Abstract
Background:
Ventilator associated pneumonia (VAP) is a major cause of poor outcome among patients in the intensive care units (ICU) world-wide. We sought to determine the factors associated with development of VAP and its prognosis among patients admitted to different ICUs of a Tertiary Care Hospital in India.
Methodology:
We did a matched case control study during October 2009 to May 2011 among patients, ≥18 years with mechanical ventilation. Patients who developed pneumonia after 48 h of ventilation were selected in the case group and those who did not develop pneumonia constituted the control group. Patients’ history, clinical and laboratory findings were recorded and analyzed.
Results:
There were 52 patients included in each group. Among cases, early onset ventilator associated pneumonia (EVAP) occurred in 27 (51.9%) and late onset ventilator associated pneumonia (LVAP) in 25 (48.1%). Drug resistant organisms contributed to 76.9% of VAP. Bacteremia (P = 0.002), prior use of steroid/immunosuppressant (P = 0.004) and re-intubations (P = 0.021) were associated with the occurrence of VAP. The association of Acinetobacter (P = 0.025) and Pseudomonas (P = 0.047) for LVAP was found to be statistically significant. Duration of mechanical ventilation (P = 0.001), ICU stay (P = 0.049) and requirement for tracheostomy (P = 0.043) were significantly higher in VAP. Among each case and control groups, 19 (36.5%) expired.
Conclusion:
We found a higher proportion of LVAP compared with EVAP and a higher proportion of drug resistant organisms among LVAP, especially Pseudomonas and Acinetobacter. Drug resistant Pseudomonas was associated with higher mortality.
Keywords: Hospital acquired pneumonia, India, multidrug resistant organism, ventilator associated pneumonia
Introduction
Development of pneumonia after 48 h in patients with mechanical ventilation is known as ventilator associated pneumonia (VAP).[1] The chance of acquiring VAP increases by 1-3%/day of mechanical ventilation.[2] In India, occurrence of VAP among intensive care unit (ICU) patients varies from 9% to 24%.[3] Global crude mortality rate of VAP ranges from 24% to 50%.[4] Mortality rate depends on aetiologic agents and susceptibility as well as other factors such as age, comorbidities and severity of disease. In VAP due to highly resistant organisms, the crude mortality can be as high as 76%.[4] The chance of VAP occurrence and its prognosis is determined by various factors viz. severity of primary illness, intubation duration, number of re-intubation and host immune competence.[5,6]
Based on the time of onset VAP is of two type, i.e., early onset/early onset ventilator associated pneumonia (EVAP) (<96 h) and late onset/late onset ventilator associated pneumonia (LVAP) (>96 h). EVAP is said to be less severe and have a better prognosis than LVAP due to the association of LVAP with antimicrobial resistant pathogens.[3] A few microorganisms such as Pseudomonas spp., Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus have been reported as the common VAP pathogens, with varying proportions.[2,7,8] The causative organisms and their resistance pattern vary among different patient population and ICUs. Thus, it is needed to identify the predominant microbial agents giving rise to VAP in different ICUs of an individual hospital.
VAP is suspected on the basis of chest radiographic infiltrates along with the presence of fever or leucocytosis or purulent tracheobronchial secretions.[1] However, chest radiographic changes can also be due to pulmonary edema, infarction, atelectasis or acute respiratory distress syndrome.[2] The clinical approach to VAP diagnosis is highly sensitive, but lacks specificity. Our primary objectives were to determine the risk factors associated with development of VAP and the outcome of patients developing VAP. The secondary objectives were to analyze the microbiological profile of organisms associated with VAP and to study their association with mortality.
Methodology
We conducted a case-control study among patients admitted to medical, surgical and trauma ICUs (2 medical, 1 surgical and 1 trauma) of a Tertiary Care Hospital in India during October 2009 to May 2011. An ethical clearance to conduct this study was obtained from institutional ethical committee prior to commencement of the study.
