Abstract
PURPOSE
To analyze the survival of CLL patients relative to age-matched individuals in the general population and determined the age-stratified utility of prognostic testing.
METHODS
All 2487 patients diagnosed with CLL between January 1995 and June 2008 and cared for in the Mayo Division of Hematology were categorized by age at diagnosis and evaluated for differences in clinical characteristics, time to first treatment(TFT), and overall survival(OS).
RESULTS
Among Rai stage 0 patients, survival was shorter than the age-matched general population for patients age<55 years(p<0.001), 55-64 years(p<0.001), and 65-74 years(p<0.001) but not those age≥75 at diagnosis(p=NS). CD38, IGHV mutation, and ZAP-70 each predicted TFT independent of stage for all age groups(all p <0.04) but had less value for predicting OS, particularly as age increased. IGHV and FISH predicted OS independent of stage for patients <age 55(p≤0.001), 55-64(p≤0.004), and 65-74(p≤0.001) but not those ≥75. CD38 and ZAP-70 each predicted OS independent of stage for only 2 of 4 age categories. Among Rai 0 patients age<75, survival was shorter than the age-matched population only for IGHV unmutated(p<0.001) patients or those with unfavorable FISH(p<0.001).
CONCLUSIONS
Survival of CLL patients age<75 is shorter than the age-matched general population regardless of disease stage. Among patients age<75, the simple combinations of stage and IGHV or stage and FISH identifies those with excess risk of death relative to the age-matched population. Although useful for predicting TFT independent of stage for patients of all ages, prognostic testing had little utility for predicting OS independent of stage among patients age≥75.
Background
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is one of the most common lymphoid malignancies accounting for approximately 11% of hematologic cancers in the Western World.1 The prevalence of CLL increases with age and the median age at the time of diagnosis is between 65 and 70 years.2-6 Recent studies suggest that the 5 year survival of CLL patients of all ages has increased over the last two decades,6-8 likely due in part to early stage at diagnosis.3,4,6,9 The absolute 10 year survival of patients with CLL has increased by ~10% for patients of all ages except those over age 80 years7,8.
While the observed improvement in the survival of CLL patients at the population level is encouraging, the clinical course of individual patients is heterogeneous. Even among individuals with early stage disease, there remains significant heterogeneity in clinical behavior and stage alone does not adequately predict the risk of progression for a given patient.10 Although numerous clinical and biologic parameters are able to predict survival and time to first treatment (TFT),10-15 the utility of these prognostic parameters may vary based on age given the higher mortality from competing health problems in older individuals.7,16,17 Indeed there remains a strong age gradient in the survival among CLL patients2,7,8,18 where the expected 10 year survival for those less than age 60 is 59% compared to 6% for those over age 80.7 Accordingly, while risk stratification using leukemia cell biomarkers (e.g. ZAP-70, FISH, IGHV testing) may provide useful information for counseling a newly diagnosed 50 year old patient with Rai stage 0 disease, its usefulness to a 75 year old patient in the same clinical circumstance is less clear13,16.
These facts have important implications for use of prognostic testing and counseling regarding life expectancy for older individuals with CLL who represent the majority of CLL patients world-wide. Most of the data on the ability of prognostic parameters to predict outcome is derived from cohorts of CLL patients with a median age <65 years10-15 and the median age of patients in many series is <60 years12,14,15. In the present study, we evaluated the clinical outcome of 2487 patients diagnosed with CLL between January 1995 and June 2008 to: i) evaluate differences in natural history based on age at diagnosis, ii) compare survival to age-matched individuals in the general population, and iii) determine the age-stratified utility of prognostic testing.
Methods
Patients
The Mayo Clinic CLL Database includes all patients with a diagnosis of CLL19,32 seen in the Division of Hematology at Mayo Clinic Rochester (MCR) who permit their records to be used for research purposes.20-26 Clinical information regarding date of diagnosis, physical examination, clinical stage (Rai), prognostic parameters, treatment history, and disease-related complications are abstracted from clinical records on all patients at the time of inclusion and maintained on an ongoing, prospective basis. For staging purposes, patients with SLL who have cytopenias at diagnosis are grouped with Rai stage III/IV patients while those with palpable lymphadenopathy without cytopenias are grouped with Rai stage I patients. Results of prognostic testing performed as part of clinical or research studies are also included in the database. This includes evaluation of absolute lymphocyte count (ALC), IGHV gene mutation analysis, ZAP-70 status, CD38 status, and cytogenetics abnormalities by interphase FISH testing using methods previously described by our group20,27-29.
With the approval of the Mayo Clinic Institutional Review Board and in accord with federal regulations and the Declaration of Helsinki, we used this database to identify all patients diagnosed with CLL between January 1995 and June 2008. All these patients had an ALC ≥5.0 × 109/L and fulfilled the 1996 criteria for CLL which were in effect throughout the study period32 and/or fulfilled the WHO criteria for CLL/SLL19. Patients were categorized by age at the time of CLL diagnosis (<55 years, 55-64 years, 65-74 years,≥75 years) with categories based in part on the previous designations of “young” CLL as individuals age ≤55 at diagnosis16,33 and stratification of patients >age 55 in 10 year intervals up to age 75. Differences in clinical characteristics, time to first treatment (TFT), and overall survival (OS) based on age at diagnosis were assessed. Since FISH can change during the course of the disease or after treatment,29 only FISH analysis obtained prior to first treatment was included in the present analysis. Based on evidence that VH 3-21 family usage is associated with poor outcome independent of mutation status 30,31, patients with VH 3-21 family usage were considered to have high risk IGHV status regardless of percent mutation.
