Abstract
Background and Purpose
l-glutamine (Gln) is an energy source for gastrointestinal (GI) epithelia and can stimulate glucagon-like peptide 1 (GLP-1) release from isolated enteroendocrine L-cells. GLP-1 and peptide YY (PYY) are co-secreted postprandially and both peptides have functional roles in glucose homeostasis and energy balance. The primary aim of this project was to establish the endogenous mechanisms underpinning Gln responses within intact GI mucosae using selective receptor antagonists.
Experimental Approach
Mouse mucosae from different GI regions were voltage-clamped and short-circuit current (Isc) was recorded to Gln added to either surface in the absence or presence of antagonists, using wild-type (WT) or PYY-/- tissues. The glucose sensitivity of Gln responses was also investigated by replacement with mannitol.
Key Results
Colonic apical and basolateral Gln responses (at 0.1 and 1 mM) were biphasic; initial increases in Isc were predominantly GLP-1 mediated. GLP-1 receptor antagonism significantly reduced the initial Gln response in the PYY-/- colon. The slower reductions in Isc to Gln were PYY-Y1 mediated as they were absent from the PYY-/- colon and were blocked selectively in WT tissue by a Y1 receptor antagonist. In jejunum mucosa, Gln stimulated monophasic Isc reductions that were PYY-Y1 receptor mediated. Gln effects were partially glucose sensitive, and Calhex 231 inhibition indicated that the calcium-sensing receptor (CaSR) was involved.
Conclusion and Implications
Gln stimulates the co-release of endogenous GLP-1 and PYY from mucosal L-cells resulting in paracrine GLP-1 and Y1 receptor-mediated electrogenic epithelial responses. This glucose-sensitive mechanism appears to be CaSR mediated and could provide a significant therapeutic strategy releasing two endogenous peptides better known for their glucose-lowering and satiating effects.
Keywords: endogenous peptide YY, Y1 receptor, enteroendocrine L-cell, PYY-/- mice, mucosal ion transport, calcium-sensing receptor
Introduction
Enteroendocrine L-cells that co-express and co-release glucagon-like peptides (GLPs) and peptide YY (PYY) are present along the length of the mammalian gastrointestinal (GI) tract, but they are more frequently observed in the distal ileum and colon (Böttcher et al., 1984; Ku et al., 2003). Glucagon-like peptide 1 (GLP-1) and PYY both mediate incretin activity and slow gastric emptying and intestinal transit, resulting in reduced postprandial glycaemia in rodents (Boey et al., 2007; Holst, 2007) and in lean as well as diabetic subjects (Greenfield et al., 2009; Samocha-Bonet et al., 2011). The capacity of L-cells to sense luminal contents has become apparent recently, aided by the ability to investigate the signalling mechanisms in enriched preparations of these relatively scarce cells, using transgenic mice expressing cell-specific fluorescence driven by a chosen gut pro-hormone promoter (Reimann et al., 2008; Egerod et al., 2012; Habib et al., 2012). These studies have identified GPCRs that are enriched selectively in different enteroendocrine cell types. A range of nutrients, including proteins, fatty acids and some of their metabolites, can stimulate L-cells to co-secrete their peptide products (Engelstoft et al., 2008; Reimann et al., 2012), while loss of L-cells has been shown recently to result in impaired glucose homeostasis (Pedersen et al., 2012). l-glutamine (Gln) is not only a widely available nutritional supplement, a major source of epithelial metabolic energy and contributor to protein synthesis in GI mucosa (particularly post-injury; Wilmore, 2001), but it is also a GLP-1 secretagogue in primary colonic cultures and murine endocrine GLUTag cells (Reimann et al., 2004; Tolhurst et al., 2011). However, to date, few studies have investigated the effects of Gln or other amino acids on intact GI mucosa where the functional consequences of endogenous GLP-1 and PYY release can be investigated pharmacologically.
GLP-1 enhances insulin release, which is beneficial in type 2 diabetes (T2D), and with PYY, these two peptides have crucial roles in slowing upper and lower GI transit (Savage et al., 1987; Drucker, 2005; Tough et al., 2011) and in signalling satiety (Anini et al., 1999; Holst, 2013). GLP-1 mimetics have been licensed to treat T2D for some time now and are additionally weight-losing (Astrup et al., 2012; Holst, 2013). Therapeutic developments are now including intestinal-sensing mechanisms and strategies that promote co-release of GLPs and PYY by activating GPCRs found to be enriched in L-cells, for example, GPR119, which is also expressed on pancreatic beta cells (Overton et al., 2008; Oshima et al., 2013). However, our understanding of the repertoire of GLP's and PYY's incretin mechanisms lags somewhat behind that of their pancreatic mechanisms.
Tolhurst et al. (2011) showed that Gln is a GLP-1 secretagogue acting via two independent signalling pathways; firstly, an increased cell excitability stimulated by a Na+-dependent elevation of intracellular calcium (Cai2+) and, secondly, an amplifying mechanism that occurred via elevated intracellular cAMP (another potent L-cell stimulus). Additionally, L-cells were shown to express a variety of amino acid transporters, which could varyingly contribute to the Na+-dependent electrogenic entry of Gln across the plasma membrane and thus trigger depolarization and GLP-1 release (Reimann et al., 2004; Tolhurst et al., 2011). L-cell activity has also been shown to be involved in amino acid-induced GLP-1 and PYY release in vivo, and that the calcium-sensing receptor (CaSR; receptor nomenclature throughout follows Alexander et al., 2011) is required for this response (Mace et al., 2012). We therefore hypothesized that in intact mucosa, Gln was likely to initiate electrogenic epithelial responses as a consequence of endogenous peptide co-release, and that if both GLP-1 and PYY were involved functionally, then their respective GPCR signalling via Gs and Gi coupling in epithelia would result in secretory and anti-secretory responses respectively.