Inclusion criteria: Case group included patients of either sex, aged ≥ 18 years with mechanical ventilation, who developed pneumonia after 48 h of ventilation.
Control group included patients of either sex, aged ≥ 18 years with mechanical ventilation without pneumonia throughout hospitalization. The cases and controls were matched based on APACHE II score (±5 points) at the time of mechanical ventilation and duration of mechanical ventilation prior to onset of VAP (controls were ventilated for at least as long as the onset of pneumonia in the case). Exclusion criteria: Patients with pneumonia prior to mechanical ventilation and those developing pneumonia within 48 h were excluded from the study.
Sample size
Sample size calculation was based on comparison of prevalence of exposure (chronic obstructive pulmonary disease (COPD) or asthma) between cases and control. We expected the prevalence of exposure in cases and controls to be 30% and 10%.To detect the minimum clinical difference of prevalence of exposure to be 20% between cases and controls with 80% power and 20% level of significance, the sample size required in each group was 39. Since this calculation is based on sample size calculation for unmatched case control design, for matched case control design we multiplied the sample size with design effect of 1.35. The final sample size was 52 in each group. The estimate of design effect 1.35 was taken from National Family Health Survey III.
Data abstraction tool was used to capture the following details of each subject included in this study: Age, sex, APACHE II score at the time of mechanical ventilation, duration of mechanical ventilation, duration of intubation and tracheostomy, duration of ICU stay, risk factors such as COPD, bronchial asthma, diabetes, smoking, alcoholism, chronic use of inhaled/oral steroids or immunosuppressant, prior use of antibiotics, re-intubation, coma (Glasgow coma scale <6), post-operative status and head injury. Prior antibiotic use was defined as intravenous antibiotic administration for >24 h during any time of the patient's hospitalization prior to and during mechanical ventilation.
Clinical examination with regard to appearance of fever, bronchial breathing and crepitations was done. Tracheobronchial secretions were assessed every day, sent for culture and sensitivity on suspicion of pneumonia and repeated if radiological clearance was delayed or there was no improvement with antibiotics. Chest X-ray and white blood cell counts were done at the time of mechanical ventilation and repeated on clinical suspicion of pneumonia.
Definitions
Development of pneumonia after 48 h in patients with mechanical ventilation was defined as VAP.[1] Clinical diagnosis of VAP was made based on the occurrence of a new and persistent radiographic infiltrate along with at least 2 of the following: Fever >38.3°C, leucocytosis >10,000 cells/mm3 and purulent tracheobronchial secretions.[1] Cases were categorized based on the occurrence of pneumonia after mechanical ventilation as early onset (<96 h) and late onset (>96 h).[3] Microbial growth showing >105 colony forming unit (CFU)/ml from tracheobronchial secretion was considered as significant.[3] Multidrug resistant (MDR) Acinetobacter spp. was defined as resistance to at least 3 among following classes of antibiotics: β-lactams and inhibitors, fluoroquinolones, aminoglycosides and carbapenams. Pan drug resistant (PDR) Acinetobacter spp. included organisms resistant to polymyxins and colistin in addition to previous classes of antibiotics. MDR Pseudomonas spp. was defined as resistance to at least three among following five classes of antibiotics: cephalosporins, β-lactam-betalactam inhibitor combinations, fluoroquinolones, aminoglycosides and carbapenams. PDR Pseudomonas spp. organisms were resistant to polymyxins and colistin as well. We didn’t find any consensus over the definition of MDR, extended spectrum drug resistance (XDR) and PDR terminology used for Gram negative microorganisms (Acinetobacter spp. and Pseudomonas spp.) most commonly isolated from VAP patients. Furthermore, colistin (Polymyxins) often remains the only active agent for MDR–Gram negative pathogens,[9] non-susceptibility to which entails it as PDR. Thus, we have kept it simple and included carbapenam resistance into MDR definition.