Statistical methods
OS was defined as the time between the date of diagnosis to the date of death or last follow-up. TFT was defined as the time between date of diagnosis and the date of initiation of first treatment or date of last follow-up at which the patient was known to be untreated. The accepted indications to initiate treatment were based on the NCI-WG 1996 criteria.32 Patients receiving early treatment as part of experimental protocols prior to meeting NCI-WG 1996 criteria to initiate therapy were censored as untreated on the date experimental therapy was administered. Estimates of survival were calculated using the Kaplan-Meier method. Cox proportional hazard models were used to model the relationship of multiple variables simultaneously including age at diagnosis with OS and TFT. Expected survival was calculated using the Cohort (Hakulinen) method34; estimates are based on the "Minnesota White" population35. Likelihood ratio tests were used to test effects of individual factors either individually or jointly. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated from the Cox models. In addition to individual cytogenetic categories, FISH results were classified as ‘unfavorable’ (17p-, 11q-) or ‘favorable’ (normal, trisomy 12, 13q-, other) for some analyses. P-values <0.05 were considered significant. All statistical analyses were performed using the SAS 9.1 software package (SAS Institute; Cary, North Carolina).
Results
There were 2487 patients who qualified for inclusion in this study. The median age at diagnosis was 64 years old. When grouped by age, 593 patients were age <55, 713 age 55-64, 748 age 65-74, and 433 age ≥75 at diagnosis. The demographic and prognostic characteristics of patients by age at diagnosis are shown in Table 1. Because CD38, ZAP-70, IGHV gene mutation status, and FISH analysis were not routinely performed during the entirety of the study period, results were not available for all patients. Patients under the age of 55 were more likely to have intermediate stage (Rai I or II) at diagnosis while those >age 65 were less likely to have an ALC>30 ×109/L; however no statistically significant differences were observed in CD38, IGHV mutation, ZAP-70 or the frequency of common cytogenetic abnormalities as identified by FISH based on age. TFT among Rai stage 0 patients was shorter for patients <55, however no difference in TFT by age was observed among Rai stage I or II patients.
Table 1.
Patient Characteristics Patient Characteristics
| Age <55 N=593 | Age 55-64 N=713 | Age 65-74 N=748 | Age ≥ 75 N=433 | P Value | |
|---|---|---|---|---|---|
| Median Age at Diagnosis | 49 | 60 | 69 | 80 | --- |
| Male | 404 (68%) | 493 (69%) | 497 (66%) | 295 (68%) | 0.739 |
| Rai Risk at diagnosis | |||||
| Low (Rai stage 0) | 251 (43%) | 383 (56%) | 408 (57%) | 245 (58%) | <0.001 |
| Intermediate (Rai stage I-II) | 308 (53%) | 257 (38%) | 269 (38%) | 129 (31%) | |
| High (Rai stage III-IV) | 21 (4%) | 40 (6%) | 40 (6%) | 46 (11%) | |
| Missing | 13 | 33 | 31 | 13 | |
| ALC (× 109/L) | |||||
| ≤30 | 449 (77%) | 550 (79%) | 631 (85%) | 370 (86%) | <0.001 |
| >30 | 133 (23%) | 148 (21%) | 109 (15%) | 60 (14%) | |
| Missing | 11 | 15 | 8 | 3 | |
| CD38 | |||||
| Negative | 294 (66%) | 376 (69%) | 365 (68%) | 195 (65%) | 0.588 |
| Positive | 152 (34%) | 169 (31%) | 172 (32%) | 105 (35%) | |
| Missing | 147 | 168 | 211 | 133 | |
| ZAP-70 | |||||
| Negative | 203 (61%) | 220 (60%) | 219 (66%) | 95 (66%) | 0.340 |
| Positive | 130 (39%) | 145 (40%) | 113 (34%) | 50 (34%) | |
| Missing | 260 | 348 | 416 | 288 | |
| IGHV | |||||
| Mutated | 150 (51%) | 172 (54%) | 170 (59%) | 64 (61%) | 0.166 |
| Unmutated | 144 (49%) | 144 (46%) | 119 (41%) | 41 (39%) | |
| Missing | 299 | 397 | 459 | 328 | |
| FISH (prior to treatment) | |||||
| 13q- | 118 (44%) | 136 (43%) | 126 (41%) | 50 (34%) | * |
| Normal | 71 (27%) | 76 (24%) | 80 (26%) | 41 (28%) | |
| +12 | 43 (16%) | 62 (20%) | 58 (19%) | 33 (23%) | |
| 11q- | 22 (8%) | 26 (8%) | 23 (7%) | 16 (11%) | |
| 17p- | 10 (4%) | 11 (3%) | 16 (5%) | 6 (4%) | |
| other | 3 (1%) | 5 (2%) | 4 (1%) | 1 (1%) | |
| Missing | 326 | 397 | 441 | 286 | |
| FISH groupings | |||||
| Normal, 13q, trisomy 12 | 232 (88%) | 274 (88%) | 264 (87%) | 124 (85%) | 0.799 |
| 11q, 17p | 32 (12%) | 37 (12%) | 39 (13%) | 22 (15%) | |
| Treated | 268 (45%) | 282 (40%) | 296 (40%) | 108 (25%) | <0.001 |
| Median TFT Rai 0 patients | 5.9 | 8.8 | 8.6 | not reached | 0.047 |
| Median TFT Rai I-II patients | 1.9 | 2.5 | 2.5 | 3.4 | 0.216 |
| Dead | 112 (19%) | 171 (24%) | 254 (34%) | 199 (46%) | <0.001 |
| Median OS (yrs) | 11.8 | 10.9 | 9.0 | 6.4 | <0.001 |
p-value not provided because chi-square test is not appropriate and calculation of Fisher’s exact p-value was not feasible (too many FISH categories).