Functional studies utilizing mouse GI and colonic mucosae have characterized fully the Y receptors involved in the long-lasting anti-secretory responses induced by PYY and neuropeptide Y (NPY), an accepted inhibitory enteric neurotransmitter. The colon appears to be most sensitive to these Y peptides and their shorter (3–36) products (Cox et al., 2001). In both the mouse and the human colon, the effects of exogenous and endogenous PYY are predominantly mediated by epithelial Y1 receptors, while NPY's effects are a combination of neuronal Y2- and epithelial Y1-mediated responses (Hyland et al., 2003; Cox & Tough, 2002, respectively; Tough et al., 2011). Notably, the cellular pharmacology of these peptide responses is the same in mouse and human colon mucosa, and thus, the mouse intestine is a valid model for testing PYY/NPY-Y receptor mechanisms further.
The primary aim of this project was therefore to investigate the mechanisms of action of Gln in intact mucosal preparations from selected areas of the mouse GI tract and to establish whether these responses were glucose sensitive. Several amino acids have been shown to secrete GLP-1 (Tolhurst et al., 2011) and these were also tested, as was the sidedness of the Gln response. The GLP-1 sensitivity of mucosae from different GI regions was included as a prelude to determining the relative contributions provided by endogenous GLP-1 and PYY to Gln responses in wild-type (WT) tissue using proven, selective GLP-1 and Y1 receptor antagonists, and comparing these Gln responses with those from the PYY-/- colon.
Methods
Tissue preparation
All experimental procedures complied with the Animals (Scientific Procedures) Act 1986 and were approved by the local ethical committee. WT and PYY null male adult mice lacking the entire PYY coding sequence (PYY-/-; on aC57Bl6-129/SvJ background; Boey et al., 2006; Karl et al., 2008) were age-matched (11–20 weeks) and had free access to standard chow and water. Mice were killed by CO2 asphyxiation or cervical dislocation and GI tissues were excised and placed immediately in fresh Krebs Henseleit (KH) with the following composition (in mM): 117 NaCl, 24.8 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2 and 11.1 d-glucose. Mucosae primarily from the jejunum or descending colon (but also other GI regions for GLP-1 sensitivity studies) were obtained by dissection under a microscope, removing the overlying smooth muscle and associated myenteric innervation, as described previously (Cox et al., 2010).
Measurement of short-circuit current (Isc) across mucosae and drug pretreatments
Adjacent mucosal preparations from a given GI area were placed individually between two halves of an Ussing chamber (exposed area 0.14 cm2) and bathed both sides with 5 mL oxygenated (95% O2, 5%CO2) KH (pH 7.4) maintained at 37°C. Mucosae from the distal colon or jejunum were voltage-clamped at 0 mV and the resulting short-circuit current (Isc, measured as μA·cm−2) was recorded continuously as described previously (Cox et al., 2010; Tough et al., 2011). Following tissue equilibration (20 min), mucosae were pretreated with 10 nM vasoactive intestinal polypeptide (VIP, an optimal secretory pretreatment upon which epithelial Gi-coupled mechanisms can be readily discerned; Cox et al., 2001, 2010; Tough et al., 2011). Once VIP-elevated Isc levels had stabilized, Gln (0.1 or 1 mM), other amino acids or glucosamine (1 mM) were added to either the basolateral or apical reservoirs, and changes in Isc were monitored for a further 20 min. All peptides, plus Y and GLP-1 receptor antagonists were added basolaterally. In pharmacological studies, selective antagonists were added prior to VIP addition, at their optimal blocking concentrations throughout, that is, 300 nM BIBO3304 {(R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate}, 1 μM BIIE0246 {(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide} (Tough et al., 2006; Cox et al., 2001 ) or 1 μM exendin (9–39) (Cox et al., 2010). Finally, PYY (10 nM) was added as an internal Y1 plus Y2 receptor agonist control. PYY-/- mouse colonic mucosa was used to determine whether the absence of this L-cell derived peptide resulted in a loss of Gln function in the absence or presence of GLP-1 blockade with 1 μM exendin (9–39). These PYY-/- mice were the same line used extensively in previous functional studies (Boey et al., 2006; Karl et al., 2008; Cox et al., 2010; Tough et al., 2011).
To investigate the involvement of the CaSR in mucosal Gln responses, we used the CaSR positive allosteric modulator R568 {2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]-benzenepropanamine hydrochloride} (Nemeth et al., 1998; Petrel et al., 2004) added apically, at a concentration shown previously to increase GLP-1 and PYY secretion from perfused rat small intestine (20 μM; Mace et al., 2012). We also used the CaSR antagonist Calhex231 (Petrel et al., 2004) at 10 μM, a concentration shown previously to selectively inhibit intestinal GLP-1 and PYY secretion (Mace et al., 2012) to determine whether it would inhibit Gln responses in colonic mucosa.
Amiloride (1 μM) and phloridzin (50 μM) were only added apically as their targets, epithelial sodium channel (ENaC) and sodium glucose co-transporter 1 (SGLT1), respectively, are selectively expressed on apical membranes. Changes in Isc following each drug addition were monitored and their effects on subsequent Gln responses were recorded.