Statistical analysis
All categorical variables were summarized using frequency and percentages. Age was summarized using mean and standard deviation and duration of ICU stay, mechanical ventilation was summarized using median and inter quartile range. McNemar's test was used to compare the association of different categorical exposure variables with the disease. Wilcoxon signed rank test was used to compare the median difference in duration of mechanical ventilation and ICU stay across the cases and controls. Paired test was used to compare the mean difference of age across cases and controls. A P < 0.05 was considered to be statistically significant. All analysis was carried out using the Statistical Package for the Social Sciences version 16.0 (SPSS, Chicago, IL, USA).
Results
A total of 52 cases and 52 controls satisfying the inclusion-exclusion criteria were enrolled in the study. Of the 52 cases that developed VAP, 48 had growth of pathogenic organisms in endotracheal (ET) culture. Remaining four cases fulfilled the clinical criteria for VAP, but of them, two cases were reported as sterile ET culture and another two cases had <105 CFU/ml culture positivity. The mean age of the case and control group was 47.12 (±15.3) and 51.87 (±16.85) years respectively. Male to female ratio among case and control group was 2.47 and 1.89 respectively. The demographic profile of our study population is shown in Table 1.
Table 1.
Demographic profile of study population
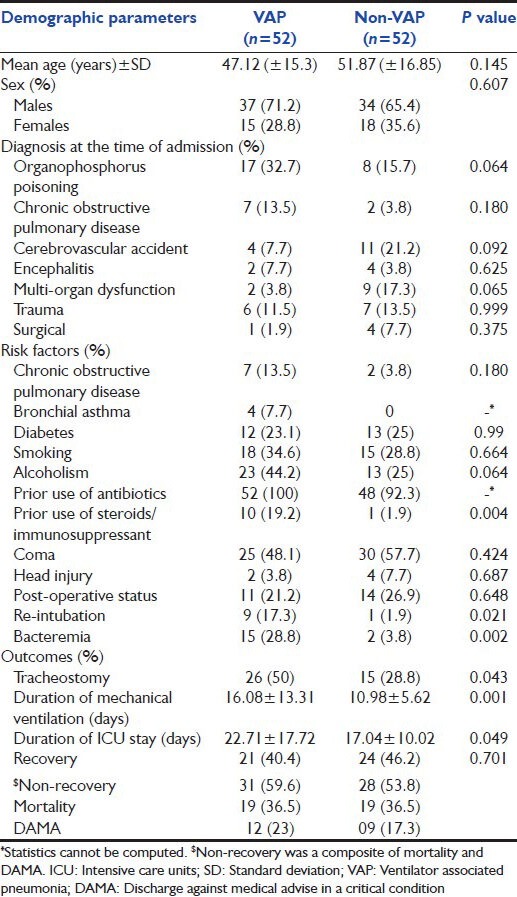
Among cases, the most common reason for admission to ICU was organophosphorus poisoning, followed by COPD. Among controls, most patients (21.2%) were admitted with cerebrovascular accident, however the casemix difference was not statistically significant. Most cases (86.5%) and controls (84.6%) were intubated for medical reasons such as respiratory failure, neuromuscular paralysis and airway protection to prevent aspiration. In other cases, intubation was done prior to surgery, which required ventilation subsequently.
On the day of admission, total 25 (48.1%) of cases were intubated. EVAP occurred in 27 (51.9%) cases whereas LVAP occurred in 25 (48.1%) cases. Most cases of VAP occurred on day 3 (26.9%) and 4 (25%). The median day of VAP onset was on day 4, inter-quartile range (3, 7.5). The mean APACHE II score was 19.5 among the cases and 20 among the controls.
Risk factors of VAP
Prior use of steroids (P = 0.004), re-intubation (P = 0.021), bacteremia (P = 0.002) were found to have a significant association with the occurrence of VAP. We found that underlying COPD was not significantly associated with development of VAP (P > 0.05).