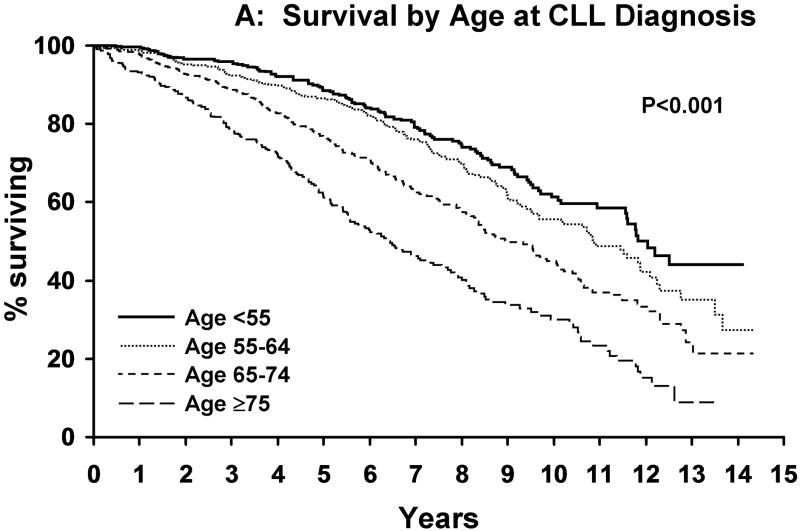
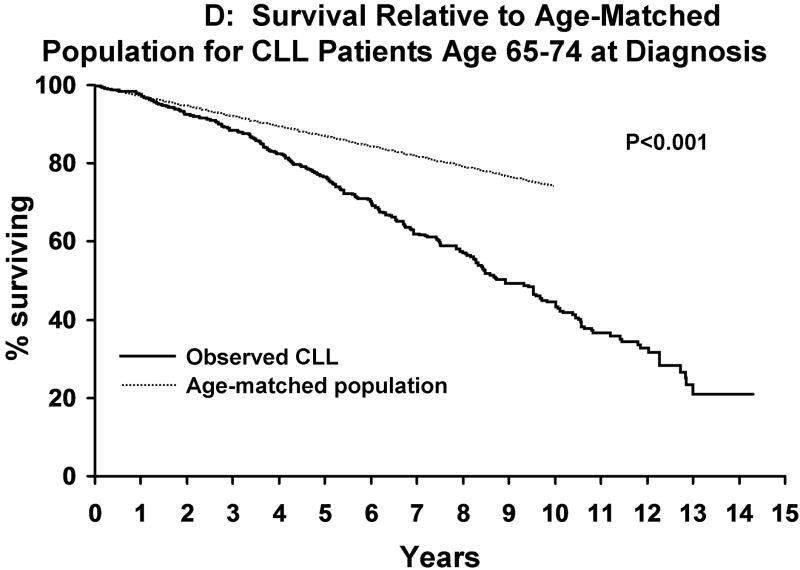
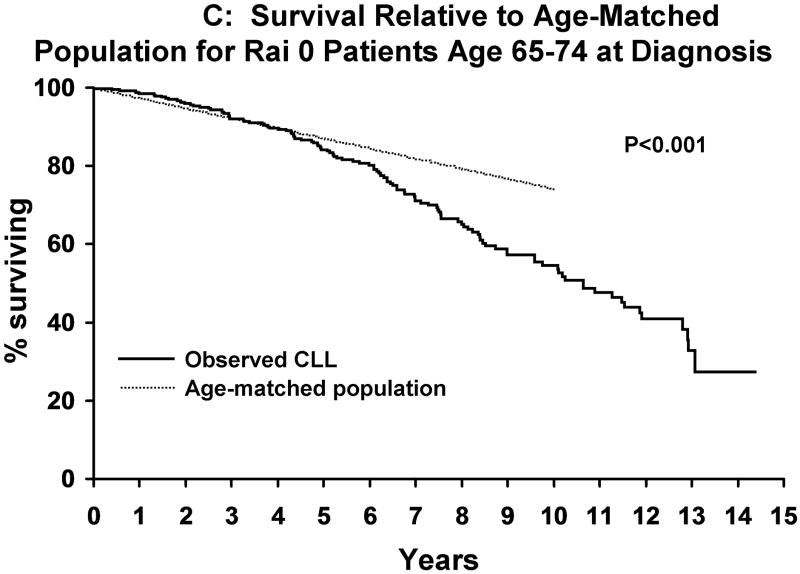
Median follow-up was 9.7 years. As of last follow-up, 954 patients have been treated and 727 patients have died. Median TFT was 4.8 years. Although survival decreased as the age at diagnosis increased (Figure 1A), the survival of CLL patients was significantly shorter than that of the age-matched general population for patients age <55 years (p<0.001), 55-64 years (p<0.001), and 65-74 years (p<0.001) at diagnosis but not those ≥75 years (p=0.14; Figure 1B-E). Among Rai stage 0 patients, survival was also shorter than that of the age-matched general population for CLL patients age <55 years (p<0.001), 55-64 years (p<0.001), and 65-74 years (p<0.001) but not those ≥ age 75 (p=0.07) at diagnosis (Figure 2A-D).
