately 15 seconds of delay beyond the time it took to just move to the keyboard and select a lexigram.
At the outset of each trial, Experimenter 2 stood in an area of the laboratory from which he could read any responses made by a chimpanzee to a lexigram keyboard on which there were 256 individual lexigrams to choose from. Experimenter 2 was unaware of what was in the container, and he did not know whether the chimpanzee had been shown the contents of the container or not. This experimenter recorded whether the chimpanzee first approached the container or the keyboard, and what lexigram was indicated on the keyboard (in all trials, the chimpanzees indicated only a single item on the keyboard). After a keyboard response, Experimenter 2 moved to the container and determined if the item inside matched what the chimpanzee had indicated on the lexigram board. If it did, that item was given to the chimpanzee. If it did not, the item was removed and the chimpanzee did not receive anything.
Each session consisted of four trials, two with the contents shown to the chimpanzee, and two with no information about item type being given to the chimpanzee. Only a subset of highly preferred food items with corresponding lexigrams were used in the experiment, including bananas, juice, oranges, M&Ms candies, apples, bread, soda, sweet potatoes, kiwis, raisins, peanuts, grapes, strawberries, and primate biscuits. Foods were chosen at random across trials. Each chimpanzee completed 10 sessions in this experiment for a total of 40 trials. Because of experimenter error, Panzee and Sherman were given one extra nonvisible trial for a total of 41 trials. Those trials were included in the analyses.
Data Analysis
We conducted a chi-square test of independence to assess the relationship between presentation condition (visible and nonvisible) and subject’s first response (to container or keyboard). We expected that subjects would visit the container first more often when they had not seen the contents (nonvisible condition), and to visit the keyboard first more often when they had seen the contents of the container (visible condition).
Results
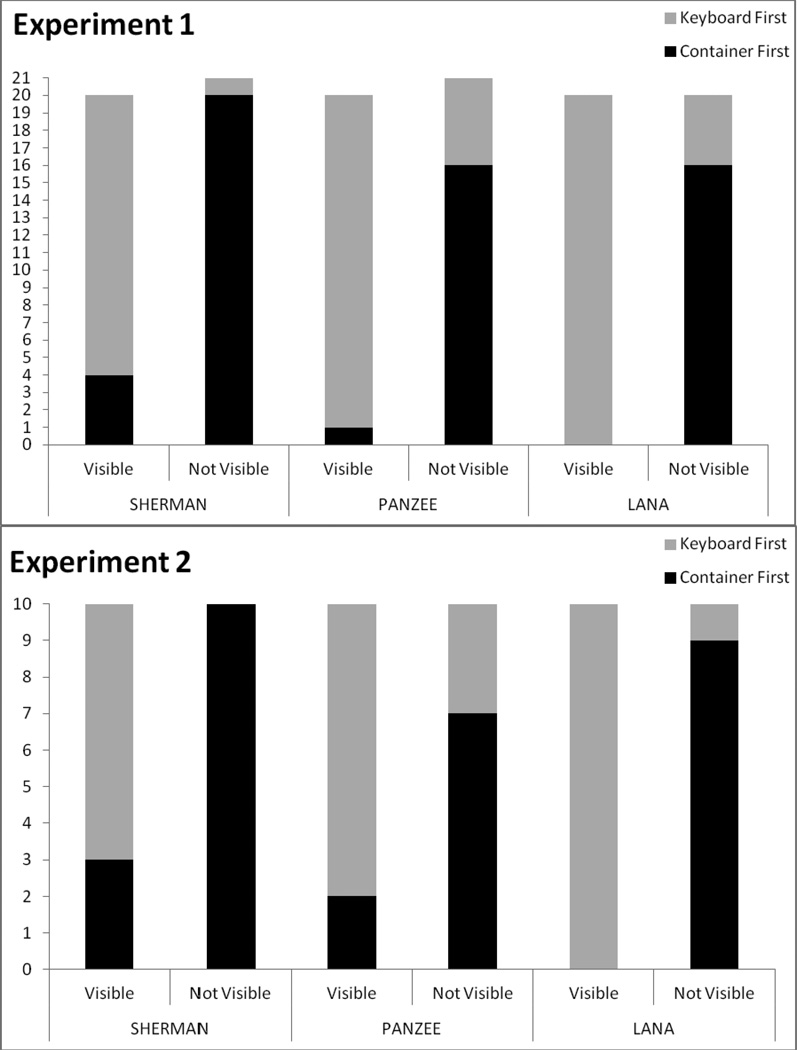
Figure 2 shows the performance of each chimpanzee in each experiment. In Experiment 1, all chimpanzees were statistically more likely to visit the keyboard and name first in the visible condition and to visit the container first in the nonvisible condition (Sherman: Χ2 (1, N = 41) = 23.89, p < .001; Panzee: Χ2 (1, N = 41) = 21.39, p < .001; Lana: Χ2 (1, N = 40) = 26.67, p < .001). This behavioral pattern emerged in the very earliest trials. For the first eight trials of Experiment 1, Lana made the optimal response in 7 of 8 trials, Panzee did so for 6 of 8 trials, and Sherman did so for 6 of 8 trials.