Organisms
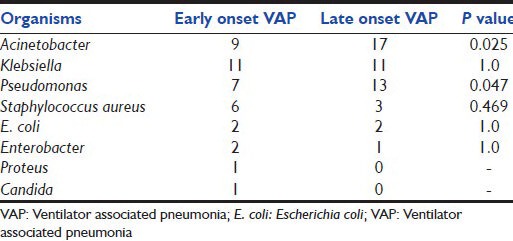
The most common organism reported was Acinetobacter (50%), followed by Klebsiella (42.3%) and Pseudomonas (40.4%). Most common organism in the EVAP was Klebsiella (40.7%), followed by Acinetobacter (33.3%), Pseudomonas (25.9%) and S. aureus (22.2%). In LVAP, most common organism was Acinetobacter, followed by Pseudomonas, Klebsiella and S. aureus. The association of LVAP was found to be statistically significant for Acinetobacter (P = 0.025) and Pseudomonas (P = 0.047) [Table 2]. Bacteremia was seen in 15 cases as compared with two patients among the control group (P = 0.001). Seven cases (13.46%) had bacteremia with the same organism as that of VAP. Eight cases (15.38%) had bacteremia with different organism. The organisms isolated from blood culture were Acinetobacter (5.8%), Klebsiella (5.8%), Enterobacter (5.8%), Candida (5.8%), S. aureus (3.8%) and Pseudomonas (1.9%).
Table 2.
Association of VAP onset with different organisms
MDR organisms
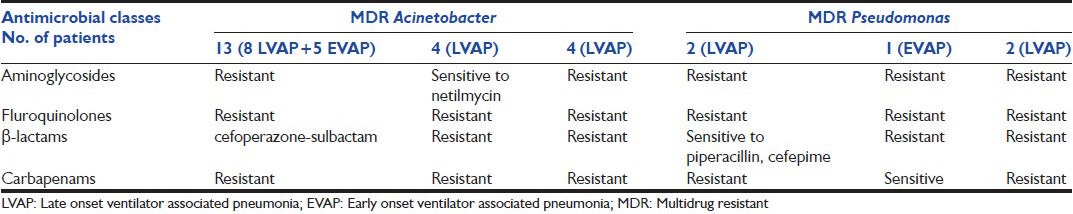
MDR organisms were seen in 40 (76.9%) patients among the cases. Among EVAPs, 19 (70.4%) of 27 cases had MDR organisms, whereas among LVAP, 21 (84%) of 25 cases had MDR organisms. MDR Acinetobacter and MDR Pseudomonas were more common among LVAP. MDR Acinetobacter was seen in 16 cases (76.2%) of LVAP and 5 cases (23.8%) of EVAP (P = 0.034). MDR Pseudomonas was seen in 4 cases of LVAP [Table 3].
Table 3.
Sensitivity profile of MDR organism
Outcome
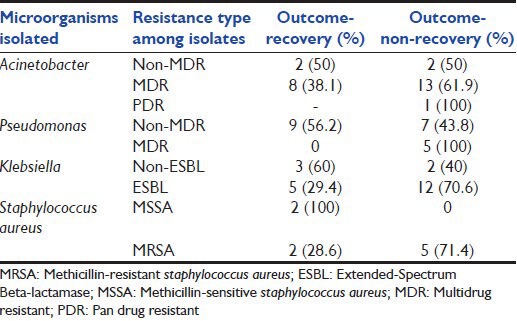
Out of 52 cases of VAP, 31 (59.6%) did not recover compared to 28 (53.8%) among controls (non-VAP). Non-recovery outcome included both mortality and discharge against medical advice. Mortality among both VAP and non-VAP categories remained 19 (36.5%). Patients with VAP had longer duration of mechanical ventilation (P = 0.001) and duration of ICU stay (P = 0.049). Among the control group, 28 (53.8%) did not recover. Non-recovery among EVAP and LVAP was recorded as 18 (66.7%) and 13 (52%) respectively. There was no significant association (P > 0.05) between APACHE II score and mortality. The mean APACHE II score among recovery and non-recovery group was 19 and 20 respectively. Among 15 bacteremic and 37 non-bacteremic VAP, non-recovery was noted as 10/15 (66.7%) and 21/37 (56.8%) respectively, which was not significant statistically (P = 0.55). Moreover, the duration of mechanical ventilation and ICU stay were not statistically different among bacteremic and non-bacteremic VAP. Organism wise outcome profile has been depicted in Table 4. While no single organism was found to be associated with mortality outcome, association of MDR and PDR Pseudomonas with mortality was statistically significant (P = 0.045).