Figure 1. Survival CLL Patients Relative To Age-Matched Individuals.
A indicates survival of CLL patients based on age at diagnosis [age <55 (n=593), age 55-64 (n=713), age 65-74 (n=748), age ≥75 (n=433)]. Figure 1B indicates the survival of CLL patients <age 55 at diagnosis (n=593) relative to the age matched population <age 55. Figure 1C indicates the survival of CLL patients age 55-64 at diagnosis(n=713) relative to the age matched population age 55-64. Figure 1D indicates the survival of CLL patients age 65-74 at diagnosis(n=748) relative to the age matched population age 65-74. Figure 1E indicates the survival of CLL patients age >75 at diagnosis(n=433) relative to the age matched population age >75.
Figure 2. Survival Rai Stage 0 CLL Patients Relative To Age-Matched Individuals.
A indicates the survival of Rai 0 CLL patients <age 55 at diagnosis (n=248) relative to the age matched population <age 55. Figure 2B indicates the survival of Rai 0 CLL patients age 55-64 at diagnosis(n=379) relative to the age matched population age 55-64. Figure 2C indicates the survival of Rai 0 CLL patients age 65-74 at diagnosis(n=405) relative to the age matched population age 65-74. Figure 2D indicates the survival of Rai 0 CLL patients age >75 at diagnosis(n=244) relative to the age matched population age >75.
We next evaluated the relationship between prognostic parameters and TFT and OS. Consistent with prior reports, stage, ALC (≤ or >30 ×109/L), CD38, IGHV mutation, ZAP-70 and cytogenetic analysis by FISH were all powerful predictors of both TFT and OS on univariate analysis (all p ≤0.003 for both TFT and OS; Table 2). In a multi-variate analysis in the 585 patients who had results for all prognostic variables, stage (HR high Rai risk vs. low Rai risk=16.1; HR intermediate Rai risk vs. low Rai risk=2.3), IGHV unmutated (HR=2.8; p<0.001), ALC >30 × 109/L (HR=2.0), and CD38 positive (HR=1.9) remained independent predictors of TFT (all p<0.001) while ZAP-70 (HR=1.3; p=0.16) and FISH (HR=1.3; p=0.264) were no longer statistically significant. With respect to OS, only high risk FISH (HR=2.9; p=0.008) and CD38 positive (HR=2.2; p=0.041) remained independent predictors of OS where IGHV (HR 2.8; p=0.072), stage (HR high Rai risk=2.8, p=0.20; HR intermediate Rai risk=1.4, p=0.37), ALC (HR 1.2; p=0.62), and ZAP-70 (HR 0.9; p=0.77) were no longer statistically significant.
Table 2.
Prognostic Parameters, Time to First Treatment (TFT), and Overall Survival (OS)
| Median TFT (years) | P value | Median OS (years) | P value | |
|---|---|---|---|---|
| Rai Risk at diagnosis (N=2397) | ||||
| Low (Rai stage 0) | 8.0 | <0.001 | 11.44 | <0.001 |
| Intermediate (Rai stage I-II) | 2.4 | 8.8 | ||
| High (Rai stage III-IV) | 0.1 | 5.2 | ||
| ALC (× 109/L) (N=2450) | ||||
| ≤30 | 5.6 | <0.001 | 10.2 | 0.003 |
| >30 | 2.4 | 8.7 | ||
| CD38 (N=1828) | ||||
| Negative | 8.0 | <0.001 | 11.9 | <0.001 |
| Positive | 2.8 | 8.5 | ||
| ZAP-70 (N=1175) | ||||
| Negative | 9.3 | <0.001 | Not reached | <0.001 |
| Positive | 3.2 | 10.8 | ||
| IgVH Mutation (N=1004) | ||||
| Mutated | 11.0 | <0.001 | Not reached | <0.001 |
| Unmutated | 2.8 | 9.7 | ||
| FISH (prior to treatment) (N=715) | ||||
| 13q- | Not reached | <0.001 | Not reached | <0.001 |
| Normal | 8.7 | Not reached | ||
| +12 | 5.4 | 10.9 | ||
| 11q- | 2.4 | 8.4 | ||
| 17p- | 5.2 | 7.6 | ||
| FISH groupings | ||||
| Normal, 13q, trisomy 12 | 8.7 | <0.001 | Not reached | <0.001 |
| 11q, 17p | 3.7 | 7.6 |
Given the variation in TFT and OS based on age at diagnosis as well as differences in the magnitude of effect of a CLL diagnosis on survival relative to age-matched controls, we next evaluated whether ALC, CD38, IGHV mutation, ZAP-70 and FISH remained useful predictors of TFT and OS for CLL patients in all age categories. Stage, ALC, CD38, ZAP-70, and IGHV remained powerful predictors of TFT for CLL patients of all ages including those age ≥75 (all p≤0.005). FISH was also a powerful predictor of TFT for CLL patients of all ages except those over age 75 where it failed to reach statistical significance (p=0.09). The hazard ratio of the individual prognostic parameter for predicting TFT was generally similar across age groups.