Figure 2.
Number of responses (moving to the keyboard first or the container first) in each condition of Experiment 1 and Experiment 2 for each chimpanzee.
When the chimpanzees either saw the food item and moved first to the keyboard, or went to the container before naming, they were correct in indicating the name of the food item on 109 of 112 trials. However, when they erroneously went to the keyboard and named without seeing the contents of the container, they correctly named the item only 4 times in 10 such trials.
Experiment 2
Methods
Participants
The same chimpanzees were tested as in Experiment 1.
Design and Procedure
The general design of Experiment 2 was the same as that of Experiment 1 in task requirements and in how the chimpanzees moved through space to make responses in naming the item in the container. The only difference occurred during the actions of Experimenter 1. Now, this experimenter first baited two opaque containers out of view of the chimpanzee with two preferred items, each of which had a corresponding lexigram. Both covered containers then were placed in front of the chimpanzee on the floor. The first container was then lifted, the lid was removed, and either the contents were shown to the chimpanzee or the container was tilted away from the chimpanzee so that the contents remained unknown. The same thing then occurred for the second container. On a given trial, one container’s contents were revealed to the chimpanzee, and the other’s were not (counterbalanced for order across trials). Then, the experimenter moved one of the two containers to the chair in the second location, as in Experiment 1. This meant that on each trial, the chimpanzee saw one food type, but the contents of one container remained unknown. Then, the chimpanzee had to attend to which container was moved to the second location so that, presumably, it knew whether it had seen the contents of that container or whether those contents remained unknown. Experimenter 1 then left the test area, and trials proceeded exactly as they had in Experiment 1, with the chimpanzee moving to the second location and either looking in the container first or selecting a lexigram on the keyboard without having looked in the container.
Each session consisted of four trials, two in which the container moved to the second area had been revealed, and two of which involved the container that had not been revealed moving to the second area. The order of these two trials types was randomized within the session. Each chimpanzee completed 5 sessions in this experiment, for a total of 20 trials, 10 of each type (contents visible or not visible).
Results
In Experiment 2, all chimpanzees were significantly more likely to visit the keyboard and name first in the visible condition and visit the container first in the nonvisible condition (Sherman: Χ2 (1, N = 20) = 10.77, p = .001; Panzee: Χ2 (1, N = 20) = 5.05, p = .025; Lana: Χ2 (1, N = 20) = 16.36, p < .001; Figure 2).
For the first eight trials of Experiment 2, Lana and Sherman made the optimal response in 7 of 8 trials, and Panzee did so for 5 of 8 trials. Thus, performance was not changing across the course of the experiments as would be expected if the chimpanzees had to learn what to do through experience.
When the chimpanzees either saw the food item and moved first to the keyboard, or went to the container before naming, they were correct in indicating the name of the food item on 55 of 56 trials. However, when they erroneously went to the keyboard and named without seeing the contents of the container, they correctly named the item only 1 time in 4 such trials.
