Abstract
Introduction:
Our aim was to evaluate the impact of hyperproteic hypocaloric enteral feeding on clinical outcomes in critically ill patients, particularly on severity of organic failure measured with the Sequential Organ Failure Assessment (SOFA).
Materials and Methods:
In a double blind clinical trial, 80 critically ill adult patients were randomized to hyperproteic hypocaloric or to isocaloric enteral nutrition; all patients completed follow-up of at least 4 days. Prescribed caloric intake was: Hyperproteic hypocaloric enteral nutrition (15 kcal/kg with 1.7 g/kg of protein) or isocaloric enteral nutrition (25 kcal/kg with 20% of the calories as protein). The main outcome was the differences in delta SOFA at 48 h. Secondary outcomes were intensive care unit (ICU) length of stay, days on ventilator, hyperglycemic events, and insulin requirements.
Results:
There were no differences in SOFA score at baseline (7.5 (standard deviation (SD) 2.9) vs 6.7 (SD 2.5) P = 0.17). The total amount of calories delivered was similarly low in both groups (12 kcal/kg in intervention group vs 14 kcal/kg in controls), but proteic delivery was significantly different (1.4 vs 0.76 g/kg, respectively P ≤ 0.0001). The intervention group showed an improvement in SOFA score at 48 h (delta SOFA 1.7 (SD 1.9) vs 0.7 (SD 2.8) P = 0.04) and less hyperglycemic episodes per day (1.0 (SD 1.3) vs 1.7 (SD 2.5) P = 0.017).
Discussion:
Enteral hyperproteic hypocaloric nutrition therapy could be associated with a decrease in multiple organ failure measured with SOFA score. We also found decreased hyperglycemia and a trend towards less mechanical ventilation days and ICU length of stay.
Keywords: Colombia, critical care, hypocaloric enteral nutrition, permissive underfeeding, randomized controlled trial
Introduction
Hospitalized patients, especially intensive care unit (ICU) patients, have a high prevalence of malnutrition. It has been estimated that 40-50% of patients admitted to the ICU in Europe and USA present some degree of malnutrition,[1] a proportion that is not much different in Colombia.[2] There is strong evidence suggesting malnutrition is associated with increased rates of complications,[3] in the same way as obesity has been related to worse prognosis in critically ill patients.[4]
Energy requirements have been estimated using formulas derived from healthy individuals,[5] generating controversy about their applicability in critically ill patients. High patient heterogeneity (surgical, medical, immunosuppressed, and polytraumatized patients), as well as diversity in stress related factors add to the problem of determining precise requirements which are changing with the patient's conditions.[6] The major metabolic alteration in the acutely and critically ill patient is a hypercatabolic state, with increased protein breakdown and decreased muscle protein synthesis, which lead to increased excretion of nitrogen, phosphorus, magnesium, potassium, and creatinine. Increased metabolic rate, fever, hyperglycemia, amino acid, and free fatty acid mobilization contribute to a net negative nitrogen balance, which, if prolonged, is detrimental for the patient.[7,8]
Nutritional therapy recommendations in the ICU are different in the American Society for Parenteral and Enteral Nutrition (ASPEN)[9] and the European Society for Parenteral and Enteral Nutrition (ESPEN)[10] guidelines, which increases uncertainty in clinical practice.[11] There is a lack of evidence of when to start nutrition therapy, as well as to the amount of calories needed. Based on the 2007 ESPEN guidelines for enteral nutrition in intensive care, enteral nutritional support should be initiated in all patients not expected to be on a full oral diet within 3 days (grade C of recommendation).[10] Furthermore, also based on expert opinion, the amount of nutrition delivered during the acute phase of critical illness should not exceed 20-25 kcal/kg/day, as this might be associated with a less favorable outcome. In addition, during the recovery phase, patients should receive a total of 25-30 kcal/kg/day.[10]
As a result, appropriate nutritional regimen includes assessment of the risk of overfeeding as well as underfeeding of the critically ill patient. Observational studies have shown deleterious clinical outcomes associated with overfeeding, especially with parenteral nutrition.[12] Likewise, the major disadvantage of enteral nutrition is the risk of underfeeding, which has also been associated with increased mortality[13] and higher risk of bacteremia.[14]