Regional variation in mucosal GLP-1 responses
Here, GI mucosae from selected areas of the mouse upper and lower GI tract were prepared as described above but were not pretreated with VIP. Instead, the effect of GLP-1 agonist exendin 4 alone (100 nM, added basolaterally) or following addition of the GLP-1 antagonist, exendin (9–39) (1 μM), was measured under basal conditions. Changes in Isc to the antagonist alone, and then to exendin 4 ± antagonism, were compared in mucosae from the duodenum, jejunum, terminal ileum and the ascending and descending colon from WT mice.
Glucose sensitivity of apical and basolateral Gln responses in mouse colon mucosa
To test whether Gln responses were glucose sensitive, colonic mucosae were bathed with KH buffer containing either glucose (11.1 mM)on both sides or by replacing glucose for mannitol (11.1 mM) on one side only, as described previously (Cox et al., 2010). Apical or basolateral addition of Gln (1 mM) after VIP pretreatment resulted in changes in Isc that were compared with controls (KH on both sides) and subsequent responses to PYY (10 nM) and the SGLT-1 inhibitor, phloridzin (50 μM), were also compared across experimental groups.
Data and statistical analysis
GraphPad Prism v5.0 (GraphPad Prism Inc., La Jolla, CA, USA) was used to analyse all the data presented. Single comparisons were performed using Student's unpaired t-test, while multiple comparisons utilized one-way anova with Dunnett's or Bonferroni's post-tests where applicable. Statistical significance was defined as a P-value ≤0.05.
Materials
PYY and VIP were obtained from Bachem Laboratories Inc. (St Helen's, UK), and aliquots were frozen and stored at −20°C, undergoing a single freeze-thaw cycle only. Gln, l-phenylalanine (Phe), l-asparagine (Asn), l-alanine (Ala), phloridzin, amiloride, dimethyl sulphoxide, d-glucose, mannitol and UK14,304 [5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine] were all purchased from Sigma (Poole, UK). BIBO3304, BIIE0246 and R568 {2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]-benzenepropanamine hydrochloride} were from Tocris Bioscience (Bristol, UK). Calhex 231 {4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]-benzamide} was from Santa Cruz (Heidelberg, Germany) and glucosamine was purchased from Alfa Aesar (Heysham, UK).
Results
Basal resistance and Isc levels, as well as secretory responses to VIP (10 nM pretreatment, unless otherwise stated) in small intestine and colonic mucosae, were within the ranges published previously (Cox et al., 2001;2010; Tough et al., 2011). Neither VIP nor Gln (at 0.1 or 1 mM) altered mucosal resistances during the course of these experiments (data not shown).
Y1 receptor activation mediates the Gln-induced reductions in Isc
Following VIP pretreatment of colonic mucosa Gln added to either the apical or basolateral reservoir resulted in a biphasic change in Isc; an initial small, transient elevation in Isc followed by a slower, longer-lasting decrease in Isc [Figure 1A, labelled as a primary (1°) or secondary (2°) component, respectively], while in jejunum mucosa, Gln triggered monophasic reductions in Isc only (Figure 1B). The Y1 receptor antagonist BIBO3304 (Wieland et al., 1998) increased Isc levels in colonic and jejunal mucosa as seen previously, indicating endogenous Y1 tone that was significant in the former tissue (Figure 1C). In Y1-blocked colonic mucosa, the 2° Gln response was abolished compared with vehicle-treated controls (***P < 0.001 for apical and *P < 0.05 for basolateral Gln responses; Figure 1D). Irrespective of the surface to which Gln was added, there was no change in the size of the 1° response ± BIBO3304 (Figure 1D). In the jejunum, apical and basolateral Gln responses were simpler, monophasic Isc reductions that were attenuated significantly by Y1 antagonism (Figure 1E). Following Gln addition, maximally effective PYY (10 nM) reduced Isc levels in a BIBO3304-sensitive manner in both colonic and jejunum mucosae (Figure 1F, G respectively).
Figure 1.

Gln-induced changes in Isc in mucosae from mouse colon and jejunum. In (A) and (B) are traces showing basolateral (bl) Gln (1.0 mM) responses (biphasic in colon, in A) and monophasic reductions in Isc in jejunum (in B). Gln responses were recorded after the addition of a near-maximal VIP concentration (10 nM) and were followed by basolateral addition of a maximal PYY concentration (10 nM). In (C), the increases in Isc observed on the addition of vehicle [dimethyl sulphoxide (DMSO), 10%] or Y1 antagonist BIBO3304 (300 nM) to mucosae from the descending colon or jejunum, revealing significant Y1 tonic activity in the former. In (D), pooled data from colonic mucosa of primary (1°) and secondary (2°) response components to Gln [apical (ap) and bl, 0.1 mM] showing significant inhibition of the reductions in Isc by pretreatment with BIBO3304 (***P < 0.001 and *P < 0.05 respectively). In (E), jejunal Gln(0.1 mM) responses were monophasic reductions in Isc that were inhibited significantly by Y1 antagonism (+ BIBO, 300 nM; *P < 0.05). In (F) and (G), reductions in Isc to exogenous PYY(10 nM, bl) show clear Y1 receptor sensitivity (plus 300 nM BIBO3304; **P < 0.01 and *P < 0.05) in colonic (F) and jejunal mucosae (G) respectively. Values are the mean ± SEM from the number of observations shown in parentheses.
