Abstract
Cellular uptake of cobalamin is facilitated by a receptor mediated endocytosis process involving transcobalamin, a plasma protein that binds cobalamin and a cell surface receptor that specifically binds transcobalamin saturated with cobalamin. Intracellular Cbl concentration is maintained by modulating the expression of the receptor, which is cell cycle associated with highest expression in actively proliferating cells and an efflux system that shunts the excess cobalamin out of the cells for mobilization to other tissues where it is most needed. This review describes the process, proteins involved and genes encoding these proteins.
Introduction
The essential role of vitamin B12 (cobalamin, Cbl) in recycling of folate for single carbon exchange reactions, purine and pyrimidine synthesis and methylation of homocysteine for the production of S-adenosylmethionine is exerted by the participation of this vitamin as methyl Cbl in the methionine synthase reaction (1). As adenosyl Cbl, it is a cofactor for methylmalonylmutase enzyme in a rearrangement reaction that converts methylmalonyl CoA to succinyl CoA (2). Cbl deficiency produces interruption of folate pathways, resulting in homocysteinemia due to inhibition of the methionine synthase pathway and methylmalonicacidemia due to inhibition of the mutase pathway (3). The anemia and hematologic changes in the form of megaloblastic bone marrow are due to abnormal DNA synthesis attributed to folate deficiency as a consequence of Cbl deficiency (4). However, the demyelination of the spinal cord and peripheral nerves seen in Cbl deficiency has not been linked to any specific pathways involving Cbl. Among the multiple causes of Cbl deficiency are dietary deficiency and genetic defects involving Cbl dependent pathways (5). The absorption, blood transport and cellular uptake of Cbl are complex processes involving multiple proteins and receptors. The gastric phase of Cbl assimilation and ileal absorption is described by Alpers et al in this issue (6). This review will address the role of two proteins, transcobalamin (TC) and the receptor for TC saturated with Cbl, in the absorption of Cbl in the gut and cellular uptake.
Early observations that there is no free Cbl in serum and that all of the Cbl is bound to proteins initiated the quest to identify these proteins and their function (7, 8). These proteins were subsequently characterized and identified as transcobalamin 1 (current nomenclature, haptocorrin, HC) and transcobalamin II (current nomenclature, transcobalamin, TC) (9, 10).
Transcobalamin
TC in Blood, Apo and holo TC
While total Cbl in serum has been used as an indicator of Cbl status, Its utility as a sensitive marker of Cbl deficiency has been questioned primarily because most of the circulating Cbl is bound to HC and this fraction is not available for cellular uptake in tissues other than the liver (11, 12). About 70 – 80% of the Cbl in serum is bound to HC and only 20 – 30% is bound to TC; however it is this latter fraction that is available for uptake into cells and constitutes newly absorbed Cbl (13, 14, 15). Orally administered Cbl appears to peak around 8 to 10 hours post ingestion (16). This represents transit time from the stomach to the distal ileum followed by absorption and release of Cbl into the circulation. Traditionally Cbl malabsorption has been diagnosed using the Shilling test, which involves the administration of radioactive B12 and collecting 24 hour urine sample (17). This test is no longer available. Some success has been achieved by monitoring the appearance of Cbl in blood following a dose of 57CoB12 (18, 19). However, radioactive Cbl for this use is no longer available. What is feasible with current technology is an accurate estimate of holo TC in serum (20, 21, 22). In theory, Cbl malabsorption could be monitored by measuring the amount of holo TC before and after an oral dose of Cbl. The available assay appears to be sufficiently sensitive to discern a change in holo TC status at peak time following a 5-10ug oral dose. Cbl on TC in the blood, appears to reach peak level in about 8 hours and is rapidly distributed to tissues (15, 18, 19). Plasma clearance of radiolabeled TC protein in the rabbit, has shown rapid clearance of the protein with a half life of ~90min (23). Therefore, following oral ingestion of dietary Cbl, the holo TC would reach a steady state and overnight fasting serum holo TC is likely to provide an accurate measure of Cbl status and decrease in holo TC may indicate chronic and sustained Cbl depletion. It is this characteristic of holo TC that may provide a more sensitive and precise indication of physiologic Cbl status. Herzlick and Herbert (24) were the first to identify the utility of measuring holo TC but the method lacked the precision and sensitivity demanded of the assay to quantify the changes in the smaller TC bound fraction of the total serum Cbl. Methodological improvements have provided a simple assay in kit form for the routine measurement of holo TC in a diagnostic laboratory setting (21, 22). Recent studies comparing serum total Cbl versus holo TC have shown that holo TC correlates better with elevated HCY and MMA as a measure of low Cbl status (25, 26). While it is generally accepted that TC-bound Cbl is taken up by all cell types, Cbl does not appear to accumulate in most tissues, rather, is recycled by an active transport mechanism (27). The ATP dependent ABCC-1 transporter involved in the translocation of Cbl absorbed in the intestine (vide infra) appears to have a role in the export of Cbl from tissue cells (28) (Figure 1). This process is at opposite poles to what happens in the liver and kidney where Cbl accumulates disproportionately. The Cbl accumulation in the kidney may be attributed to binding of TC-Cbl to the highly expressed megalin, involved in the reabsorption of a number of proteins including TC-Cbl (29, 30). Megalin expression is very low in the liver and therefore, could not account for the TC-Cbl sequestration. In the human liver, HC-bound Cbl uptake by the asialoglycoprotein receptor has been purported to be the likely mechanism for Cbl accumulation (11). This could not account for the Cbl accumulation in the mouse liver since mouse has no HC like protein in the blood and all of the Cbl is carried on TC (31). The Cbl binding proteins such as HC and TC cannot retain Cbl in tissues such as the liver and kidney since they are destroyed and the Cbl released during uptake into cells. The only known proteins likely to retain Cbl in cells are the two enzymes MS and MMU (27, 32). The saturation state of these enzymes and total enzyme activity in liver and kidney can account for only a fraction of the Cbl in liver and kidney. Therefore, a second look at Cbl accumulation in these tissues is warranted.
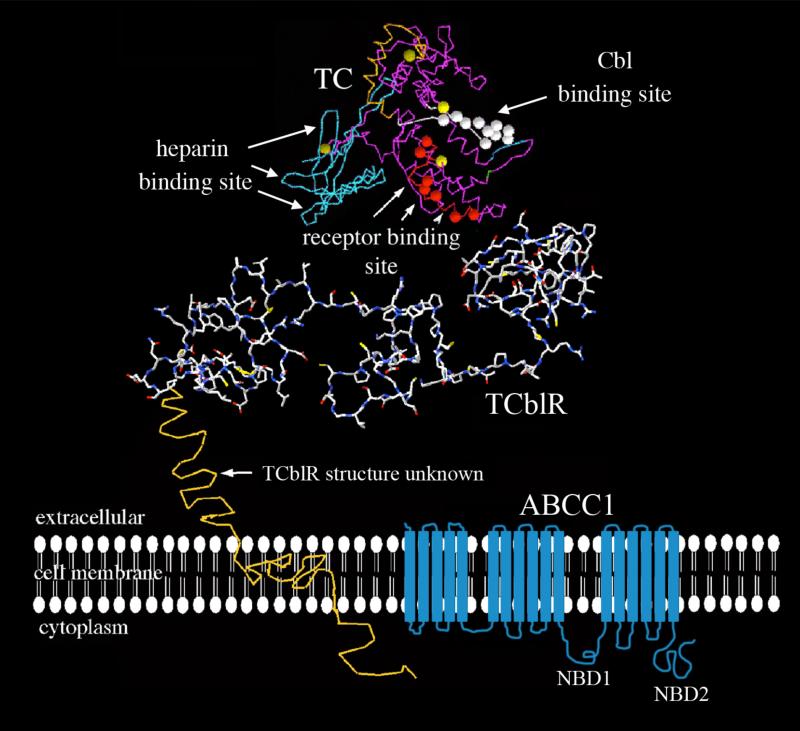
Figure 1.
The structure of proteins involved in cobalamin transport. The white circles on the TC molecule represent the Cbl binding region and the orange circles represent the receptor binding region as determined by epitope specific monoclonal antibodies that block these functions; the blue region represents the heparin-binding region likely involved in receptor binding. The TCblR molecule, with a partial theoretical 3-dimensional shape (Swiss Model) is shown oriented in the plasma membrane along with the transmembrane and cytoplasmic domains. The structure of the two LDLR-type A domains of TCblR is derived from the known structure of the LDL receptor. The two LDLR-A domains with regions involved in calcium binding are necessary for binding to holo-TC. The ABCC1 transporter involved in efflux of cobalamin is depicted in blue with its numerous transmembrane domains and nucleotide binding domains (NBD1 and NBD2).
Source of TC in blood
Having identified the function of TC in the cellular uptake of Cbl, the search was on to locate the source of this protein. Early studies suggested the liver as the source because liver perfusate contained TC and this was affected by liver damage (33, 34). This notion was soon dismissed when studies showed that total hepatectomy did not affect TC level in blood (35). TC synthesis in vitro by primary and established cell lines in culture suggested that most cell types could contribute to TC in circulation (36). We were the first to conduct a detailed study of TC synthesis in human umbilical vein endothelial cells (HUVEC) in culture and show that copious amounts of TC is secreted by these cells in culture. We could also show that the intact umbilical vein in the umbilical cord could synthesize and secrete TC (37). The rate of TC synthesis and the substantial endothelial surface could produce sufficient TC to maintain the concentration in blood. The relatively short half-life of holo TC in circulation of 60- 90 min and the proximity of the vascular endothelium to the circulating TC, seemed ideally suited to maintaining the concentration of this protein in circulation (37).