In contrast to the near uniform value of ALC, CD38, IGHV mutation, ZAP-70 and FISH for predicting TFT in all age categories, their ability to predict OS was less consistent. Stage, CD38, and IGHV were statistically significant predictors of OS in CLL patients of all age categories including those ≥age 75 (all p<0.05). FISH predicted OS in all age categories <age 75 (all p≤0.003) but not patients age ≥75 (p=0.34). ZAP-70 predicted OS for patients who were <55 (p=0.007) and 55-64 (p=0.004) but not patients_age 65-74 (p=0.28) or >75 (p=0.97). ALC>30 ×109/L was only a significant predictor of OS for patients in the 65-74 year old group.
Next, we evaluated the ability of ALC, CD38, IGHV mutation, ZAP-70 and FISH to predict TFS and OS in each age category after adjusting for stage. With respect to TFT, CD38, IGHV mutation, and ZAP-70, each predicted TFT independent of stage for all age categories (Top Table 3). FISH predicted TFT independent of stage for patients age<55, 55-64 and 65-74 but not for those age ≥75 (p=0.08). The utility of these parameters for predicting OS independent of stage was less consistent and varied by age. Both IGHV and FISH predicted OS independent of stage for patients <age 55, 55-64, and 65-74 but not those ≥75 (Bottom Table 3). CD38 and ZAP-70 each predicted survival independent of stage for only 2 of the 4 age categories (ZAP-70 for age <55 and 55-64; CD38 for 55-64 and ≥75).
Table 3.
Multivariate Models
| TIME TO FIRST TREATMENT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Prognostic Factor | Age <55 | Age 55-64 | Age 65-74 | Age ≥ 75 | ||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| Rai Stage: High vs Low | 7.7 | <0.001 | 9.0 | <0.001 | 6.7 | <0.001 | 10.6 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.5 | <0.001 | 2.4 | <0.001 | 2.8 | <0.001 | 4.3 | <0.001 |
| ALC (>30 vs ≤30) | 1.3 | 0.070 | 1.8 | <0.001 | 2.2 | <0.001 | 2.9 | <0.001 |
| Rai Stage: High vs Low | 14.6 | <0.001 | 16.0 | <0.001 | 6.9 | <0.001 | 11.3 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.4 | <0.001 | 2.4 | <0.001 | 2.6 | <0.001 | 2.6 | <0.001 |
| CD38 (Positive vs negative) | 2.3 | <0.001 | 2.8 | <0.001 | 1.7 | <0.001 | 1.7 | 0.037 |
| Rai Stage: High vs Low | 19.7 | <0.001 | 9.7 | <0.001 | 15.0 | <0.001 | 6.8 | 0.001 |
| Rai Stage: Intermediate vs Low | 2.3 | <0.001 | 2.2 | <0.001 | 3.6 | <0.001 | 1.1 | 0.802 |
| ZAP-70 (Positive vs negative) | 2.0 | <0.001 | 1.9 | <0.001 | 2.8 | <0.001 | 5.6 | <0.001 |
| Rai Stage: High vs Low | 16.1 | <0.001 | 8.6 | <0.001 | 30.0 | <0.001 | 11.7 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.7 | <0.001 | 2.1 | <0.001 | 2.5 | <0.001 | 1.3 | 0.587 |
| IgVH Mutation (UM vs. M) | 3.8 | <0.001 | 3.6 | <0.001 | 4.7 | <0.001 | 5.4 | 0.002 |
| Rai Stage: High vs Low | 6.2 | <0.001 | 6.2 | <0.001 | 10.0 | <0.001 | 13.2 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.8 | <0.001 | 2.1 | 0.002 | 4.9 | <0.001 | 3.2 | 0.003 |
| FISH (17p, 11q vs normal, 13q, trisomy 12) | 2.7 | 0.001 | 3.0 | <0.001 | 2.8 | <0.001 | 2.0 | 0.078 |
| OVERALL SURVIVAL | ||||||||
| Prognostic Factor | Age <55 | Age 55-64 | Age 65-74 | Age ≥ 75 | ||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| Rai Stage: High vs Low | 3.6 | 0.002 | 5.0 | <0.001 | 2.6 | <0.001 | 3.8 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.2 | <0.001 | 1.9 | <0.001 | 1.9 | <0.001 | 1.8 | <0.001 |
| ALC | 1.4 | 0.126 | 1.5 | 0.027 | 1.7 | 0.001 | 1.5 | 0.052 |
| Rai Stage: High vs Low | 11.8 | <0.001 | 4.4 | <0.001 | 2.1 | 0.047 | 3.2 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.3 | 0.008 | 1.7 | 0.021 | 1.9 | 0.001 | 2.0 | 0.003 |
| CD38 (Positive vs negative) | 1.6 | 0.077 | 2.2 | <0.001 | 1.3 | 0.138 | 1.5 | 0.038 |