General Discussion
In both experiments, the behavioral patterns of the chimpanzees were clear and consistent. In Experiment 1, when they saw the food early in the trial, they named it right away without looking again in the container. When they had not seen the item at the start of the trial, they approached the container, viewed its contents, and then went to the keyboard and named it. This occurred from the earliest sessions, and thus the chimpanzees spontaneously reacted to the test with proficient responses, on the basis of what information they had (or did not have) when they left their start position and moved to the response area.
As noted, it was possible that a simple behavioral rule could have underlain this performance, even from the early trials. Because these chimpanzees routinely request and name items (and, most often, food items) during their daily interactions with humans, they may have learned over time that they should not name things they had not seen. An argument against this is that the chimpanzees most often use lexigrams with humans (mainly caregivers) to request foods that are not visible, and so it is not uncommon at all for them to make keyboard responses without food present. But, given the novel requirement of moving from one cage to another to name an item in a container, it was possible.
Experiment 2 discounted this possibility. Now, the chimpanzees always saw a food item that they could name. But, that item was not always in the container that moved to the response area, and so they had to track and remember whether the container that was moved contained the item they could name, or whether they needed to look into the container first. And, again, they responded proficiently and from the earliest trials. Thus, seeing food (or not seeing food) could not serve as a cue to which behavioral response to make, and yet chimpanzees effectively sought information when it was needed or made a response immediately when information was already available.
These results indicate that chimpanzees know what they have seen, and know when they can or cannot accurately name an item that is otherwise not visible at response time. This form of information seeking behavior on trials where they did not see exactly what food was in the relevant container required the chimpanzees to leave the initial area and, when the item was unknown, undertake a relatively high cost response of moving to the container and looking in it, given that such movements more than doubled the time it took to name and obtain the item in the container. That the chimpanzees made this response only when they did not know what was in the container suggests that they recognized that they had no way to name the item without first viewing it, and then rectified that lack of information.
The present study made use of the special ability of these chimpanzees to use symbols that represent, in this case, food items to indicate what was in a container once they knew that information. These chimpanzees show perhaps the strongest evidence for representational symbol use in any nonhuman animals given their unique rearing histories (see Rumbaugh & Washburn, 2003), but this level of “language training” may not be requisite for giving nonhuman animals this kind of test. Our paradigm could be adapted for any species that had even a small number of associations between arbitrary symbols and real world items provided those species were trained to indicate the “label” that went with each specific item once they could discern what that item was through their own information-seeking behaviors.
The outcome of this study supports previous arguments (e.g., Call, 2010; Call & Carpenter, 2001) that great apes, and perhaps other species, share with humans a metacognitive capacity for dealing with incomplete information. The information-seeking behavior of great apes, and perhaps other species, is indicative of metacognition rather than learned rules for responding, although the metacognitive capacities of nonhumans are likely not equivalent to the varied forms of information-seeking behaviors and other responses that humans make when they are doubtful or uncertain. That said, the existence of any metacognitive capacities in nonhuman animals has important implications for understanding the evolution of other aspects of “mind.” For example, there is an ongoing debate about the relative emergence of metacognition and theory of mind in human evolution, sparking the contrast between those who argue that metacognition likely emerged prior to theory of mind (e.g., Smith et al., 2008) and those who argue that mindreading (theory of mind) is prior (e.g., Carruthers, 2008). Independent of which view of the relative emergence of these capacities one takes, however, the present data suggest that the metacognitive processes that support information seeking behavior are shared across species, and that some species can engage controlled, decisional cognitive processes in the face of difficult problems for which only incomplete information is available.
Acknowledgements
This research was supported by grants HD-061455 and HD-060563 from the National Institute of Child Health and Human Development and by grants BCS-0956993 and BCS-0924811 from the National Science Foundation.