Role of TC in intestinal absorption of Cbl
With the identification of cubilin as the IF receptor (38) and amnionless as the membrane protein involved in internalizing cubilin and its cargo proteins, the process of apical docking of IF-Cbl in the intestinal lumen and uptake into the ileal enterocyte is well defined (39, 40). It is well accepted that dissociation of IF and release of Cbl occurs in the enterocyte but the subsequent steps in the translocation of Cbl into circulation had not been clearly defined. However, it was generally accepted that translocation of the Cbl cargo from IF to TC occurred in the villi of the intestine based on the following observations. Orally administered Cbl appeared in the blood bound to newly synthesized TC (13); in the isolated guinea pig ileum, IF mediated absorbed Cbl appeared in the medium bound to TC (14). The early studies that identified TC bound Cbl in the intestinal lumen, suggested this tissue as a potential source of TC. The distribution of TC mRNA throughout the villous contributed by the endothelium of the microvasculature would support in situ synthesis of TC that would be readily available to bind the newly absorbed Cbl (15). The direction of TC secretion in the ileum appears to be towards the serosal side and would favor the binding of free Cbl by the nascent TC in the villous (15). This process requires an active transport system for efflux of free Cbl and such a system was recently identified as the ATP-binding cassette (ABCC1/MRP1) transporter expressed in the basolateral surface of the intestinal epithelial cell (41). An active transport system for exit of free Cbl from cells was previously shown in mouse L-1210 cells in culture (27). This efflux of free Cbl, is also facilitated by the ABCC1 cassette (28). Inhibiting this transporter leads to accumulation of Cbl in the intestinal lumen and decreases systemic Cbl levels (41). The role of TC in carrying the Cbl from its site of absorption into circulation is also evident from the decreased absorption of Cbl reported in children with congenital TC deficiency (42, 43). This process has been studied in some detail using in vitro cultures of Caco2 and HT29 colon adenocarcinoma cell lines (44-46). Even though these can serve as models for IF-Cbl uptake and transcytosis of Cbl, it should be noted that these cells are not of ileal origin where absorption of Cbl takes place and as transformed cells, may have acquired unique properties such as uptake and secretion of TC via the apical surface following weeks in culture (45, 46). There is no evidence for TCblR expression on the apical surface of these polarized cells and therefore, TC-Cbl uptake likely represents uptake via megalin.
Transplacental transport and polymorphisms of TC
Fetal Cbl requirement has to be met by the maternal transport systems that provide all nutrients across the placental barrier. Very early studies had identified preferential and rapid delivery of newly absorbed Cbl to the fetus in animal models (47, 48). In humans, there appears to be a delay in the translocation of Cbl from the placenta to the fetus (49). The increased Cbl requirement in the developing fetus has to be met by maternal TC-Cbl and this is reflected in higher total Cbl, holo HC and holo TC in the cord blood (50). Congenital TC deficiency is not lethal to the fetus. These fetuses develop normally and are carried to term but develop metabolic and clinical picture of Cbl deficiency within months after birth (42, 51). An alternate system must fulfill the function of TC in the fetus. Even though direct proof is lacking, megalin is highly expressed in embryonic tissue and is a likely candidate receptor for TC-Cbl during fetal development. While there is no clear consensus on the association of maternal low Cbl status and birth defects, most studies have shown some albeit, weak correlation with low Cbl status and elevated matabolites such as HCY and MMA (52-55). A stronger correlation was observed with a decrease in the level of maternal holo TC with increased risk for birth defects. The TC259P to R polymorphism of the TC gene has been associated with decreased holo TC and increased HCY and MMA, however its link with birth defects remains weak (56, 57). Differences have been reported in the expression of TC259P and R polymorphisms with higher expression of TC259P associated with elevated apo TC and elevated HCY in TC259P/R heterozygotes (58). No correlation of HCY or MMA with TC genotype was observed in an elderly population (59) whereas higher holo TC and lower MMA were associated with TC259P genotype in another study (60). In an elderly latino population with higher prevalence of TC259R, the risk of Cbl deficiency appeared to be higher (61). The TC genotype may influence Cbl availability and cellular uptake, but direct experimental proof is lacking. Even though TC genotype by itself may not pose a major risk factor for fetal and adult Cbl disorders, in combination with polymorphisms of other Cbl and folate pathway proteins, it is likely to have adverse metabolic consequences (62, 63).
Binding properties and structure of TC
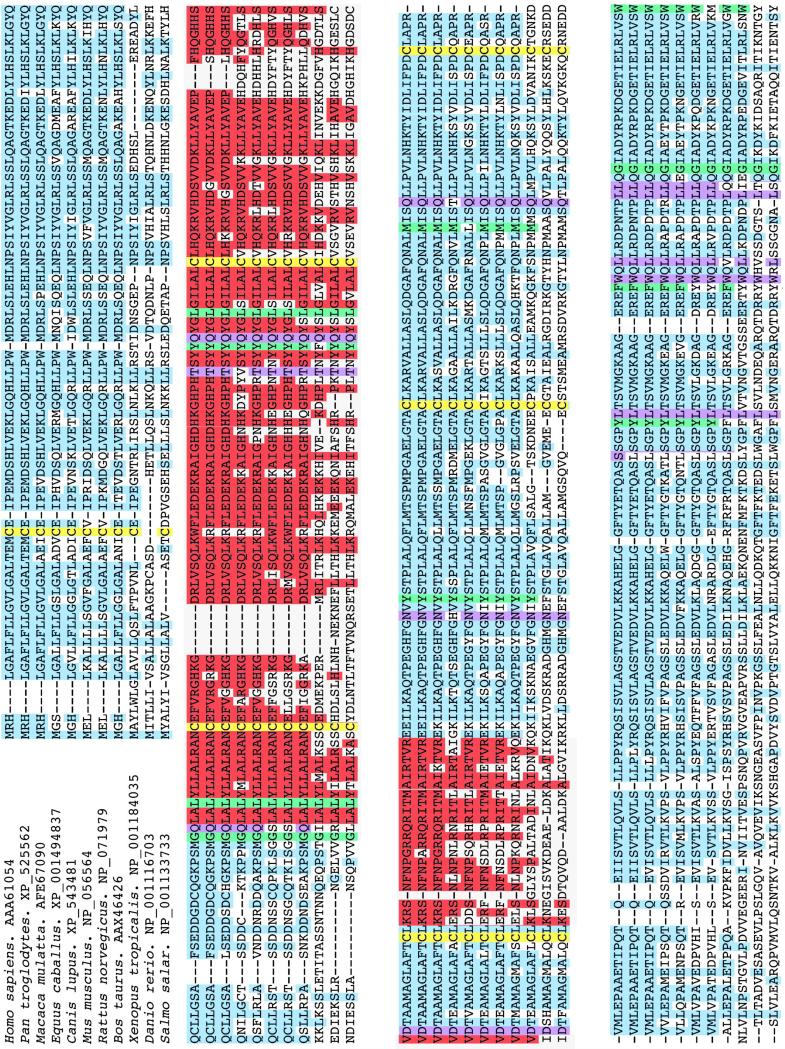
Among the Cbl binding proteins, HC has the highest affinity for Cbl followed by TC and IF. This contrasts with the specificity, with IF having the highest specificity and HC, the least (64). Thus TC appears to have greater specificity for Cbl than HC. This discrimination is more pronounced in recombinant human TC produced in insect cells (Quadros EV, unpublished data) suggesting that the folding of the protein and tertiary structure may influence the specificity for Cbl. Their two domain structure, the conserved cysteine residues and most of the amino acids involved in Cbl binding are all conserved among these proteins and therefore, slight differences in the folding of the proteins to encase the Cbl molecule, and the manner of interaction with the nucleotide portion of the Cbl molecule is likely to dictate the specificity for Cbl and binding studies support this conclusion (64). TC is expressed in all mammalian species and its expression can be traced down to worms and fish (Figure 2). The amino acids and regions highly conserved across species appear to be involved in binding Cbl and to the receptor. Based on the primary sequence, amino acids involved in Cbl binding and the cysteine residues involved in disulfide bonds (Cys 3 - 249; 98 - 291; 147- 187) are highly conserved across species. Evidence in support of this is now available from the crystallographic data of bovine and human TC (65). Specifically, six amino acids each in the alpha and beta domains directly interact by hydrogen bonding and all other side chains of the corrin ring interact by solvent mediated hydrogen bonding. In addition the beta domain hydrogen bonds with oxygen and nitrogen molecules of the nucleotide portion. Additionally, hydrophobic interactions take place at Met 270 to ring A, Gly 390 to ring B, Phe 376 and Try 409 to ring C, Try 137 to ring D. His 173 can coordinate with the upper axial position of cobalt if this position is occupied by H2O. This interaction results in folding of the protein into an amino terminal major domain and a carboxy terminal minor domain to form a pocket in which the Cbl molecule is enclosed (65). The amide side chains on the corrin ring, especially at the b, c and d position are critical for tight binding. The amide side chain at the e position is amenable to modification for attaching other ligands without a substantial loss in affinity (66). The two positions on the Cbl molecule that are ideal for coupling drugs and toxins for delivery via the TC/TCblR pathway are the upper axial position on the central cobalt and the ribose moiety of the lower nucleotide position. Attaching ligands at these positions does not affect the function of TC (66). Coupling to the central cobalt would result in cleavage and release of the ligand within the cell and coupling to the ribose would provide a non-cleavable linkage. The folded conformation of TC saturated with Cbl also presents the epitopes for high affinity interaction of the TC-Cbl complex with TCblR. Mapping the functional domains of TC with monoclonal antibodies that block the binding of TC-Cbl with TCblR has identified peptide regions involving amino acids 103 to 159 and the positively charged heparin binding residues 207 to 227 as regions most likely to interact with the receptor (67) (Figure 1). Crystallographic data identifies these domains within the solvent exposed structure of the holo protein involving Tyr 54 – leu 67 (alpha helix 3), Asn 97 –His 133 (a 5), His 149 – 166 (a 7), Lys 189- Thr 207 (end of a helix 8 to 9).