| Rai Stage: High vs Low | 22.8 | 0.005 | 9.1 | <0.001 | 1.0 | 0.982 | 9.1 | <0.001 |
| Rai Stage: Intermediate vs Low | 2.8 | 0.013 | 1.4 | 0.333 | 2.1 | 0.024 | 4.1 | <0.001 |
| ZAP-70 (Positive vs negative) | 2.6 | 0.011 | 2.2 | 0.025 | 1.2 | 0.578 | 0.7 | 0.426 |
| Rai Stage: High vs Low | 34.0 | <0.001 | 1.7 | 0.475 | 2.1 | 0.319 | 2.7 | 0.358 |
| Rai Stage: Intermediate vs Low | 2.2 | 0.074 | 1.5 | 0.225 | 2.0 | 0.019 | 1.8 | 0.120 |
| IgVH Mutation (UM vs. M) | 6.2 | <0.001 | 2.8 | 0.004 | 3.2 | <0.001 | 2.0 | 0.073 |
| Rai Stage: High vs Low | * | 4.5 | 0.007 | 7.4 | <0.001 | 3.7 | 0.002 | |
| Rai Stage: Intermediate vs Low | 1.7 | 0.294 | 1.0 | 0.936 | 2.3 | 0.007 | 2.7 | 0.008 |
| FISH (17p, 11q vs normal, 13q, trisomy 12) | 5.2 | 0.001 | 4.6 | 0.001 | 3.0 | 0.001 | 1.4 | 0.372 |
Too few events to estimate.
Finally, since OS was only shorter than the age matched population for CLL patients <age 75, we evaluated whether prognostic testing could identify which Rai 0 CLL patients <age 75 had a survival shorter than the age matched population. IGHV and FISH testing were used for this analysis based on the ability of these tests to identify CLL patients with shorter OS independent of stage in all age categories <75. The survival of Rai 0 IGHV unmutated CLL patients <age 75 was shorter than that of the age-matched general population (p<0.001) while Rai 0 IGHV M CLL patients had a survival similar to the population (Figure 3A and B). Similarly, the survival of Rai 0 CLL patients <age 75 with unfavorable FISH was shorter than that of the age-matched general population (p<0.001) while Rai 0 CLL patients with favorable FISH had a survival similar to the age-matched general population (Figure 3C and D).
Figure 3. Survival of Rai 0 Patients Age <75 Relative To Age-Matched Individuals Based on IGHV Mutation Status and Cytogenetic Analysis By FISH.
A: Survival of Rai 0, IGHV mutated CLL patients Age <75 (n=344) relative to the age-matched population. Figure 3B: Survival of Rai 0, IGHV unmutated CLL patients Age <75 (n=160) relative to the age-matched population. Figure 3C: Survival of Rai 0, CLL patients Age <75 (n=317) with favorable FISH (no 17p- or 11q-) relative to the age-matched population. Figure 3D: Survival of Rai 0, CLL patients Age <75 (n=27) with unfavorable FISH (e.g. 17p- or 11q-) relative to the age-matched population.
Discussion
Age has repeatedly been shown to be an independent predictor of survival in CLL patients16,17,36-38 and has the potential to alter the utility of prognostic testing given the higher mortality from competing health problems in older individuals. While the majority of patients with CLL are over age 65 at the time of diagnosis and have early stage disease,3,4,6,9 most of the published data on prognostic parameters is derived from younger patient cohorts. This incongruity has led to uncertainty regarding if and when to use prognostic testing in routine clinical practice for patients with CLL. In the present study, the survival of CLL patients < age 75 at diagnosis was shorter than that of the age-matched general population regardless of disease stage. In contrast, survival did not differ from the age-matched general population among CLL patients ≥age 75 at diagnosis. Prognostic testing using CD38, IGHV mutation, and ZAP-70 was useful for predicting TFT independent of stage for CLL patients of all ages (including those ≥age 75) but had less value for predicting OS, particularly as the age at diagnosis increased. Among Rai 0 patients <age 75, survival was shorter than the age-matched general population only for IGHV unmutated patients or those with unfavorable FISH.