Table 4.
Outcome profile of different microorganisms
Discussion
The results of our study showed that use of steroids and immunosuppressant was commoner among cases, which is attributable to the suppressive effects of steroids on innate and acquired immunity. A previous study[10] showed that steroid use was higher among the cases, but the association was not statistically significant. We found a significant association between re-intubation and development of VAP (P = 0.021), which is in accordance with previous study.[11] Re-intubation is associated with transfer of organisms from upper respiratory to lower respiratory tract, which could explain the higher incidence of VAP among such cases. We also found bacteremia to be significantly associated with VAP (P = 0.002); however, we cannot be sure whether it was a cause or effect with our study design.
EVAP was as frequent as LVAP, which is in contrast to previous studies, which indicated fewer EVAP than LVAP.[12] Gram-negative organisms were the most common ones in both early and LVAP. Though, the American thoracic society guidelines for VAP states that EVAP is associated with less virulent organisms such as methicillin-sensitive Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumonia, our study has shown higher prevalence of virulent organisms even with EVAP. The changing microbial pattern with a shift toward more gram negative pathogens in EVAP is very evident. There were no cases of S. pneumonia and H. influenzae in the present study and the higher rates of mortality among EVAP (11, 28.9%) than in LVAP (08, 21.1%) is attributable to the higher rates of virulent and MDR pathogens among EVAP in our study.
In the current study, majority (76.9%) of the organisms isolated was MDR. In the present study, the proportion of MDR organism among EVAP was higher compared with previous studies.[13,14] According to the American thoracic society guidelines for VAP the incidence of MDR[15] organisms is greater in LVAP as patients are exposed to risk factors for MDR pathogens such as prior antibiotic therapy, current hospitalization of 5 days or more. It is evident that patients of EVAP in our study were exposed to risk factors for MDR pathogens, especially prior antibiotics. The prevalence of multidrug resistance among the Acinetobacter group was 84.5% including one case of pan drug resistance, which is similar to a previous study.[3] This clearly demonstrates the prohibitive prevalence of MDR Acinetobacter in our ICUs.
Though VAP resulted in increased resource utilization as evident by prolonged mechanical ventilation (P = 0.001) and ICU stay (P = 0.049) compared with the controls, there was no significant difference in mortality (19, 36.5% in both VAP and non-VAP) of patients. Mortality rates among both groups were 36.5%. This may be attributed to the fact that the mortality among cases and controls may have been due to causes other than VAP, such as acute coronary event, poly-trauma, multiorgan dysfunction etc., We did not find any difference in outcome among bacteremic and non-bacteremic VAP, which is in contrast with a previous study.[16] Although VAP in general and any microorganism in particular was not associated with higher mortality, drug resistant Pseudomonas was associated with higher mortality. Most of the cases were tracheostomized after the onset of VAP. The higher rates of tracheostomy among cases are probably due to the longer duration of mechanical ventilation among cases as compared with the controls.
Strengths of our study include, case-control design to decipher the risk factors and APACHE II score matching among cases and controls at the time of mechanical ventilation. We believe that, APACHE II score matching at the time of mechanical ventilation[17,18] rather than on admission, for the assessment of risk factors associated with development of VAP and outcomes would better represent the true differences. Single centre setting of our study and failure to capture information by ICU category limit the generalizability of our findings. Another limitation in our study is non-inclusion of XDR category for defining drug resistant isolates. Risk of prior use of antibiotics, which has been shown to be associated with increased risk of VAP in another study[11] could not be assessed as all patients among the cases and most patients in the control group had received antibiotics before enrollment. This may partially explain the higher incidence of MDR organisms even in EVAP as compared with previous studies.