Figure 2.
Amino acid sequence homology of TC from various species analyzed using the CLUSTAL 2.0.5 alignment. The cysteines forming disulfide bridges are indicated in yellow; known hydrophobic interactions with cobalamin are indicated in green and hydrogen bonding with cobalamin is indicated in purple; the putative receptor binding regions are indicated in red. The amino acids that bind cobalamin and the highly conserved regions in all species are likely to be involved in interaction with the receptor.
TC gene and genetic abnormalities
The isolation of highly purified and intact TC from cohn fraction III of human plasma (68) enabled us to identify the N-terminal protein sequence and generate monoclonal antibodies against this protein (69). These tools ultimately enabled us to clone the cDNA encoding human TC from a HUVEC cDNA library (70, 71). The 1866 base pair cDNA codes for a 409 amino acids (AA) secreted protein and an 18 AA signal peptide. The cDNA also contained 37 nucleotides (nt) in the 5’ and 548nt in the 3’ untranslated regions with a polyadenylation signal located 510nt downstream of the stop codon (71). The cloning of the gene for TC has identified the transcription start site at 158nt upstream of the ATG start codon, thus providing a size of 1987nt for the full length mRNA (72). Additional cDNA clones isolated by other investigators identified cDNA clones that differed in codon sequences at positions 198, 219, 259 and 376 (73). These authors concluded that the multiple protein bands observed by isoelectric focusing may represent polymorphisms due to these substitutions. This observation and the previously reported polymorphisms of the TC protein (74) took on a different twist, when we for the first time, showed that recombinant human TC expressed from a single cDNA clone in SF9 insect cells, separated as two proteins by isoelectric focusing (75). The identical two bands were obtained when purified human TC and serum from different subjects was analyzed by isoelectric focusing. An explanation for the apparent two bands was evident when the amino terminal sequence of recombinant protein yielded two sequences, one missing the first two, methionine and glutamic acid residues. This phenomenon was attributed to alternative splicing of the leader sequence (66). Therefore, earlier reports of multiple isoproteins of TC may well be due to TC interaction with plasma proteins and artifact of the methods used. The human TC gene is located on chromosome 22 between 22q11 and 22q13.1 (76). The gene spans 18kb and contains 9 coding exons and 8 introns (72, 77). The farthest transcription site identified is located 157nt upstream of the ATG start site (78). The TC gene is remarkably similar in structure to the other two Cbl binding proteins namely HC (79) and IF (80) in the number and size of each exon pointing their origin to a common ancestral gene, likely on chromosome 11 where HC and IF are located. TC appears to be constitutively secreted by the endothelial cells, however, some evidence points to inducible expression (81). The lack of consensus TATA and CCAAT boxes in the promotor region, a characteristic of house keeping genes, favors the constitutive expression of TC. However, upregulation of TC secretion in inflammation and certain cancers would suggest induction of TC synthesis in response to specific stimuli (82, 83). The 5’ region of TC gene spanning 1000bp upstream of the ATG codon, contains a number of positive and negative regulatory elements that function in concert to regulate TC expression (84). Specific elements within the promoter region such as the GC box at −568, the GC/GT box at −179 that interact with SP1 and SP3 and an E box at -589 to −559 may be involved in tissue specific expression of TC (85). In addition, the C and G tetramers that are motifs for ets transcription factors, the GC rich sequence, and the CACGTG sequence for c-myc oncogene product, all point to potential up regulation of TC synthesis in cancers and inflammatory disorders (86).
TC deficiency presents as the clinical phenotype of Cbl deficiency with megaloblastic bone marrow, homocysteinuria and methylmalonicaciduria (87-89). These abnormalities have ranged from complete absence of TC protein to a non-functional protein. To date more than 30 cases have been identified and the gene defects have ranged from frame-shift mutations to deletions and splicing defects (Table 1). Early diagnosis and frequent injections of B12 may be beneficial in treating these patients.
Table 1.
Mutations identified in human TC and TCblR genes
| TC Mutations | ||
|---|---|---|
| Gene mutation | Effect | Reference |
| r.31C>G | p.Leu11Val | Qian et al, 2002 (130) |
| r.62G>A | p.Cys21Tyr | Qian et al, 2002 |
| r.79G>A | p.Asp27Asn | Qian et al, 2002 |
| c.110insC | frameshift | Ratschmann et al, 2009 (121) |
| r.145C>T | p.His49Tyr | Qian et al, 2002 |
| c.173delC | frameshift | Li et al, 1994b (123) |
| r.254T>A | p.Leu85Gln | Qian et al, 2002 |
| r.257G>A | p.Gly86Glu | Qian et al, 2002 |
| c.387delA | frameshift | Li et al, 1994b (123) |
| r.472G>T | p.Gly158Cys | Qian et al, 2002 |
| c.497_498del | frameshift | Schiff et al, 2010(124) |
| c.501_503del | frameshift | Schiff et al, 2010 |
| c.580+1G>C | splice (loss of exon 4) | Namour et al, 2003 (125); Schiff et al, 2010 |
| c.580+624A>T | insertion | Häberle et al, 2009 (126) |
| c.927_930delTCTG | frameshift | Li et al, 1994a (122) |
| c.940+303_1106+746del21252 | insCTGG | Häberle et al, 2009 |
| c.1106+1G>A | P.M315fsX326 | Nissen et al, 2010 (127) |
| c.1110T>G | p.Tyr370X | Li et al, 1994b |
| c. (Exon 8 deletion) | p.E371fsX372 | Nissen et al, 2010 |
| c.1115_1116del | frameshift | Schiff et al, 2010 |
| c.1139dupA | frameshift | Schiff et al, 2010 |
| c.1195C>T | p.R399X | Prasad et al, 2008 |
| c.1236_1237del | frameshift | Schiff et al, 2010 |
| c.IVS3T>G | splice | Namour et al, 2003 |
6. The receptor for transcobalamin- bound cobalamin
Cellular uptake of cobalamin
While it is generally accepted that bulk of the Cbl in the body is stored in the liver and kidney, the mechanism for uptake in the liver is thought to occur via the asialoglycoprotein receptor (11, 12) and in the kidney via megalin (30). In all tissue cells, the uptake occurs via a cell surface receptor that specifically recognizes TC-bound Cbl. This process has been studied in isolated cells in culture (90). The cellular uptake appeared to be a biphasic process and involved initial binding of TC-Cbl to the cell surface that could occur at 4°C followed internalization of the TC-Cbl complex which required metabolic energy and occurred best at 37°C. The process also required divalent cation Ca++ as indicated by the inhibition of uptake by EDTA or EGTA. The lysosomal accumulation of the TC-Cbl suggested receptor mediated endocytosis as the likely mechanism (91). The receptors appear to be segregated in discrete microvilli and appear to be directed to clathrin coated pits during endocytosis (92, 93). The expression of TCblR appears to be cell cycle associated with most receptor expression during log phase of growth in actively proliferating cells and drastically down-regulated in resting cells (94-96). The existence of this membrane attached receptor was known for more that half a century but the purification and structural characterization of this protein proved to be difficult due to the low amounts of the receptor and the ligand specific affinity chromatography required to purify the protein. Numerous attempts were made to study the properties of this receptor and obtain pure protein. The first effort using TC-Cbl affinity matrix yielded substantial enrichment based on TC-Cbl binding of the detergent soluble receptor from human placenta but was not pure (97). A series of publications by another group claimed to have purified the receptor for TC-Cbl (98-100). In light of our current knowledge of this receptor, it is highly unlikely that the protein they had purified, was the receptor. Our own attempts to purify this receptor from human placenta identified a number of methodological problems that had to be addressed if we were to succeed in purifying this protein. Therefore, a detailed characterization of the functional activity of the membrane bound as well as soluble receptor from human placenta was attempted. The binding kinetics of membrane bound receptor showed saturation kinetics with an association constant of 0.26 – 1.1 nM−1. The soluble receptor bound TC-Cbl with a similar affinity but the fraction of membrane bound receptor that could be rendered soluble and recovered as functional protein, was low. However receptor-ligand crosslinking and deglycosylation studies provided information on the size of the receptor protein and carbohydrate content. Based on these studies we assigned a molecular weight of 58 kDa for the soluble receptor and 41 kDa to the core peptide. The carbohydrate content accounted for approximately 29% of the molecular weight and consisted primarily of sialic acid (47%) and the remainder consists of N and O linked sugars in the form of N-acetylglucosamine and terminal X-linked mannose, galactose and N-acetylgalactosamine (101, 102). These estimates are likely to be an approximation due to incomplete deglycosylation or aberrant migration of the protein in SDS-PAGE since the cDNA encodes a 282aa peptide and a 252aa membrane attached receptor (103).