Patiño et al., studied the use of a high protein, hypocaloric parenteral nutrition for critically ill patients (glucose 150-200 g/day and protein 1.5-2 g/kg/day) instead of a high glucose load regimen. Their observations suggested that this kind of strategy is more physiologic and beneficial to overcome the initial phase of the critically illness, avoiding the increase in the stress-related hyperglycemia.[15] There is also a recent observational study that concludes that higher provision of protein and amino acids correlates with decreased mortality in critically ill patients, even after adjusting for baseline prognostic variables as the Acute Physiology and Chronic Health Evaluation (APACHE) and the Sequential Organ Failure Assessment (SOFA).[16]
Different studies have shown a relationship between multiple organ dysfunction, morbidity and mortality in the ICU setting,[17,18] and the importance of organ dysfunction scoring systems as prognostic predictors. SOFA score[19] is an objective and relatively simple surveillance instrument. It assesses six organ systems (cardiovascular, renal, central nervous system, hematological, liver, and respiratory); assigning a 4-level scale of organ dysfunction for each organ system, which are then aggregated; higher scores represent higher dysfunction. There is a positive correlation between total maximum SOFA score and mortality in critically ill patients.[17] Delta SOFA, the change in total SOFA score at 48 and 96 h, as compared to admission score, has shown to be a good predictor of patient outcome, including mortality.[20]
The objective of this study was to compare two enteral nutritional regimens in the critically ill patient, and their impact in the development of severe organic failure, as measured with the SOFA.
Materials and Methods
Study population
This randomized, double blind clinical trial was performed at the 30-bed ICU of a tertiary-level university hospital in Bogota, Colombia. Patients were recruited during the 12-month period between August 2011 and July 2012. Study population consisted of adult patients (18 years or older) admitted in the ICU, who were expected to require enteral nutrition through nasoenteric tube for at least 96 h. Patients that had received previous nutritional support were excluded, as were patients that received concomitant parenteral nutrition, pregnant women, patients in transplantation program, those with contraindications for enteral nutrition, chronic renal failure, uremic encephalopathy, diabetes, morbid obesity, or do-not-resuscitate orders on admission.
Randomization
Randomization was performed using dark sealed envelopes with computer-generated random allocations. Only patients who completed 96 h of follow-up were considered for the analysis; patients who did not fulfill the follow-up period were excluded, and the envelope was returned to the sequence for patient replacement, until completion of the sample size (40 in each group). Study patients were prospectively followed-up until they were discharged from ICU, completed a follow-up of 21 days, or died, whichever occurred first. Only one of the members of the team (JDR) knew patient allocation, prescribed the formulations, and supervised the administration of the regimens; but ICU staff, who decided on daily care patient, was blind to patient allocation.
Intervention
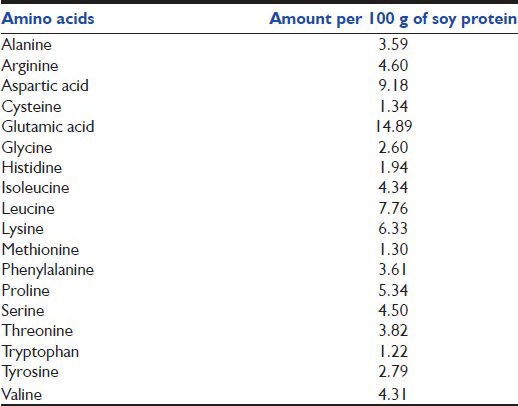
Patients were allocated to two groups. The intervention group received hyperproteic hypocaloric enteral nutrition, defined as a goal of 15 kcal/kg/day, with more than 1.5 g of protein per kg of body weight. Control group received standard nutritional regimen with a goal of 25 kcal/kg/day. To achieve this goal we used an enteral formula in continuous feeding for both groups; the composition of the formula is shown in Table 1. To reach the protein goal, the study group regimen was enriched with additional protein modules, based on soy protein diluted in water and administered in two daily boluses [Table 2]. Patients in the study group received hyperproteic regimen until day 7, if they needed any further enteral nutrition they were switched to standard nutritional regimen with a goal of 25 kcal/kg/day without protein boluses.