These findings have a number of important implications for the use of prognostic tests in patients with CLL as well as the use of test results to select patients for clinical trials testing the value of early treatment. First, although the life expectancy of CLL patients <age 75 is substantially shorter than that of the age matched general population, the simple combination of stage and IGHV mutation status or stage and FISH can identify those with excess risk of death. Second, with regard to TFT, CD38, ZAP-70, IGHV, and FISH each provide useful information for CLL patients of all ages independent of stage. Third, prognostic markers appear to be a sound basis upon which to select “high risk” early stage patients <age 75 for clinical trials of early intervention. In the present cohort, both IGHV mutation status and FISH predicted OS independent of stage with relatively large hazard ratios (range 2.8-6.2) among patients in all age categories < age 75. Fourth, while useful for predicting TFT among patients ≥age75, prognostic testing appears to have more limited utility for predicting OS independent of stage among patients in this age category. Accordingly, it does not appear appropriate to enroll patients >age 75 in clinical trials of early intervention based on prognostic testing if the aim is to improve OS.
Several other aspects of this analysis are noteworthy. The study included comprehensive multi-variate analysis of both traditional (age, stage, ALC) and biologic (ZAP-70, CD38, FISH, IGHV) parameters in a large cohort of CLL patients. Consistent with prior reports demonstrating that the biologic parameters contain complementary prognostic information,14,39,40 multiple biologic prognostic parameters were independent predictors of TFT and/or OS in multivariate analyses including traditional parameters (stage, ALC). It is also notable that the distribution of biologic prognostic parameter results did not differ by age at diagnosis arguing against the notion that CLL in younger patients is more biologically aggressive.
How do these results relate to previous studies? Although age has repeatedly been shown to be an independent predictor of survival in CLL patients,7,8,16,17 few prior studies have evaluated interactions between age and the utility of prognostic testing. Mauro and colleagues previously demonstrated that lymphocyte doubling time is a predictor of OS among CLL patients both age ≤55 and over age 55 but provided no further age stratification of those over 55.33 Dohner and colleagues observed that the presence of del(11q22) was a profound stratifier of survival in patients <age 55 (median survival del(11q22)=64 months vs. no del(11q22)=209 months; p<0.001) but not patients > age 55 (median survival del(11q22)=94 months vs. no del(11q22)=111 months; p=0.82).16 Due to the limited data demonstrating utility in older patients, many hematologist/oncologists do not routinely use prognostic testing for older individuals with early stage CLL which limits the accuracy of counseling on natural history and life expectancy for these patients. The present study provides more comprehensive data regarding the utility of prognostic testing for classifying risk among CLL patients over age 65. To our knowledge, it also is the first study to evaluate how prognostic test results can be used to stratify the risk of death among patients with CLL relative to the age-matched general population.
Our study has several important limitations. Given that the natural history of CLL is changing,7,8 the fact that all patients in the current cohort were diagnosed in the last 15 years is a strength of the study. However, not all patients had all the molecular/biologic prognostic parameters measured since they were only discovered/used routinely in the last 5-10 years.10-14 Second, the diagnosis of CLL was based on the 1996 criteria for CLL32 which were in effect throughout the study interval but recently underwent revision41. Third, as in other analyses of clinical outcome,36,42-44 we evaluated overall survival rather than disease-specific survival. Because overall survival is the outcome of greatest interest to patients, we believe that this is the most appropriate outcome for survival analysis with use of TFT as a secondary measure of disease specific outcomes. Fourth, our study focused on the ability of prognostic tests to predict TFT and OS. The utility of these assays for predicting other outcomes, such as response to treatment, was not the focus of the current study. Finally, the study represents a single center experience that requires validation in independent series of patients monitored prospectively.
In aggregate, these findings suggest that survival of CLL patients <age 75 at diagnosis is shorter than that of the age-matched general population regardless of disease stage. Prognostic testing had little utility for predicting OS independent of stage among patients age ≥75, although it remained useful for predicting TFT. In settings where the goal is to identify patients with excess risk of death relative to the age matched population, it appears that clinical staging is the only test necessary for patients ≥age 75 at diagnosis while limited testing with a combination of stage and IGHV or stage and FISH are appropriate strategies for most patients <age 75.
Acknowledgments
Support through grants from the National Cancer Institute (CA 113408 to T.D. Shanafelt and CA136591 to D.F. Jelinek) and Gabrielle’s Angel Foundation for Cancer Research (T.D. Shanafelt) are gratefully acknowledged.