Determination of functional receptor activity
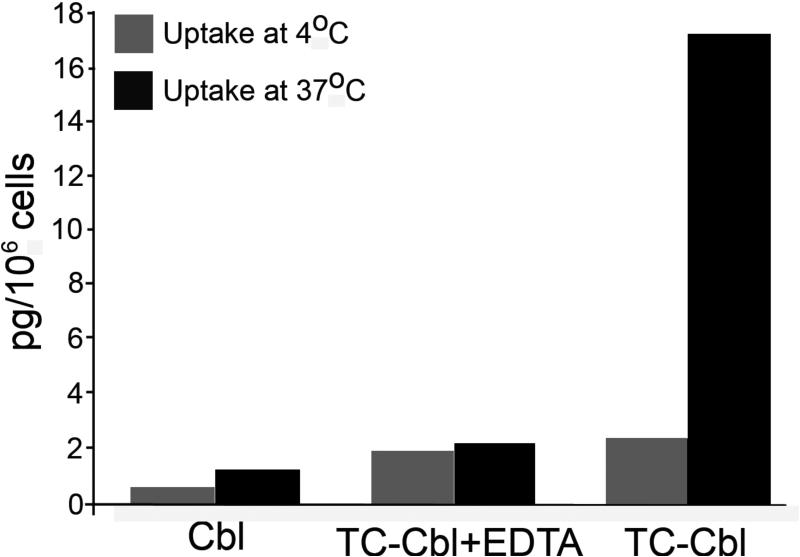
Functional receptor activity in whole cells can be readily determined by incubating cells with 57CoB12 labeled TC. In a typical assay, 1×106 cells are suspended in 1ml buffer or culture medium containing 10,000 to 20,000 CPM of radiolabeled TC for 1hr at 4°C, or 37°C. An identical tube containing 10mM EGTA or EDTA is included to show blocking of the uptake. After 1 hour incubation, cells are pelleted by centrifugation at 2000g for 5 min. washed once with buffer and counted for radioactivity (Figure 3). To discriminate between cell surface bound and internalized TC-Cbl, cells may be incubated with 0.25M trypsin / 2mM EGTA for 5 min at 37°C and the trypsin releasable and cell associated TC-Cbl determined. Typically uptake at 4°C represents TC-Cbl binding to cell surface receptors and uptake at 37°C represents binding and internalization of the TC-Cbl. In reality some internalization does occur at 4°C and uptake at 37°C is considerably higher due to new receptors appearing on the cell surface. In practical terms, trypsin/EDTA releasable radioactivity represents TC-Cbl bound to the cell surface receptor and radioactivity in the cell pellet represents TC-Cbl internalized. This simple assay using live cells provides a reliable measure of receptors expressed in different cell types.
Figure 3.
TC-Cbl uptake in human umbilical vein endothelial cells established in culture. Cells are seeded at 0.2-0.5 × 106 cells/2 ml in six-well plates overnight. For uptake of Cbl, [57Co]cyanocobalamin-TC is added in 1ml DMEM and incubated at 4°C or 37°C. Following removal of the medium and washing, cells are detached by incubating with 0.5 ml 0.05% trypsin/EDTA at 37°C for 5 min. The radioactivity in the trypsin/EDTA solution and the cells is determined as a measure of TCblR-mediated binding and uptake of TC-Cbl. Suspension cultures may be used at a density of 0.5 -1 × 106 / ml for uptake studies. Specific uptake may be blocked by including 10 mM EGTA or EDTA in the incubation medium.
TC-Cbl binding to membrane preparations can be measured using protocols described for the preparation and assay of placental membranes (97, 101). Soluble receptor activity can be monitored by size exclusion chromatography on Sephacryl S-200 or similar matrix following incubating soluble receptor with radio-labeled TC-Cbl. The receptor –TC-Cbl complex and free TC-Cbl separate as distinct peaks and the receptor binding of TC-Cbl can be quantified by determining the radioactivity associated with the higher molecular weight peak (101). In purified fractions of the soluble receptor, functional activity can be monitored using a lectin-agarose matrix to separate receptor-bound TC-Cbl from free TC-Cbl. Both Concavalin A and Wheat germ agglutinin are suitable lectins for this assay (102, 103).
Structural and genetic aspects of the TCblR/CD320 receptor
The abundant availability of human placentas provided the raw material to solubilize and purify the receptor protein by conventional purification techniques. Methodological refinements and multiple affinity purification steps were needed to obtain the protein in pure form. The protein and the gene encoding this protein was finally identified from the peptide sequences of the pure protein. The cDNA encodes a 282aa peptide that includes a 30aa signal peptide. The 252aa membrane attached receptor consists of a 32aa cytoplasmic domain, a 21aa transmembrane domain and a 199aa extracellular domain with two LDLR type A domains separated by a cysteine rich epidermal growth factor (EGF) sequence found in other LDL receptor family of proteins (104) (Figure 1). SDS-PAGE analysis of the proein indicates a size of 58kDa, far in excess of the peptide size. This suggests extensive glycosylation with N-glycosylation sites at residues 126, 195, and 213 and the numerous serine/threonine sites that could serve for O-glycosylations. The 282aa full-length protein binds ot wheat germ agglutinin (WGA) as well as concalavin A (ConA), whereas, the extracellular domains does not bind ConA suggesting gamma linked mannose residues in the cytoplasmic domain and terminal N-acetyl glucosamine in the extracellular domain. Based on what is known about the LDLR-A domains and their specicity for Ca++ binding, the two LDLR-A domains likely play a crucial role in the binding of TC-Cbl (103, 105). The cDNA encoding this protein was isolated earlier by another group and was named 8D6 antigen. They had suggested a potential role for this protein as a signaling molecule involved in maturation of B-cells (106). This observation was based on the blocking of B cell maturation by a monoclonal antibody to the protein. Whether this effect was due to blocking of Cbl uptake, needs to be determined.
We have generated monoclonal antibodies to the extracellular domain of TCblR and have mapped their epitope specificity using recombinant fragments expressed in HEK 293 cells (105). The amino terminal region of the second LDLR-A appears to highly antigenic in that many of the antibodies isolated bound to this region. A single antibody that bound to the carboxy end of the second LDLR-A domain was identified as blocking Ab, due to its ability to block TC-Cbl uptake into cells (105). All of the antibodies including the blocking Ab, irrespective of their epitope recognition site, are effectively internalized by the receptor. These antibodies could have therapeutic utility in delivering drugs and toxins to cancer cells and other target cells. The cell cycle associated expression of TCblR with sustained or increased expression in certain cancers, could provide the increased targeting needed to reduce systemic toxicity. Preliminary studies using Saporin-conjugated secondary antibody have shown internalization of the Saporin conjugate and inhibition of proliferation in many cancer cell lines with minimal effect on normal primary cell lines (107). Our previous studies have shown that targeting TC to block Cbl uptake or to deliver drugs would also be effective (108). Other studies have shown that drugs or fluorescent compounds conjugated to B12 could accumulate in tumors for targeting and imaging (109, 110). A summary of the interactions of TC and TCblR in Cbl uptake is shown in figure 1.
The gene encoding human CD320 contains 5 coding exons and 4 introns, spans 6.224kb and is located on the short arm of chromosome 19 at p13.2. This location is also shared in primates but not many other species. The mouse ortholog of CD320 spans a length of 6.792 kb and is located on chromosome 17B1. Most of the gene structure and the flanking DNA appear to be highly conserved among species(Figure 4). The 1000bp region immediate 5’ of the ATG start site contains all of the promotor activity to regulate TCblR expression. Specifically, the region −668 to −455 is involved in up regulation of TCblR expression. Two transcription factors, myeloid zinc finger 1 (MZF1) and the Ras responsive element binding protein 1 (RREB1) are involved in upregulation of TCblR expression (111). The former is involved in growth, proliferation, differentiation and apoptosis and the latter may exert its effect via the Ras/Raf pathway. In concert with the above, transcription factors C/EBP enhancer binding protein, HNF-3B hepatocyte nuclear factor 3 and AP-1 activator protein 1 appear to suppress TCblR expression. These proteins together may modulate cell cycle associated expression and sustained up regulation of TCblR in certain cancers.
Figure 4.