Contributor Information
Michael J. Beran, Georgia State University
J. David Smith, University at Buffalo.
Bonnie M. Perdue, Georgia State University
References
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Savage-Rumbaugh ES, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees (Pan troglodytes) Developmental Psychobiology. 1999;34:119–127. doi: 10.1002/(sici)1098-2302(199903)34:2<119::aid-dev5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Redford JS, Washburn DA. Rhesus macaques (Macaca mulatta) monitor uncertainty during numerosity judgments. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:111–119. doi: 10.1037/0097-7403.32.2.111. [DOI] [PubMed] [Google Scholar]
- Brakke KE, Savage-Rumbaugh ES. The development of language skills in bonobo and chimpanzee: I. Comprehension. Language and Communication. 1995;15:121–148. [Google Scholar]
- Bräuer J, Call J, Tomasello M. Visual perspective taking in dogs(Canis familiaris)in the presence of barriers. Applied Animal Behaviour Science. 2004;88:299–317. [Google Scholar]
- Call J. Do apes know that they can be wrong? Animal Cognition. 2010;13:689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Carruthers P. Meta-cognition in Animals: a skeptical Look. Mind and Language. 2008;23:58–89. [Google Scholar]
- Carruthers P. How we know our own minds: The relationship between mindreading and metacognition. Behavioral and Brain Sciences. 2009;32:121–138. doi: 10.1017/S0140525X09000545. [DOI] [PubMed] [Google Scholar]
- Couchman JJ, Countinho MVC, Beran MJ, Smith JD. Metacognition is prior. Behavioral and Brain Sciences. 2009;32:142. [Google Scholar]
- Crystal JD, Foote AL. Metacognition in animals. Comparative Cognition and Behavior Reviews. 2009;4:1–16. doi: 10.3819/ccbr.2009.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Foote AL. Evaluating information-seeking approaches to metacognition. Current Zoology. 2011;57:531–542. doi: 10.1093/czoolo/57.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Foote AL, Crystal JD. “Play it Again“: A new method for testing metacognition in animals. Animal Cognition. 2012;15:187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–254. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Heimbauer LA, Beran MJ, Owren MJ. A chimpanzee recognizes synthetic speech with significantly reduced acoustic cues to phonetic content. Current Biology. 2011;21:1210–1214. doi: 10.1016/j.cub.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling of knowing. Psychological Review. 1993;100:609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son L, Terrace H. Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Menzel CR. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology. 1999;113:426–434. doi: 10.1037/0735-7036.113.4.426. [DOI] [PubMed] [Google Scholar]
- Nelson TO, editor. Metacognition: Core readings. Toronto: Allyn and Bacon; 1992. [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E, Petter M. Do pigeons (Columba livia) study for a test? Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM. Language learning by a chimpanzee: The LANA Project. New York: Academic Press; 1977. [Google Scholar]
- Rumbaugh DM, Washburn DA. Intelligence of apes and other rational beings. New Haven: Yale University Press; 2003. [Google Scholar]
- Savage-Rumbaugh ES. Ape language: From conditioned response to symbol. New York: Columbia University Press; 1986. [Google Scholar]
- Schwartz BL. Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychonomic Bulletin and Review. 1994;1:357–375. doi: 10.3758/BF03213977. [DOI] [PubMed] [Google Scholar]
- Smith JD. The study of animal metacognition. Trends in Cognitive Sciences. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Couchman JJ, Coutinho MVC, Boomer JB. Animal metacognition: Problems and prospects. Comparative Cognition & Behavior Reviews. 2009;4:33–46. [Google Scholar]
- Smith JD, Beran MJ, Coutinho MVC, Couchman JC. The comparative study of metacognition: Sharper paradigms, safer inferences. Psychonomic Bulletin and Review. 2008;15:679–691. doi: 10.3758/pbr.15.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty states and reinforcement signals in the comparative study of metacognition. Journal of Experimental Psychology: General. 2006;135:282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Smith JD, Couchman JJ, Beran MJ. The highs and lows of theoretical interpretation in animal-metacognition research. Philosophical Transactions of the Royal Society B. 2012;367:1297–1309. doi: 10.1098/rstb.2011.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) Journal of Experimental Psychology: General. 1995;124:391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Allendoerfer KR, Washburn WA. Memory monitoring by animals and humans. Journal of Experimental Psychology: General. 1998;127:227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Schull J, Washburn DA. The uncertain response in humans and animals. Cognition. 1997;62:75–97. doi: 10.1016/s0010-0277(96)00726-3. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Jozefowiez J, Cerutti D. Metacognition in animals: How do we know that they know? Comparative Cognition and Behavior Reviews. 2009;4:29–39. [Google Scholar]