Conclusion
Proportion of LVAP remained higher than EVAP. MDR organisms especially Acinetobacter, Klebsiella and Pseudomonas are associated with majority of VAP cases. Proportion of MDR among LVAP remains more than EVAP. Bacteremia, prior use of steroid/immunosuppressant and re-intubations were associated with occurrence of VAP. Although, VAP was not associated with higher mortality it was associated with increased requirement for tracheostomy, longer duration of mechanical ventilation and ICU stay.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Coppadoro A, Bittner E, Berra L. Novel preventive strategies for ventilator-associated pneumonia. Crit Care. 2012;16:210. doi: 10.1186/cc11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakshit P, Nagar VS, Deshpande AK. Incidence, clinical outcome, and risk stratification of ventilator-associated pneumonia: A prospective cohort study. Indian J Crit Care Med. 2005;9:211–6. [Google Scholar]
- 3.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4:218–25. doi: 10.3855/jidc.634. [DOI] [PubMed] [Google Scholar]
- 4.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 5.Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82:172–8. doi: 10.1136/pgmj.2005.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morehead RS, Pinto SJ. Ventilator-associated pneumonia. Arch Intern Med. 2000;160:1926–36. doi: 10.1001/archinte.160.13.1926. [DOI] [PubMed] [Google Scholar]
- 7.Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months’ prospective study. Ann Thorac Med. 2007;2:52–7. doi: 10.4103/1817-1737.32230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S, Choudhary J, Ahmed M, Arora V, Ali S. Treatment of ventilator-associated pneumonia with piperacillin-tazobactum and amikacin vs. cefepime and levofloxacin: A randomized prospective study. Indian J Crit Care Med. 2007;11:117–21. [Google Scholar]
- 9.Lu Q, Luo R, Bodin L, Yang J, Zahr N, Aubry A, et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology. 2012;117:1335–47. doi: 10.1097/ALN.0b013e31827515de. [DOI] [PubMed] [Google Scholar]
- 10.Türkoğlu MA, Iskit AT. Ventilator-associated pneumonia caused by high risk microorganisms: A matched case-control study. Tuberk Toraks. 2008;56:139–49. [PubMed] [Google Scholar]
- 11.Noor A, Hussain SF. Risk factors associated with development of ventilator associated pneumonia. J Coll Physicians Surg Pak. 2005;15:92–5. [PubMed] [Google Scholar]
- 12.Gadani H, Vyas A, Kar AK. A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth. 2010;54:535–40. doi: 10.4103/0019-5049.72643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craven DE. Epidemiology of ventilator-associated pneumonia. Chest. 2000;117:186S–7S. doi: 10.1378/chest.117.4_suppl_2.186s. [DOI] [PubMed] [Google Scholar]
- 14.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Agbaht K, Diaz E, Muñoz E, Lisboa T, Gomez F, Depuydt PO, et al. Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: A study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med. 2007;35:2064–70. doi: 10.1097/01.CCM.0000277042.31524.66. [DOI] [PubMed] [Google Scholar]
- 17.Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, et al. Effect of ventilator-associated pneumonia on mortality and morbidity. Am J Respir Crit Care Med. 1996;154:91–7. doi: 10.1164/ajrccm.154.1.8680705. [DOI] [PubMed] [Google Scholar]
- 18.Kofteridis DP, Alexopoulou C, Valachis A, Maraki S, Dimopoulou D, Georgopoulos D, et al. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: A matched case-control study. Clin Infect Dis. 2010;51:1238–44. doi: 10.1086/657242. [DOI] [PubMed] [Google Scholar]