Comparision of human TCblR gene structure with the receptor from other mammalian species. The intron-exon boundaries and genomic organization of the TCblR gene was compiled from sequence information available from gene data banks (Reproduced from reference 94)
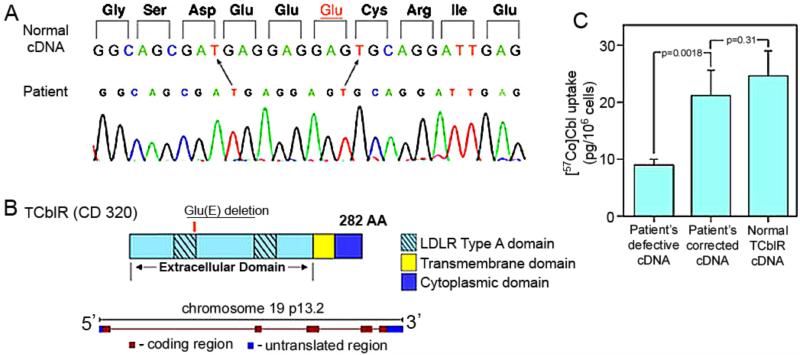
Functional defects or congenital abnormalities of TCblR were not identified previously. However, when a recent newborn presented with elevated C3 acylcarnitine along with moderate methylmalonic academia and methylcitrate, the infant was investigated for inborn errors of Cbl metabolism. Complementation analysis failed to identify any of the previously known defects in the Cbl metabolic pathways. The only defect identified was a decreased uptake of TC-Cbl by the patient's fibroblasts in culture. Based on this observation, the patient was further investigated for a potential defect in the CD320 gene. A single codon deletion (c.262_264GAG) in the Ca++ binding motif of the first LDLR-A domain was identified that accounted for a 50% decrease in Cbl uptake into cells (Figure 5). A search of the fibroblast repository at the Department of Human Genetics, McGill University, identified 5 additional cases that had elevated MMA that had the identical gene defect in CD320 (112). This gene defect appears to have been missed until now because the gene encoding TCblR was not identified.
Figure 5.
The nucleotide sequence of the region corresponding to the codon deletion in the first LDLR type A domain in the patient. B: The organization of the protein and the gene encoding TCblR. C: Normalization of holo-TC uptake in cells transfected with patient's cDNA that has the missing codon (GAG) inserted by site directed mutagenesis. The cDNA in pcDNA3.1 plasmid (4 μg) was transfected into HEK 293 cells seeded at a density of 0.6 °— 106/well in six-well plates. Binding and uptake of holo-TC was determined 48 hr after transfection for 1 hr at 37°C as described for figure 3. (Reproduced from reference 103)
Numerous single nucleotide polymorphisms of CD320 have been identified. In investigating the association of these SNPs with neural tube defect pregnancy in an Irish population, no difference in the frequency of most of these SNPs was observed among controls, NTD mothers and NTD children. However, about 4% of this population was heterozygous and 1% was homozygous for the E88 deletion. In light of our finding of elevated MMA in newborns homozygous for this deletion, undetected and untreated, this deletion could contribute to lifelong elevated MMA and its pathological consequences. Two linked variants, rs2336573 (G220R) and rs8426 were identified as associated with a 6 fold increase in NTD risk (113).
A century of clinical and basic science research started with the identification of the cause and treatment of pernicious anemia (114, 115), the isolation of vitamin B12 (116, 117) and elucidation of the structure of cobalamin (118). The advances in biology have led to the identification of pathways, proteins and genes involved (119). The structural and functional characterization of proteins involved and the metabolic consequences of gene defects have provided a better understanding of clinical disorders of Cbl metabolism (120) to implement strategies to manage these disorders.
This review provides a comprehensive account of the process of cellular uptake of vitamin B12 and the proteins involved.
Provides information on transcobalamin, the plasma transporter of cobalamin
Provides information on the structure and function of the receptor for transcobalamin-bound cobalamin.
Identifies gene defects of transcobalamin and its receptor.
Acknowledgements
This work was supported by NIH grant DK064732 to EVQ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The voyage of discovery is not in seeking new landscapes but in having new eyes. Marcel Proust
References
- Taylor RT, Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Propylation characteristics with the use of a chemical reducing system and purified enzyme. The Journal of biological chemistry. 1967;242:1509–1516. [PubMed] [Google Scholar]
- Cannata JJ, Focesi A, Jr., Mazumder R, Warner RC, Ochoa S. Metabolism of Propionic Acid in Animal Tissues. Xii. Properties of Mammalian Methylmalonyl Coenzyme a Mutase. The Journal of biological chemistry. 1965;240:3249–3257. [PubMed] [Google Scholar]
- Green R. Metabolite assays in cobalamin and folate deficiency. Bailliere's clinical haematology. 1995;8:533–566. doi: 10.1016/s0950-3536(05)80220-3. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SN. Morphology, biology and biochemistry of cobalamin- and folate-deficient bone marrow cells. Bailliere's clinical haematology. 1995;8:441–459. doi: 10.1016/s0950-3536(05)80215-x. [DOI] [PubMed] [Google Scholar]
- Watkins D, Whitehead VM, Rosenblatt DS. Megaloblastic Anemia. In: Orkin SH, Nathan DG, editors. In Nathan and Oski's hematology of infancy and childhood. Saunders/Elsevier; Philadelphia: 2009. pp. xxvipp. 487–520. [Google Scholar]
- Alpers DA. In this issue.
- Miller A, Sullivan JF. Electrophoretic studies of the vitamin B12-binding protein of normal and chronic myelogenous leukemia serum. The Journal of clinical investigation. 1959;38:2135–2143. doi: 10.1172/JCI103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CA, Finkler AE. In vivo plasma vitamin B12 binding in B12 deficient and nondeficient subjects. The Journal of laboratory and clinical medicine. 1962;60:765–776. [PubMed] [Google Scholar]
- Hall CA. Transcobalamins I and II as natural transport proteins of vitamin B12. The Journal of clinical investigation. 1975;56:1125–1131. doi: 10.1172/JCI108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RH. Human vitamin B12 transport proteins. Progress in hematology. 1975;9:57–84. [PubMed] [Google Scholar]
- Ashwell G, Morell A. The dual role of sialic acid in the hepatic recognition and catabolism of serum glycoproteins. Biochemical Society symposium. 1974:117–124. [PubMed] [Google Scholar]
- Burger RL, Schneider RJ, Mehlman CS, Allen RH. Human plasma R-type vitamin B12-binding proteins. II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. The Journal of biological chemistry. 1975;250:7707–7713. [PubMed] [Google Scholar]
- Chanarin I, Muir M, Hughes A, Hoffbrand AV. Evidence for intestinal origin of transcobalamin II during vitamin B12 absorption. British medical journal. 1978;1:1453–1455. doi: 10.1136/bmj.1.6125.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SP, Weiss JP, Cotter R. Formation of transcobalamin II--vitamin B12 complex by guinea-pig ileal mucosa in organ culture after in vivo incubation with intrinsic factor--vitamin B12. British journal of haematology. 1978;40:401–414. doi: 10.1111/j.1365-2141.1978.tb05812.x. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Regec AL, Khan KM, Quadros E, Rothenberg SP. Transcobalamin II synthesized in the intestinal villi facilitates transfer of cobalamin to the portal blood. The American journal of physiology. 1999;277:G161–166. doi: 10.1152/ajpgi.1999.277.1.G161. [DOI] [PubMed] [Google Scholar]
- Doscherholmen A, Hagen PS. Radioactive vitamin B12 absorption studies: results of direct measurement of radioactivity in the blood. Blood. 1957;12:336–346. [PubMed] [Google Scholar]
- Schilling RF. Intrinsic factor studies: II. The effect of gastric juice on the urinary excretion of radioactivity after the oral administration of radioactive vitamin B(12) The Journal of laboratory and clinical medicine. 2004;144:268–272. doi: 10.1016/j.lab.2004.09.001. discussion 225-266. [DOI] [PubMed] [Google Scholar]
- Forshaw J, Harwood L. Measurement of intestinal absorption of 57-Co vitamin B-12 by serum counting. Journal of clinical pathology. 1966;19:606–609. doi: 10.1136/jcp.19.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D, Lascelles PT. An assessment of serum 57Co cyanocobalamin as an index of vitamin B12 absorption. Journal of clinical pathology. 1968;21:792. doi: 10.1136/jcp.21.6.792-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor MV, Cetin M, Aytac S, Altay C, Nexo E. Nonradioactive vitamin B12 absorption test evaluated in controls and in patients with inherited malabsorption of vitamin B12. Clinical chemistry. 2005;51:2151–2155. doi: 10.1373/clinchem.2005.055509. [DOI] [PubMed] [Google Scholar]
- Orning L, Rian A, Campbell A, Brady J, Fedosov SN, Bramlage B, Thompson K, Quadros EV. Characterization of a monoclonal antibody with specificity for holo-transcobalamin. Nutrition & metabolism. 2006;3:3. doi: 10.1186/1743-7075-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clinical chemistry. 2008;54:567–573. doi: 10.1373/clinchem.2007.096784. [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Burger RL, Mehlman CS, Allen RH. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. The Journal of clinical investigation. 1976;57:27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlich B, Herbert V. Depletion of serum holotranscobalamin II. An early sign of negative vitamin B12 balance. Laboratory investigation; a journal of technical methods and pathology. 1988;58:332–337. [PubMed] [Google Scholar]