Colonic Isc responses to different l-amino acids added apically or basolaterally
Three L-amino acids (each added at 1 mM), namely Gln, Phe and Asn, exhibited similarly sized biphasic Isc responses in colonic mucosa, while Ala (1 mM) responses were smaller (but not significantly, compared with Gln). The amino sugar, glucosamine was inactive, significantly so after apical and basolateral additions (Figure 2A,B). Subsequent control PYY responses were no different from those observed without prior amino acid addition (data not shown). The relative activity we observed with these five amino acids was similar to their ability to release GLP-1 from primary colonic cultures (Tolhurst et al., 2011).
Figure 2.
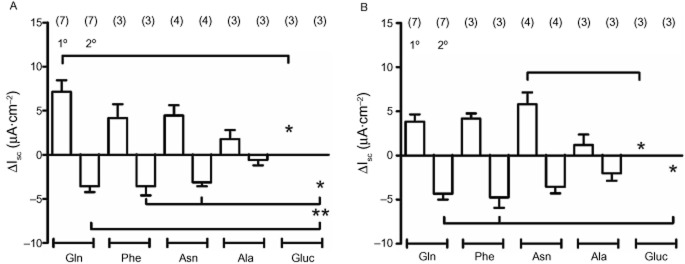
Responses to different amino acids and glucosamine (1 mM throughout) added either apically (in A) or basolaterally (in B) to mouse descending colon mucosa. Each amino acid induced biphasic changes in Isc with the exception of glucosamine (Gluc, *P < 0.05 or **P < 0.01, compared with specific responses as indicated). Values are the mean ± SEM from the number of observations shown in parentheses.
Regional variation in GLP-1 mediated increases in Isc in mouse GI tract mucosae
In order to determine whether exogenous GLP-1 activated epithelial Isc responses directly (prior to investigating whether the 1° Gln effects were mediated by endogenous GLP-1), we used the stable GLP-1 agonist, exendin 4 (100 nM), in the absence or presence of the GLP-1 receptor antagonist, exendin (9–39) (1 μM). The antagonist alone (added basolaterally) reduced basal Isc levels with an onset of 1 min and maximal effect within 5 min and in all GI regions tested, indicating endogenous GLP-1 activity along the length of the mouse GI tract (Figure 3). Exendin 4 added basolaterally to naive tissue increased Isc levels (presumably via epithelial Gs-linked GLP-1 receptors) with the largest responses in ascending colon and all exendin 4 responses were abolished by pretreatment with exendin (9–39) (Figure 3).
Figure 3.
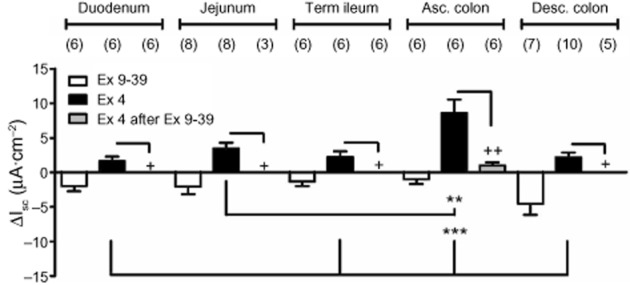
Regional GLP-1 responses to basolateral addition of the GLP-1 antagonist [exendin (9–39), 1 μM] alone; to exendin 4 (Ex 4, 100 nM) in the absence or presence of exendin (9–39) (1 μM). Values are the mean ± SEM from the number of observations shown in parentheses. In all GI areas tested, Ex 4-induced increases in Isc were significantly inhibited by the GLP-1 receptor antagonist (Ex 9–39) (++P < 0.01 and + P < 0.05, Student's t-test). Multiple comparisons showed the greatest GLP-1 responses in ascending colon mucosa compared with other GI areas (***P < 0.001 and **P < 0.01).
Glucose sensitivity of Gln response
GPCR mechanisms in L-cells are often glucose sensitive, while epithelial GPCR-mediated responses are not (e.g. L-cell GPR119 agonism is glucose sensitive, while consequent PYY-Y1 receptor-mediated epithelial responses are glucose insensitive; Cox et al., 2010). In keeping with these different mechanisms, apical replacement of glucose by mannitol resulted in a significant inhibition of the 2° Gln response, and although there was also ∼60% inhibition of Gln's 1° response, this was not statistically significant (Figure 4A). Basolateral glucose removal had no significant effect on either phase of the apical Gln response (Figure 4A). Interestingly, when basolateral glucose was removed, the basolateral Gln response (specifically the 2° phase) was significantly reduced, while the initial small increases in Isc were apparently glucose insensitive (Figure 4B). Subsequent basolateral PYY responses were not glucose sensitive (Figure 4C). Finally, the SGLT1 inhibitor, phloridzin, was used as an internal control to confirm selective loss of apical SGLT1 activity when glucose had been replaced by mannitol in this reservoir. Here, apical mannitol rendered phloridzin significantly less effective as shown by the smaller phloridzin-induced reductions in Isc compared with SGLT1 block in control conditions or after basolateral mannitol (Figure 4D).
Figure 4.