Footnotes
Author Contributions
Concept: Tait Shanafelt, Neil Kay, Tim Call, Clive Zent
Designed research: Tait Shanafelt, Kari Rabe, Neil Kay, Tim Call, Clive Zent, Diane Jelinek
Performed research: Tait Shanafelt, Diane Jelinek, Susan Schwager, Curt Hanson
Analyzed and interpreted data: Kari Rabe, Megan Reinalda, Susan Slager, Tait Shanafelt, Neil Kay, Clive Zent, Diane Jelinek, Debbie Bowen, Curt Hanson, Tim Call
Wrote the paper: Tait Shanafelt
Critical review and revision of paper: Neil Kay, Kari Rabe, Tim Call, Clive Zent, Diane Jelinek, Debbie Bowen, Susan Slager, Curt Hanson
To be presented at the American Society of Hematology, December 2009, New Orleans
The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Diehl LF, Karnell LH, Menck HR The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer. 1999;86:2684–92. [PubMed] [Google Scholar]
- 3.Call T, Phyilky R, Noel P, et al. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clinic Proceedings. 1994;69:323–328. doi: 10.1016/s0025-6196(12)62215-0. [DOI] [PubMed] [Google Scholar]
- 4.Rozman C, Bosch F, Montserrat E. Chronic lymphocytic leukemia: a changing natural history? Leukemia. 1997;11:775–778. doi: 10.1038/sj.leu.2400679. [DOI] [PubMed] [Google Scholar]
- 5.Molica S, Levato D. What is changing in the natural history of chronic lymphocytic leukemia. Haematologica. 2001;86:8–12. [PubMed] [Google Scholar]
- 6.Abrisqueta P, Pereira A, Rozman C, et al. Improving survival in patients with chronic lymphocytic leukemia (1980-2008): the Hospital Clinic of Barcelona experience. Blood. 2009 doi: 10.1182/blood-2009-04-214346. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111:4916–21. doi: 10.1182/blood-2007-12-129379. [DOI] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Dickman PW, Wilson WH, et al. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973-2003 in Sweden. Haematologica. 2009;94:1259–65. doi: 10.3324/haematol.2009.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molica S, Levato D, Dattilo A. Natural history of early chronic lymphocytic leukemia. A single institution study with emphasis on the impact of disease-progression on overall survival. Haematologica. 841999:1094–9. [PubMed] [Google Scholar]
- 10.Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 11.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7. [PubMed] [Google Scholar]
- 12.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 13.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 14.Rassenti LZ, Jain S, Keating MJ, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann MA, Eichhorst BF, Busch R, et al. Prospective Evalution of Prognostic Parameters in Early Stage Chronic Lymphocytic Leukemia (CLL): Results of teh CLL1-Protocol of the German CLL Study Group (GCLLSG) Blood. 2007;110 Abstract 625. [Google Scholar]
- 16.Dohner H, Stilgenbauer S, James MR, et al. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89:2516–22. [PubMed] [Google Scholar]
- 17.Cavotsky D, Fooks J, Richards S. Prognostic factors in chronic lymphocytic leukaemia: the importance of age, sex and response to treatment in survival. Br J Haematol. 1989;72:141–149. doi: 10.1111/j.1365-2141.1989.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 18.Carli PM, Coebergh JW, Verdecchia A. Variation in survival of adult patients with haematological malignancies in Europe since 1978. Eur J Cancer. 1998;34:2253–2263. doi: 10.1016/s0959-8049(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Kay NE, Jenkins G, et al. B-cell count and survival:Differentiating chronic lymphocytic leukemia (CLL) from monoclonal B-cell lymphocytosis (MBL) based on clinical outcome. Blood. 2008 doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanafelt TD, Jelinek D, Tschumper R, et al. Cytogenetic abnormalities can change during the course of the disease process in chronic lymphocytic leukemia. Journal of Clinical Oncology. 2006;24:3218–9. doi: 10.1200/JCO.2006.06.1077. [DOI] [PubMed] [Google Scholar]
- 22.Zent CS, Ding W, Schwager SM, et al. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br J Haematol. 2008;141:615–21. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddocks-Christianson K, Slager SL, Zent CS, et al. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br J Haematol. 2007;139:398–404. doi: 10.1111/j.1365-2141.2007.06801.x. [DOI] [PubMed] [Google Scholar]
- 24.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 25.Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma. 2007;48:2412–7. doi: 10.1080/10428190701724801. [DOI] [PubMed] [Google Scholar]
- 26.Palmer S, Hanson CA, Zent CS, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141:607–14. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewald G, Brockman S, Paternoster S, et al. Chromosome anomalies detected by interphase fluorscence in hybridization: correlation with significant biological features of chronic lymphocytic leukemia. British Journal of Haematology. 2003;121:287–95. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 28.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–61. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 29.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–41. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 30.Tobin G, Thunberg U, Johnson A, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted V{lambda} 2-14 gene usage and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;13:13. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 31.Tobin G, Thunberg U, Johnson A, et al. Somatically mutated Ig V(H)3-21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99:2262–4. doi: 10.1182/blood.v99.6.2262. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group Guidelines fo Chronic Lymphocytic Leukemia: Reised Guidelines for Diagnosis and Treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 33.Mauro FR, Foa R, Giannarelli D, et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94:448–54. [PubMed] [Google Scholar]
- 34.Therneau T, Grambasch P. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 35.Therneau T, Offord JR. Technical Report Number 63: Expected Survival Based on Hazard Rates, Technical Reports. Rochester: Mayo Clinic; 1999. pp. 1–26. [Google Scholar]
- 36.Wierda WG, OșBrien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–85. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 37.Shanafelt TD, Jenkins G, Call TG, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer. 2009;115:363–72. doi: 10.1002/cncr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Dixon DO, Kantarjian HM, et al. Prognosis of chronic lymphocytic leukemia: a multivariate regression analysis of 325 untreated patients. Blood. 1987;69:929–36. [PubMed] [Google Scholar]
- 39.Krober A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100:1410–6. [PubMed] [Google Scholar]
- 40.Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99:1023–9. doi: 10.1182/blood.v99.3.1023. [DOI] [PubMed] [Google Scholar]
- 41.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia. Blood; a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines; 2008. pp. 5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 43.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 44.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]