- Morkbak AL, Heimdal RM, Emmens K, Molloy A, Hvas AM, Schneede J, Clarke R, Scott JM, Ueland PM, Nexo E. Evaluation of the technical performance of novel holotranscobalamin (holoTC) assays in a multicenter European demonstration project. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2005;43:1058–1064. doi: 10.1515/CCLM.2005.185. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Molloy AM, Ueland PM, Fernandez-Ballart JD, Schneede J, Arija V, Scott JM. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. The Journal of nutrition. 2007;137:1863–1867. doi: 10.1093/jn/137.8.1863. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Jacobsen DW. The dynamics of cobalamin utilization in L-1210 mouse leukemia cells: a model of cellular cobalamin metabolism. Biochimica et biophysica acta. 1995;1244:395–403. doi: 10.1016/0304-4165(95)00037-c. [DOI] [PubMed] [Google Scholar]
- Beedholm-Ebsen R, van de Wetering K, Hardlei T, Nexo E, Borst P, Moestrup SK. Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood. 2010;115:1632–1639. doi: 10.1182/blood-2009-07-232587. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Verroust PJ. Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annual review of nutrition. 2001;21:407–428. doi: 10.1146/annurev.nutr.21.1.407. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Birn H, Fischer PB, Petersen CM, Verroust PJ, Sim RB, Christensen EI, Nexo E. Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8612–8617. doi: 10.1073/pnas.93.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygum K, Lildballe DL, Greibe EH, Morkbak AL, Poulsen SS, Sorensen BS, Petersen TE, Nexo E. Mouse transcobalamin has features resembling both human transcobalamin and haptocorrin. PloS One. 2011;6:e20638. doi: 10.1371/journal.pone.0020638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Osborne ML, Kolhouse JF, Binder MJ, Podell ER, Utley CS, Abrams RS, Allen RH. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. The Journal of clinical investigation. 1981;67:1270–1283. doi: 10.1172/JCI110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Hansen HJ. Studies on the site of synthesis of transcobalamin-II, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1968;127:740–744. doi: 10.3181/00379727-127-32789. [DOI] [PubMed] [Google Scholar]
- England JM, Down MC, Tavill AS, Clarke HG, Chanarin I. The origin and function of the transcobalamins. British journal of haematology. 1973;25:544. [PubMed] [Google Scholar]
- Sonneborn DW, Abouna G, Mendez-Picon G. Synthesis of transcobalamin II in totally hepatectomized dogs. Biochimica et biophysica acta. 1972;273:283–286. doi: 10.1016/0304-4165(72)90218-8. [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M, Porck HJ, Erten J, Muller MR, Steinmann B, Kierat L, Arwert F. Synthesis and secretion of the human vitamin B12-binding protein, transcobalamin II, by cultured skin fibroblasts and by bone marrow cells. Biochimica et biophysica acta. 1985;845:421–427. doi: 10.1016/0167-4889(85)90207-1. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Rothenberg SP, Jaffe EA. Endothelial cells from human umbilical vein secrete functional transcobalamin II. The American journal of physiology. 1989;256:C296–303. doi: 10.1152/ajpcell.1989.256.2.C296. [DOI] [PubMed] [Google Scholar]
- Kozyraki R, Kristiansen M, Silahtaroglu A, Hansen C, Jacobsen C, Tommerup N, Verroust PJ, Moestrup SK. The human intrinsic factor-vitamin B12 receptor, cubilin: molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. Blood. 1998;91:3593–3600. [PubMed] [Google Scholar]
- Fyfe JC, Madsen M, Hojrup P, Christensen EI, Tanner SM, de la Chapelle A, He Q, Moestrup SK. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- Kozyraki R, Cases O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie. 2012 doi: 10.1016/j.biochi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Nielsen MJ, Rasmussen MR, Andersen CB, Nexo E, Moestrup SK. Vitamin B12 transport from food to the body's cells--a sophisticated, multistep pathway, Nature reviews. Gastroenterology & hepatology. 2012;9:345–354. doi: 10.1038/nrgastro.2012.76. [DOI] [PubMed] [Google Scholar]
- Burman JF, Mollin DL, Sourial NA, Sladden RA. Inherited lack of transcobalamin II in serum and megaloblastic anaemia: a further patient. British journal of haematology. 1979;43:27–38. doi: 10.1111/j.1365-2141.1979.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Seligman PA, Steiner LL, Allen RH. Studies of a patient with megaloblastic anemia and an abnormal transcobalamin II. The New England journal of medicine. 1980;303:1209–1212. doi: 10.1056/NEJM198011203032105. [DOI] [PubMed] [Google Scholar]
- Dix CJ, Hassan IF, Obray HY, Shah R, Wilson G. The transport of vitamin B12 through polarized monolayers of Caco-2 cells. Gastroenterology. 1990;98:1272–1279. doi: 10.1016/0016-5085(90)90344-z. [DOI] [PubMed] [Google Scholar]
- Bose S, Seetharam S, Dahms NM, Seetharam B. Bipolar functional expression of transcobalamin II receptor in human intestinal epithelial Caco-2 cells. J Biol Chem. 1997;272:3538–3543. doi: 10.1074/jbc.272.6.3538. [DOI] [PubMed] [Google Scholar]
- Pons L, Guy M, Lambert D, Hatier R, Gu√©ant J. Transcytosis and coenzymatic conversion of [(57)Co]cobalamin bound to either endogenous transcobalamin II or exogenous intrinsic factor in caco-2 cells. Cell PhysiolBiochem. 10(2000):135–148. doi: 10.1159/000016344. [DOI] [PubMed] [Google Scholar]
- Hellegers A, Okuda K, Nesbitt RE, Jr., Smith DW, Chow BF. Vitamin B12 absorption in pregnancy and in the newborn. The American journal of clinical nutrition. 1957;5:327–331. doi: 10.1093/ajcn/5.3.327. [DOI] [PubMed] [Google Scholar]
- Luhby AL, Cooperman JM, Donnenfeld AM. Placental transfer and biological half-life of radioactive vitamin B12 in the dog, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1959;100:214–217. doi: 10.3181/00379727-100-24576. [DOI] [PubMed] [Google Scholar]
- Prystowsky H, Hellegers AE, Ranke E, Ranke B, Chow BF. Further observations on the metabolism of vitamin B12 in human pregnancy. American journal of obstetrics and gynecology. 1959;77:1–5. doi: 10.1016/0002-9378(59)90260-1. [DOI] [PubMed] [Google Scholar]
- Obeid R, Morkbak AL, Munz W, Nexo E, Herrmann W. The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clinical chemistry. 2006;52:263–269. doi: 10.1373/clinchem.2005.057810. [DOI] [PubMed] [Google Scholar]
- Prasad C, Rosenblatt DS, Corley K, Cairney AE, Rupar CA. Transcobalamin (TC) deficiency--potential cause of bone marrow failure in childhood. J Inherit Metab Dis. Suppl. 2008;2:s287–s292. doi: 10.1007/s10545-008-0864-3. [DOI] [PubMed] [Google Scholar]
- Riedel BM, Molloy AM, Meyer K, Fredriksen A, Ulvik A, Schneede J, Nexo E, Hoff G, Ueland PM. Transcobalamin polymorphism 67A->G, but not 776C->G, affects serum holotranscobalamin in a cohort of healthy middle-aged men and women. The Journal of nutrition. 2011;141:1784–1790. doi: 10.3945/jn.111.141960. [DOI] [PubMed] [Google Scholar]
- Stanislawska-Sachadyn A, Woodside JV, Sayers CM, Yarnell JW, Young IS, Evans AE, Mitchell LE, Whitehead AS. The transcobalamin (TCN2) 776C>G polymorphism affects homocysteine concentrations among subjects with low vitamin B(12) status. European journal of clinical nutrition. 2010;64:1338–1343. doi: 10.1038/ejcn.2010.157. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food and nutrition bulletin. 2008;29:S101–111. doi: 10.1177/15648265080292S114. discussion S112-105. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics. 2009;123:917–923. doi: 10.1542/peds.2008-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DA, Pangilinan F, Mills JL, Kirke PN, Conley M, Weiler A, Frey T, Parle-McDermott A, O'Leary VB, Seltzer RR, Moynihan KA, Molloy AM, Burke H, Scott JM, Brody LC. Evaluation of transcobalamin II polymorphisms as neural tube defect risk factors in an Irish population. Birth defects research. Part A, Clinical and molecular teratology. 2005;73:239–244. doi: 10.1002/bdra.20122. [DOI] [PubMed] [Google Scholar]
- Gueant JL, Chabi NW, Gueant-Rodriguez RM, Mutchinick OM, Debard R, Payet C, Lu X, Villaume C, Bronowicki JP, Quadros EV, Sanni A, Amouzou E, Xia B, Chen M, Anello G, Bosco P, Romano C, Arrieta HR, Sanchez BE, Romano A, Herbeth B, Anwar W, Namour F. Environmental influence on the worldwide prevalence of a 776C->G variant in the transcobalamin gene (TCN2) Journal of medical genetics. 2007;44:363–367. doi: 10.1136/jmg.2006.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namour F, Guy M, Aimone-Gastin I, de Nonancourt M, Mrabet N, Gueant JL. Isoelectrofocusing phenotype and relative concentration of transcobalaminIIisoproteins related to the codon259 Arg/Pro polymorphism. BiochemBiophysResCommun. 1998;251:769–774. doi: 10.1006/bbrc.1998.9463. [DOI] [PubMed] [Google Scholar]