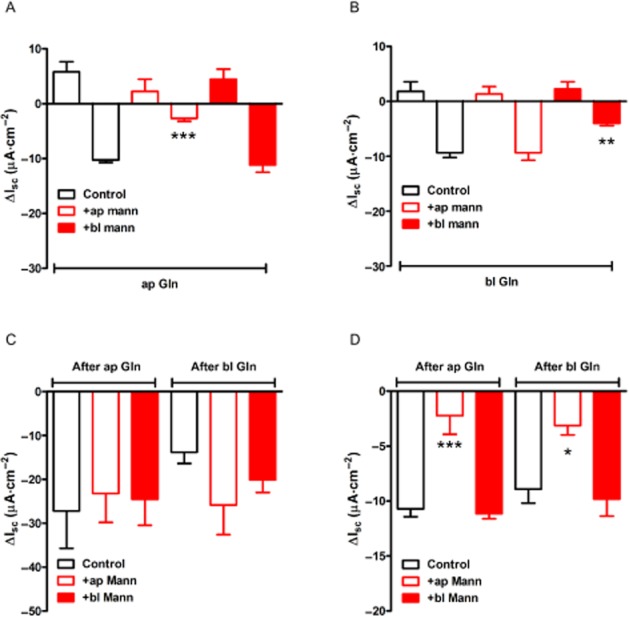
Glucose sensitivity of Gln but not PYY responses in mouse colon mucosa. In (A) and (B), the effect of replacing glucose for mannitol in the apical (+ap mann) or basolateral (+bl mann) reservoirs on responses to Gln added to either the apical (A) or basolateral (B) reservoir. The absence of glucose from either side did not appear to alter the size of the small 1° increases in Isc, but it did reduce the Gln-induced reductions in Isc significantly (***P < 0.001 and **P < 0.01). Subsequent PYY (10 nM) responses were not glucose sensitive (in C), while in (D), SGLT1 inhibition by phloridzin (50 μM, in all bars) was significantly reduced when apical glucose was replaced by mannitol (***P < 0.001 and *P < 0.05, compared with controls). Values are the mean ± SEM from four observations throughout.
Involvement of the CaSR but not ENaC in colonic Gln responses
To test whether CaSR mechanisms were involved in the amino acid responses observed in colonic mucosa, we utilized R568, a CaSR positive allosteric modulator (Nemeth et al., 1998) and the selective CaSR antagonist, Calhex 231 (Petrel et al., 2004), at optimal concentrations previously shown to enhance or inhibit, respectively, GLP-1 and PYY secretion from rat small intestine (Mace et al., 2012). Firstly, the 1° and 2° components of colonic Gln responses were enhanced significantly by pretreatment with R568 (Figure 5). Secondly, Calhex 231 pretreatment reduced the size of both components of the Gln response, but only the reductions in Isc were significantly inhibited (Figure 5). Neither CaSR modulator altered Isc levels per se at these concentrations (data not shown).
Figure 5.
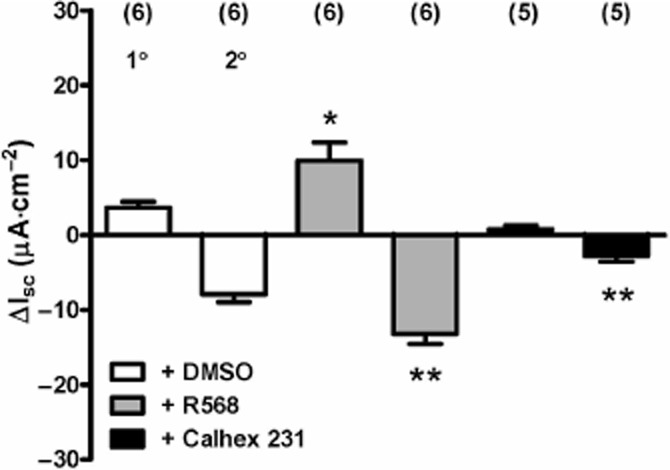
The effect of CaSR modulators R568 (20 μM) and Calhex 231 (10 μM) on Gln (1 mM) responses in mouse descending colon mucosa. All additions were made to the apical reservoir only and values are the mean ± SEM from the observation numbers shown in parentheses. Statistical comparisons compared with the vehicle (≤0.2% DMSO) controls revealed significant differences, *P < 0.05 for the 1° component of the Gln response, and **P < 0.01 for comparison of control and pretreated 2° component Gln responses.
As Na+ absorption via apical ENaC is significant in colon mucosa and may account for a proportion, particularly of the Gln 1° response, we tested whether the ENaC inhibitor amiloride affected either of the components of the apical Gln response (1 mM). Apical amiloride (1 μM) reduced Isc levels per se as expected (–8.3 ± 2.1 μA·cm−2, n = 3), after which, the Gln response was 2.6 ± 0.9 μA·cm−2 (n = 3 for the 1° component) and −2.0 ± 0.8 μA·cm−2 (n = 3 for the 2° component) compared with control Gln responses (1°, 6.1 ± 1.5 μA·cm−2, P = 0.19; 2°, −3.1 ± 0.7 μA·cm−2, n = 7, P = 0.44). There were no statistical differences between either of the components of the Gln response.
Loss of Gln function in PYY-/- colon mucosa treated with a GLP-1 antagonist
Having established that Y1 receptor antagonism blocked the Gln-induced reductions in Isc in jejunal and colonic mucosae, we next set out to confirm that colonic Gln responses involved a combination of endogenous PYY and GLP-1. We did this by comparing Gln responses in WT and PYY-/- tissue in the absence or presence of the GLP-1 antagonist, exendin (9–39). In WT colon, Gln induced 1° responses were reduced by exendin (9–39); however, this was not statistically significant (Figure 6A; Student's t-test, P = 0.15), while in PYY-/- tissue, the 1° response was abolished by exendin (9–39) (***P < 0.001) indicating significant mediation by endogenous GLP-1 (Figure 6B). Notably, apical and basolateral Gln-induced reductions in Isc were absent from PYY-/- colon (significantly so compared with WT tissue) and indicating the requirement for PYY in this component of the Gln response.