- McCaddon A, Blennow K, Hudson P, Regland B, Hill D. Transcobalamin polymorphism and homocysteine. Blood. 2001;98:3497–3499. doi: 10.1182/blood.v98.12.3497. [DOI] [PubMed] [Google Scholar]
- Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R. Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood. 2002;100:718–720. doi: 10.1182/blood-2002-01-0209. [DOI] [PubMed] [Google Scholar]
- Garrod MG, Allen LH, Haan MN, Green R, Miller JW. Transcobalamin C776G genotype modifies the association between vitamin B12 and homocysteine in older Hispanics. Eur J ClinNutr. 2010;64:503–509. doi: 10.1038/ejcn.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueant-Rodriguez RM, Rendeli C, Namour B, Venuti L, Romano A, Anello G, Bosco P, Debard R, Gerard P, Viola M, Salvaggio E, Gueant JL. Transcobalamin and methioninesynthasereductase mutated polymorphisms aggravate the risk of neural tube defects in humans. NeurosciLett. 2003;344:189–192. doi: 10.1016/s0304-3940(03)00468-3. [DOI] [PubMed] [Google Scholar]
- Zetterberg H. Methylenetetrahydrofolatereductase and transcobalamin genetic polymorphisms in human spontaneous abortion: biological and clinical implications. ReprodBiolEndocrinol. 2004;2:7. doi: 10.1186/1477-7827-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedosov SN, Berglund L, Fedosova NU, Nexo E, Petersen TE. Comparative analysis of cobalamin binding kinetics and ligand protection for intrinsic factor, transcobalamin, and haptocorrin. The Journal of biological chemistry. 2002;277:9989–9996. doi: 10.1074/jbc.M111399200. [DOI] [PubMed] [Google Scholar]
- Wuerges J, Geremia S, Randaccio L. Structural study on ligand specificity of human vitamin B12 transporters. The Biochemical journal. 2007;403:431–440. doi: 10.1042/BJ20061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare PM, Wilbur DS, Heusser S, Quadros EV, McLoughlin P, Morgan AC. Synthesis of cobalamin-biotin conjugates that vary in the position of cobalamin coupling. Evaluation of cobalamin derivative binding to transcobalamin II. Bioconjugate chemistry. 1996;7:217–232. doi: 10.1021/bc9600022. [DOI] [PubMed] [Google Scholar]
- Fedosov SN, Orning L, Lovli T, Quadros EV, Thompson K, Berglund L, Petersen TE. Mapping the functional domains of human transcobalamin using monoclonal antibodies. The FEBS journal. 2005;272:3887–3898. doi: 10.1111/j.1742-4658.2005.04805.x. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Rothenberg SP, Pan YC, Stein S. Purification and molecular characterization of human transcobalamin II. The Journal of biological chemistry. 1986;261:15455–15460. [PubMed] [Google Scholar]
- Quadros EV, Rothenberg SP, McLoughlin P. Characterization of monoclonal antibodies to epitopes of human transcobalamin II. Biochemical and biophysical research communications. 1996;222:149–154. doi: 10.1006/bbrc.1996.0713. [DOI] [PubMed] [Google Scholar]
- Platica O, Geneczko R, Regec A, Quadros EV, Rothenberg SP. Isolation of the complementary DNA for human transcobalamin II, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1989;192:95–97. doi: 10.3181/00379727-192-1-rc1. [DOI] [PubMed] [Google Scholar]
- Platica O, Janeczko R, Quadros EV, Regec A, Romain R, Rothenberg SP. The cDNA sequence and the deduced amino acid sequence of human transcobalamin II show homology with rat intrinsic factor and human transcobalamin I. The Journal of biological chemistry. 1991;266:7860–7863. [PubMed] [Google Scholar]
- Regec A, Quadros EV, Platica O, Rothenberg SP. The cloning and characterization of the human transcobalamin II gene. Blood. 1995;85:2711–2719. [PubMed] [Google Scholar]
- Li N, Seetharam S, Lindemans J, Alpers DH, Arwert F, Seetharam B. Isolation and sequence analysis of variant forms of human transcobalamin II. Biochimica et biophysica acta. 1993;1172:21–30. doi: 10.1016/0167-4781(93)90264-e. [DOI] [PubMed] [Google Scholar]
- Porck HJ, Frater-Schroder M, Frants RR, Kierat L, Eriksson AW. Genetic evidence for fetal origin of transcobalamin II in human cord blood. Blood. 1983;62:234–237. [PubMed] [Google Scholar]
- Quadros EV, Sai P, Rothenberg SP. Functional human transcobalamin II isoproteins are secreted by insect cells using the baculovirus expression system. Blood. 1993;81:1239–1245. [PubMed] [Google Scholar]
- Arwert F, Porck HJ, Frater-Schroder M, Brahe C, Geurts van Kessel A, Westerveld A, Meera Khan P, Zang K, Frants RR, Kortbeek HT, et al. Assignment of human transcobalamin II (TC2) to chromosome 22 using somatic cell hybrids and monosomic meningioma cells. Human genetics. 1986;74:378–381. doi: 10.1007/BF00280489. [DOI] [PubMed] [Google Scholar]
- Li N, Seetharam S, Seetharam B. Genomic structure of human transcobalamin II: comparison to human intrinsic factor and transcobalamin I. BiochemBiophys Res Commun. 1995;208:756–764. doi: 10.1006/bbrc.1995.1402. [DOI] [PubMed] [Google Scholar]
- Rothenberg SP, Quadros EV, Regec A, Transcobalamin A., II . In: In Vitamin B12. Bannerjii R, editor. John Wiley and Sons Publ; 1999. [Google Scholar]
- Johnston J, Yang-Feng T, Berliner N. Genomic structure and mapping of the chromosomal gene for transcobalamin I (TCN1): comparison to human intrinsic factor. Genomics. 1992;12:459–464. doi: 10.1016/0888-7543(92)90435-u. [DOI] [PubMed] [Google Scholar]
- Hewitt JE, Gordon MM, Taggart RT, Mohandas TK, Alpers DH. Human gastric intrinsic factor: characterization of cDNA and genomic clones and localization to human chromosome 11. Genomics. 1991;10:432–440. doi: 10.1016/0888-7543(91)90329-d. [DOI] [PubMed] [Google Scholar]
- Donaldson RM, Jr., Brand M, Serfilippi D. Changes in circulating transcobalamin II after injection of cyanocobalamin. The New England journal of medicine. 1977;296:1427–1430. doi: 10.1056/NEJM197706232962502. [DOI] [PubMed] [Google Scholar]
- Jensen HS, Gimsing P, Pedersen F, Hippe E. Transcobalamin II as an indicator of activity in metastatic renal adenocarcinoma. Cancer. 1983 Nov 1;52(9):1700–4. doi: 10.1002/1097-0142(19831101)52:9<1700::aid-cncr2820520925>3.0.co;2-v. PubMed PMID: 6616421. [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M, Hitzig WH, Grob PJ, Kenny AB. Increased unsaturated transcobalamin II in active autoimmune disease. Lancet. 1978;2:238–239. doi: 10.1016/s0140-6736(78)91747-6. [DOI] [PubMed] [Google Scholar]
- Regec AL, Quadros EV, Rothenberg SP. Transcobalamin II expression is regulated by transcription factor(s) binding to a hexameric sequence (TGGTCC) in the promoter region of the gene. Archives of biochemistry and biophysics. 2002;407:202–208. doi: 10.1016/s0003-9861(02)00495-2. [DOI] [PubMed] [Google Scholar]
- Li N, Seetharam S, Seetharam B. Characterization of the human transcobalamin II promoter. A proximal GC/GT box is a dominant negative element. The Journal of biological chemistry. 1998;273:16104–16111. doi: 10.1074/jbc.273.26.16104. [DOI] [PubMed] [Google Scholar]
- Li N, Seetharam B. A 69-base pair fragment derived from human transcobalamin II promoter is sufficient for high bidirectional activity in the absence of a TATA box and an initiator element in transfected cells. Role of an E box in transcriptional activity. J Biol Chem. 1998;273:28170–28177. doi: 10.1074/jbc.273.43.28170. [DOI] [PubMed] [Google Scholar]
- Hakami N, Neiman PE, Canellos GP, Lazerson J. Neonatal megaloblastic anemia due to inherited transcobalamin II deficiency in two siblings. The New England journal of medicine. 1971;285:1163–1170. doi: 10.1056/NEJM197111182852103. [DOI] [PubMed] [Google Scholar]
- Hitzig WH, Dohmann U, Pluss HJ, Vischer D. Hereditary transcobalamin II deficiency: clinical findings in a new family. The Journal of pediatrics. 1974;85:622–628. doi: 10.1016/s0022-3476(74)80503-2. [DOI] [PubMed] [Google Scholar]
- Linnell JC, Quadros EV, Elliott PG, Malleson P. Defective adenosylcobalamin synthesis in a case of transcobalamin II deficiency. Journal of inherited metabolic disease. 1980;3:95–96. doi: 10.1007/BF02312538. [DOI] [PubMed] [Google Scholar]
- DiGirolamo PM, Huennekens FM. Transport of vitamin B12 into mouse leukemia cells. Archives of biochemistry and biophysics. 1975;168:386–393. doi: 10.1016/0003-9861(75)90267-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tavassoli M, Jacobsen DW. Receptor binding and internalization of immobilized transcobalamin II by mouse leukaemia cells. Nature. 1980;288:713–715. doi: 10.1038/288713a0. [DOI] [PubMed] [Google Scholar]
- Soda R, Tavassoli M, Jacobsen DW. Receptor distribution and the endothelial uptake of transcobalamin II in liver cell suspensions. Blood. 1985;65:795–802. [PubMed] [Google Scholar]
- Kishimoto T, Tavassoli M, Green R, Jacobsen DW. Receptors for transferrin and transcobalamin II display segregated distribution on microvilli of leukemia L1210 cells. Biochemical and biophysical research communications. 1987;146:1102–1108. doi: 10.1016/0006-291x(87)90761-3. [DOI] [PubMed] [Google Scholar]