Figure 6.
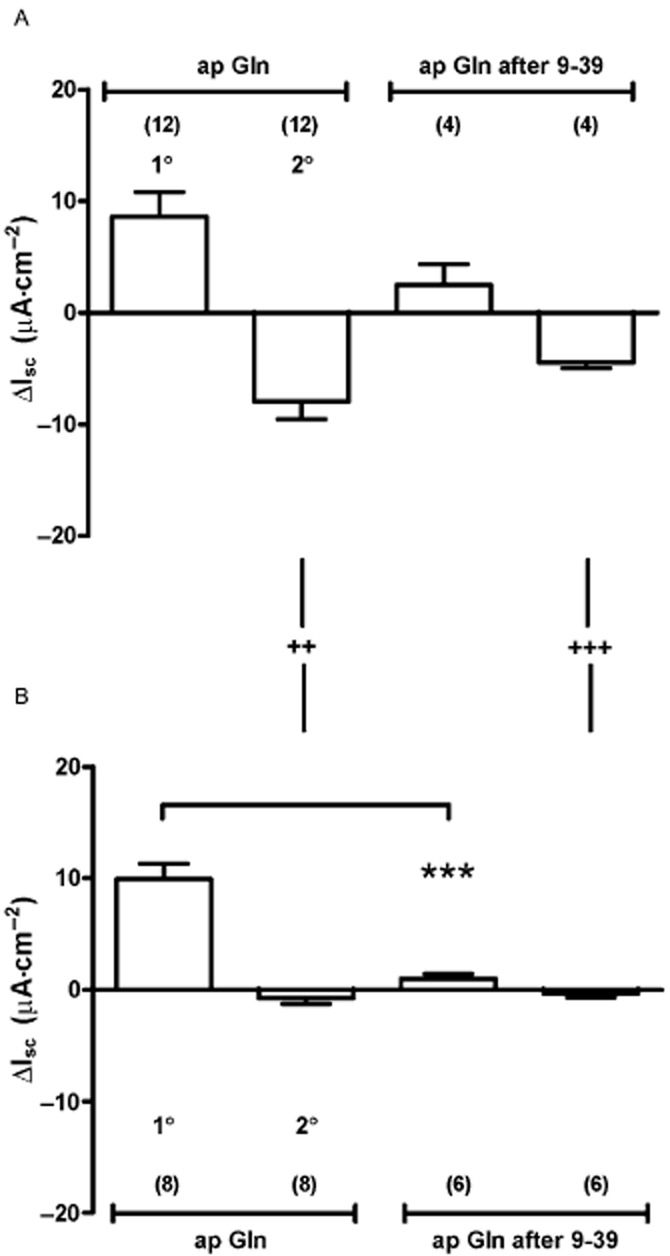
The effect of GLP-1 receptor inhibition with exendin (9–39) (1 μM) and absence of PYY upon colonic responses to apical Gln. Comparison of biphasic Gln responses showing 1° increases followed by 2° decreases in Isc in WT (A) and PYY-/- tissue (B) shows that GLP-1 antagonism significantly reduced the 1° response compared with untreated Gln responses (*** P < 0.001, in B). In WT tissue (A), the 1° increase in Isc to Gln was partially sensitive to GLP-1 receptor blockade but this was not statistically significant (P = 0.15, Student's t-test). PYY ablation results in loss of the 2° component of control ap Gln responses (++P < 0.01) and after GLP-1 blockade (+++P < 0.001). Values are the mean ± SEM from observation numbers shown in parentheses.
Discussion
The reductions in Isc observed to apical or basolateral addition of Gln in jejunal and colonic mucosae were Y1 receptor mediated and epithelial in origin, based on their Y1 antagonist, BIBO3304 sensitivity. Previous functional studies have established that PYY-mediated Y1 receptor responses are direct and epithelial, while Y2 responses are indirect and neuronally mediated (Hyland et al., 2003; Tough et al., 2006;2011). The biphasic changes in Isc stimulated by Gln addition (0.1 and 1.0 mM) to colonic preparations were replicated by other L-amino acids (at 1 mM). Plasma concentrations of Gln are within the 0.1–1.0 mM range and an oral dose (30 g) of Gln raises plasma levels after 60 min to 1.3–2.7 mM in lean humans with co-incident elevations in plasma GLP-1 (Greenfield et al., 2009). As a major product of protein digestion and a widely used nutritional supplement, similar luminal Gln concentrations have been measured postprandially (1.0–4.0 mM; Rhoads and Wu, 2009), so the use of a 1.0 mM Gln in the present study is physiologically relevant. Phe, Asn and, to a lesser degree, Ala mimicked Gln effects, while the amino sugar, glucosamine, was inactive (on both sides) and this activity series resembled their relative GLP-1 secretory capacities (Tolhurst et al., 2011). Glucosamine can additionally be transported directly into L-cells via the facilitative transporter glucose transporter 2 (Reimann et al., 2008), and because the amino sugar is uncharged, this process remains electroneutral and would thus not alter Isc levels per se.
Tolhurst et al. (2011) found that Gln induced L-cells to release GLP-1 with an EC50 value of ∼0.1 mM and Emax of ∼1 mM, and we provide evidence that apical and basolateral Gln has the capacity to stimulate rapid changes in Isc across mucosal preparations at the same low mM concentrations. The involvement of GLP-1 in the initial transient increases in Isc seen in colonic mucosa was most clearly revealed in PYY-/- tissue, where with GLP-1 receptor blockade, subsequent Gln responses were abolished. In WT colon, the GLP-1 antagonist exendin (9–39) reduced the initial Isc elevation seen after apical Gln but not significantly, and we conclude that another electrogenic mechanism may contribute to this albeit small electrogenic response. It is notable that certain Na+-dependent neutral amino acid transporters are highly enriched in L-cells (e.g. SNAT2 and bOAT1; Tolhurst et al., 2011), but whether these or alternative epithelial-derived processes contribute to the initial transient Gln Isc response in colon remains to be resolved. Using exendin (9–39), we observed low, but significant levels of tonic GLP-1-mediated secretory activity present along the length of the mouse small and large intestine, and marginally greatest in the descending colon mucosa. To our knowledge, this is the first evidence of such tonic activity. Responses to the addition of a stable GLP-1 mimetic exendin 4 were significantly larger in ascending colon reflecting a higher GLP-1 receptor expression in this region. In all the GI areas tested, these responses were abolished by GLP-1 receptor blockade. Few studies have determined whether GLP-1 has the capacity to elicit epithelial transport responses directly, but the present study provides evidence that it can in mouse colon, and a previous study showed similar GLP-1 tonic activity was also present in human colon mucosa (and more obvious when Y receptors were blocked; Cox et al., 2010), and we presume these increases in Isc occur via Gs signalling in epithelial cells because they are rapid in onset and thus unlikely to be indirect.
In colonic mucosa, the reductions in Isc (PYY-Y1 mediated) to apical Gln were glucose sensitive, while the smaller initial Gln response appeared to be partially glucose sensitive. If a proportion of the latter was mediated by a non-GLP-1, electrogenic transport mechanism, then that could result in the sensitivity pattern we observed with apical Gln. It was also interesting to note that basolateral Gln reduced Isc in a partially glucose-sensitive manner and this is unlikely to be SGLT1 mediated as the transporter is located apically. Instead, a basolateral glucose-sensitive electrogenic mechanism appears to be involved here and may be one of the transporters or exchangers highlighted by Tolhurst et al. (2011). Further studies with selective inhibitors or sided Na+-replacement studies may possibly help clarify this, as well as the smaller initial glucose-insensitive basolateral Gln response.
Mucosal Gln responses appear to be mediated by the CaSR, a class C GPCR member proposed to function as an L-amino acid sensor in the gut. Our in vitro findings in native tissue complement those of Mace et al. (2012) who showed that several L amino acids, including Gln, cause release of PYY and GLP-1 from rat perfused small intestine, which was sensitive to the selective CaSR antagonist Calhex 231, while K-cell derived gluco-insulinotropic peptide release was unaffected. Although widely expressed, CaSR are present notably in pancreatic beta cells (Rasschaert and Malaisse, 1999) and also gastrin-secreting antral G-cells (Ray et al., 1997), implicating functional roles in insulin and gastrin secretion. In normal human colon, immunohistochemical studies have shown CaSR immunoreactivity on L-cells (Sheinin et al., 2000) and CaSR can mediate L-Phe stimulated CCK release from mouse duodenal I-cells (Liou et al., 2011), but these sensing receptors do exhibit a wider, epithelial expression pattern in certain species (Sheinin et al., 2000; Geibel and Hebert, 2009), making mechanistic studies challenging. CCK does not alter Isc levels significantly in mouse small intestine mucosa (Cox et al., unpublished), and although Gln responses were small in this gut area compared with colonic responses, they were blocked by BIBO3304 and thus were PYY-Y1 receptor mediated. Therefore, we conclude that I-cell derived CCK does not have a significant role to play in the electrogenic mucosal responses we measured to Gln.
We provide evidence for the first time that Gln-induced CaSR responses on either mucosal surface were glucose sensitive (whereas the subsequent PYY-Y1 effects were not) and this appears to be a common feature of L-cell GPCR mechanisms. Glucose sensitivity is exhibited by the acylethanolamine receptor GPR119, which is enriched on L-cells (Chu et al., 2008) and upon stimulation in mouse and human colon mucosa results in PYY-release and Y1 receptor-mediated paracrine activity with evidence of coincident GLP-1-mediated mucosal responses (Cox et al., 2010). These L-cell-specific GPCR activities, in combination with GPCR-induced pancreatic endocrine function (Engelstoft et al., 2008), raise their profile as targets for future development of novel anti-diabetic and anti-obesity therapeutics. In addition, PYY and GLP-1 appear to contribute significantly to the weight loss observed following Roux-en-Y bariatric surgery (Chronaiou et al., 2012; Dirksen et al., 2013), and thus, dual agonism afforded by upstream stimulation of, for example, GPR119 or similarly enriched L-cell GPCRs, should offer improved therapeutic potential as treatments for T2D and potentially as ‘knifeless’ alternatives to bariatric surgery. Thus, understanding the detailed mechanisms by which Gln (and other amino acids) mediate the release of endogenous incretins, such as GLP-1 and PYY in native mucosae, could help validate the use of Gln supplementation as an adjunct therapy for treating T2D and obesity.