- Meyer LM, Miller IF, Gizis E, Tripp E, Hoffbrand AV. Delivery of vitamin B12 to human lymphocytes by transcobalamins I, II and 3. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1974;146:747–750. doi: 10.3181/00379727-146-38185. [DOI] [PubMed] [Google Scholar]
- Hall CA. The uptake of vitamin B12 by human lymphocytes and the relationships to the cell cycle. The Journal of laboratory and clinical medicine. 1984;103:70–81. [PubMed] [Google Scholar]
- Hall CA, Colligan PD, Begley JA. Cyclic activity of the receptors of cobalamin bound to transcobalamin II. Journal of cellular physiology. 1987;133:187–191. doi: 10.1002/jcp.1041330125. [DOI] [PubMed] [Google Scholar]
- Seligman PA, Allen RH. Characterization of the receptor for transcobalamin II isolated from human placenta. The Journal of biological chemistry. 1978;253:1766–1772. [PubMed] [Google Scholar]
- Bose S, Seetharam S, Seetharam B. Membrane expression and interactions of human transcobalamin II receptor. The Journal of biological chemistry. 1995;270:8152–8157. doi: 10.1074/jbc.270.14.8152. [DOI] [PubMed] [Google Scholar]
- Bose S, Feix J, Seetharam S, Seetharam B. Dimerization of transcobalamin II receptor. Requirement of a structurally ordered lipid bilayer. The Journal of biological chemistry. 1996;271:11718–11725. doi: 10.1074/jbc.271.20.11718. [DOI] [PubMed] [Google Scholar]
- Vanamala SK, Seetharam S, Yammani RR, Seetharam B. Human transcobalamin II receptor binds to Staphylococcus aureus protein A: implications as to its structure and function. Archives of biochemistry and biophysics. 2003;411:204–214. doi: 10.1016/s0003-9861(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Sai P, Rothenberg SP. Characterization of the human placental membrane receptor for transcobalamin II-cobalamin. Archives of biochemistry and biophysics. 1994;308:192–199. doi: 10.1006/abbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochemical and biophysical research communications. 2005;327:1006–1010. doi: 10.1016/j.bbrc.2004.12.103. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood. 2009;113:186–192. doi: 10.1182/blood-2008-05-158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. Receptors of the low density lipoprotein (LDL) receptor family in man. Multiple functions of the large family members via interaction with complex ligands. Biological chemistry. 1998;379:951–964. [PubMed] [Google Scholar]
- Jiang W, Nakayama Y, Sequeira JM, Quadros EV. Characterizing monoclonal antibodies to antigenic domains of TCblR/CD320, the receptor for cellular uptake of transcobalamin-bound cobalamin. Drug delivery. 2011;18:74–78. doi: 10.3109/10717544.2010.509745. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, Choi YS. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. The Journal of experimental medicine. 2000;191:1077–1084. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. Targeted delivery of saporin toxin by monoclonal antibody to the transcobalamin receptor, TCblR/CD320. Molecular cancer therapeutics. 2010;9:3033–3040. doi: 10.1158/1535-7163.MCT-10-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GR, Quadros EV, Rothenberg SP, Morgan AC, Schrader JW, Ziltener HJ. Antibodies to transcobalamin II block in vitro proliferation of leukemic cells. Blood. 1997;89:235–242. [PubMed] [Google Scholar]
- Collins DA, Hogenkamp HP. Transcobalamin II receptor imaging via radiolabeled diethylenetriaminepentaacetate cobalamin analogs, Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 1997;38:717–723. [PubMed] [Google Scholar]
- Lee M, Grissom CB. Design, synthesis, and characterization of fluorescent cobalamin analogues with high quantum efficiencies. Organic letters. 2009;11:2499–2502. doi: 10.1021/ol900401z. [DOI] [PubMed] [Google Scholar]
- Jiang W, Sequeira JM, Nakayama Y, Lai SC, Quadros EV. Characterization of the promoter region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene. 2010;466:49–55. doi: 10.1016/j.gene.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros EV, Lai SC, Nakayama Y, Sequeira JM, Hannibal L, Wang S, Jacobsen DW, Fedosov S, Wright E, Gallagher RC, Anastasio N, Watkins D, Rosenblatt DS. Positive newborn screen for methylmalonic aciduria identifies the first mutation in TCblR/CD320, the gene for cellular uptake of transcobalamin-bound vitamin B(12) Human mutation. 2010;31:924–929. doi: 10.1002/humu.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangilinan F, Mitchell A, VanderMeer J, Molloy AM, Troendle J, Conley M, Kirke PN, Sutton M, Sequeira JM, Quadros EV, Scott JM, Mills JL, Brody LC. Transcobalamin II receptor polymorphisms are associated with increased risk for neural tube defects. Journal of medical genetics. 2010;47:677–685. doi: 10.1136/jmg.2009.073775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot GR, Murphy WP. Treatment of pernicious anemia by a special diet. JAMA. 1926;87:470–476. doi: 10.1001/jama.250.24.3328. [DOI] [PubMed] [Google Scholar]
- Castle WB. The Aetiological Relationship of Achylia Gastrica to Pernicious Anaemia. Proceedings of the Royal Society of Medicine. 1929;22:1214–1216. [PMC free article] [PubMed] [Google Scholar]
- Rickes EL, Brink NG, Koniuszy FR, Wood TR, Folkers K. Comparative Data on Vitamin B12 From Liver and From a New Source, Streptomyces griseus. Science. 1948;108:634–635. doi: 10.1126/science.108.2814.634-a. [DOI] [PubMed] [Google Scholar]
- Smith EL, Parker LF. Purification of anti-pernicious anaemia factor. The Biochemical journal. 1948;43:viii. [PubMed] [Google Scholar]
- Moore FM, Willis BT, Hodgkin DC. Structure of a monocarboxylic acid derivative of vitamin B 12. Crystal and molecular structure from neutron diffraction analysis. Nature. 1967;214:130–133. doi: 10.1038/214130a0. [DOI] [PubMed] [Google Scholar]
- Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. British journal of haematology. 2010;148:195–204. doi: 10.1111/j.1365-2141.2009.07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins D, Rosenblatt DS. Inborn errors of cobalamin absorption and metabolism, American journal of medical genetics. Part C. Seminars in medical genetics. 2011;157:33–44. doi: 10.1002/ajmg.c.30288. [DOI] [PubMed] [Google Scholar]
- Ratschmann R, Minkov M, Kis A, Hung C, Rupar T, Muhl A, Fowler B, Nexo E, Bodamer OA. Transcobalamin II deficiency at birth. Molecular genetics and metabolism. 2009;98:285–288. doi: 10.1016/j.ymgme.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Li N, Rosenblatt DS, Kamen BA, Seetharam S, Seetharam B. Identification of two mutant alleles of transcobalamin II in an affected family. Human molecular genetics. 1994;3:1835–1840. doi: 10.1093/hmg/3.10.1835. [DOI] [PubMed] [Google Scholar]
- Li N, Rosenblatt DS, Seetharam B. Nonsense mutations in human transcobalamin II deficiency. Biochemical and biophysical research communications. 1994;204:1111–1118. doi: 10.1006/bbrc.1994.2577. [DOI] [PubMed] [Google Scholar]
- Schiff M, Ogier de Baulny H, Bard G, Barlogis V, Hamel C, Moat SJ, Odent S, Shortland G, Touati G, Giraudier S. Should transcobalamin deficiency be treated aggressively? Journal of inherited metabolic disease. 2010;33:223–229. doi: 10.1007/s10545-010-9074-x. [DOI] [PubMed] [Google Scholar]
- Namour F, Helfer AC, Quadros EV, Alberto JM, Bibi HM, Orning L, Rosenblatt DS, Jean-Louis G. Transcobalamin deficiency due to activation of an intra exonic cryptic splice site. British journal of haematology. 2003;123:915–920. doi: 10.1046/j.1365-2141.2003.04685.x. [DOI] [PubMed] [Google Scholar]
- Haberle J, Pauli S, Berning C, Koch HG, Linnebank M. TC II deficiency: avoidance of false-negative molecular genetics by RNA-based investigations. Journal of human genetics. 2009;54:331–334. doi: 10.1038/jhg.2009.34. [DOI] [PubMed] [Google Scholar]
- Nissen PH, Nordwall M, Hoffmann-Lucke E, Sorensen BS, Nexo E. Transcobalamin deficiency caused by compound heterozygosity for two novel mutations in the TCN2 gene: a study of two affected siblings, their brother, and their parents. Journal of inherited metabolic disease. 2010 doi: 10.1007/s10545-010-9145-z. [DOI] [PubMed] [Google Scholar]
- Anastasio N, Watkin D, Vezina L, Dempsey-Nunez L, Reichman L, Quadros EV, Rosenblatt DS. Mol.Genet.Metab. 2009;98:122. (Abstract) [Google Scholar]
- Karth P, Singh R, Kim J, Costakos D. Bilateral central retinal artery occlusions in an infant with hyperhomocysteinemia. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2012;16:398–400. doi: 10.1016/j.jaapos.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Qian L, Quadros EV, Regec A, Zittoun J, Rothenberg SP. Congenital transcobalamin II deficiency due to errors in RNA editing. Blood cells, molecules & diseases. 2002;28:134–142. doi: 10.1006/bcmd.2002.0499. discussion 143-135. [DOI] [PubMed] [Google Scholar]