Acknowledgments
S. J. was a BSc Hons student in Biomedical Sciences at KCL and some of the data presented in this manuscript formed part of her final year Pharmacology Research Project. The work was partly funded by the EU [7th Framework Programme, Grant agreement no. 223057 (GIPIO; H. M. C.)] and Wellcome Trust (Ref; 079291/Z/06/Z, H. M. C.). We thank Prof H Herzog (Neuroscience Program, Garvan Institute of Medical Research, Sydney, Australia) for providing the WT and PYY-/- mice used in this study, and Ms A E-Mazid for contributing some data included in Figure 6 (part of her MSc Pharmacology Research Project).
Glossary
- Ala
l-alanine
- Asn
l-asparagine
- BIBO3304
(R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide
- Cai2+
intracellular calcium
- Calhex 231
4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]-benzamide
- CaSR
calcium-sensing receptor
- ENaC
epithelial sodium channel
- GI
gastrointestinal
- Gln
l-glutamine
- GLP-1
glucagon-like peptide 1
- Isc
short-circuit current
- KH
Krebs Henseleit
- NPY
neuropeptide Y
- Phe
l-phenylalanine
- PYY
peptide YY
- PYY-/-
PYY knockout
- R568
2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]-benzenepropanamine hydrochloride
- SGLT1
sodium glucose co-transporter 1
- T2D
type 2 diabetes
- VIP
vasoactive intestinal polypeptide
- WT
wild-type
Conflicts of interests
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (5) 2011;164(Suppl.1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roze C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch. 1999;438:299–306. doi: 10.1007/s004240050913. [DOI] [PubMed] [Google Scholar]
- Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean MEJ, et al. Safety, tolerability and sustained weight loss over 2 years with a once-daily human GLP-1 analogue, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–1370. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- Boey D, Sainsbury A, Herzog H. The role of peptide YY in regulating glucose homeostasis. Peptides. 2007;28:390–395. doi: 10.1016/j.peptides.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Böttcher G, Sjölund K, Ekblad E, Håkanson R, Schwartz TW, Sundler F. Co-existence of PYY and glicentin in endocrine cells of the gut. Regul Pept. 1984;8:261–266. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK. Lower ghrelin levels and exaggerated postprandial PYY, GLP-1 and insulin responses after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22:1761–1770. doi: 10.1007/s11695-012-0738-5. [DOI] [PubMed] [Google Scholar]
- Chu Z-L, Carroll C, Alfonson J, Guierrez V, He H, Lucman A, et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology. 2008;149:2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HM, Pollock EL, Tough IR, Herzog H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Cox HM, Tough IR, Woolston AM, Zhang L, Nguyen AD, Sainsbury S, et al. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11:532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen C, Damgaard M, Bojsen-Moller KN, Jorgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25:346–e255. doi: 10.1111/nmo.12087. doi: 10.1038/ijo.2013.15. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab. 2005;1:22–31. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling of obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi: 10.1146/annurev.physiol.010908.163128. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AM, Richards P, Cairns LS, Roger GJ, Bannon CAM Parker HE, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Holst JJ. Incretin hormones and the satiation signal. Int J Obes (Lond) 2013;37:1161–1168. doi: 10.1038/ijo.2012.208. doi: 10.1038/ijo.2012.208. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland NP, Sjöberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Ku SK, Lee HS, Lee JH. An immunohistochemical study of the gastrointestinal endocrine cells in C57BL/6 mice. Anat Histol Embryol. 2003;32:21–28. doi: 10.1046/j.1439-0264.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity bu GLUT2 and the calcium-sensing receptor CaSR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Van Wagenen BC, DelMar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Yoshida S, Ohishi T, Matsui T, Tanaka H, Yonetoku Y, et al. Novel GPR119 agonist AS1669058 potentiates insulin secretion from rat islets and has potent anti-diabetic effect in ICR and diabetic db/db mice. Life Sci. 2013;92:167–173. doi: 10.1016/j.lfs.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Overton HA, Fyfe MCT, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153:576–581. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J, Ugleholdt RK, Jorgensen SM, Windelov JA, Grunddal KV, Schwartz TW, et al. Glucose metabolism is altered after loss of L cells and α cells but not influenced by loss of K cells. Am J Physiol Endocrinol Metab. 2012;304:E60–E73. doi: 10.1152/ajpendo.00547.2011. [DOI] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem. 2004;279:18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- Rasschaert J, Malaisse WJ. Expression of the calcium-sensing receptor in pancreatic islet β-cells. Biochem Biophys Res Commun. 1999;264:615–618. doi: 10.1006/bbrc.1999.1577. [DOI] [PubMed] [Google Scholar]
- Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest. 1997;99:2328–2333. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Williams L, Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion fro GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Rhoads JM, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–122. doi: 10.1007/s00726-008-0225-4. [DOI] [PubMed] [Google Scholar]
- Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, et al. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in Type 2 diabetic patients. J Nutr. 2011;141:1233–1238. doi: 10.3945/jn.111.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AP, Adrian TE, Carolan G, Chaterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem. 2000;48:595–601. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough IR, Holliday ND, Cox HM. Y4 receptors mediate the inhibitory responses of pancreatic polypeptide in human and mouse colon mucosa. J Pharmacol Exp Ther. 2006;319:20–30. doi: 10.1124/jpet.106.106500. [DOI] [PubMed] [Google Scholar]
- Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol. 2011;164:66–79. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131:2543S–2549S. doi: 10.1093/jn/131.9.2543S. [DOI] [PubMed] [Google Scholar]