Table 1.
Composition of enteral nutritional used in both groups
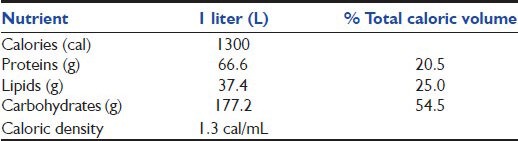
Table 2.
Composition of the soy protein preparation used in the intervention group
Endpoints
SOFA score was measured at randomization (baseline) and then every 48 h. The primary endpoint for the study was change in SOFA score at 48 h (delta SOFA). Secondary endpoints were: Change in SOFA score at 96 h, total SOFA score, insulin requirements, frequency of hyperglycemia or hypoglycemia, length of ICU stay, days on ventilator, and mortality.
Statistical analysis
Using TAMAMU software (Pontificia Universidad Javeriana, Bogota, Colombia), we estimated the sample size needed to provide 80% power with an alpha error of 0.05, to detect an absolute difference in the SOFA score between the two measurements of 15% (8.0 expected total score and 1.2 for expected delta SOFA) and a standard deviation (SD) between the difference of the means of 3.0. A one-sided Student's t-test was used for the primary outcome, since its reduction was the primary hypothesis of our study; two-sided t-test was used for all other endpoints. On the basis of these calculations, our sample was estimated to be 80 patients. All values were expressed as mean ± SD for continuous variables or proportions for categorical variables. We considered statistically significant a P value of 0.05 or less.
Ethical considerations
Written informed consent before enrollment in the study was provided by relatives. The study was approved by the Ethics Committee of the Pontificia Universidad Javeriana (Acta No. 18-2010) and complied with the provisions of the Good Clinical Practice guidelines, the Declaration of Helsinki, and local regulations. This trial has been registered in ClinicalTrials.gov, number NCT01531335.
Role of the funding source
The study sponsor provided an unrestricted grant and was not involved in any of the stages of the study. All authors had full access to the data and the corresponding author had final responsibility to submit the manuscript for publication.
Results
In total, 115 potential patients met the initial inclusion criteria for enrollment, but only 80 completed the follow-up and were included in the per protocol analysis. The main reason for exclusion and patient replacement was early discharge from the ICU [Figure 1]. Table 3 shows the characteristics of the final study population.
Figure 1.
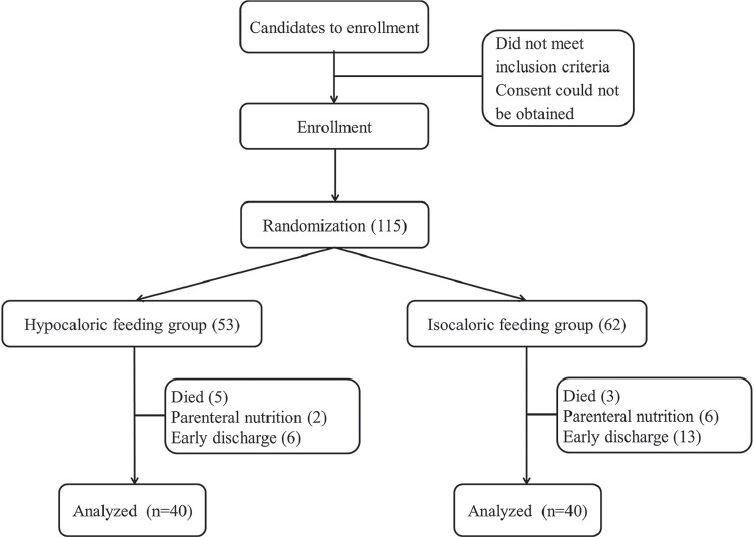
Flow chart of the the patient cohort randomized. Early discharge was less than 48 h after admission
Table 3.
Characteristics of the subjects included in the per protocol analysis
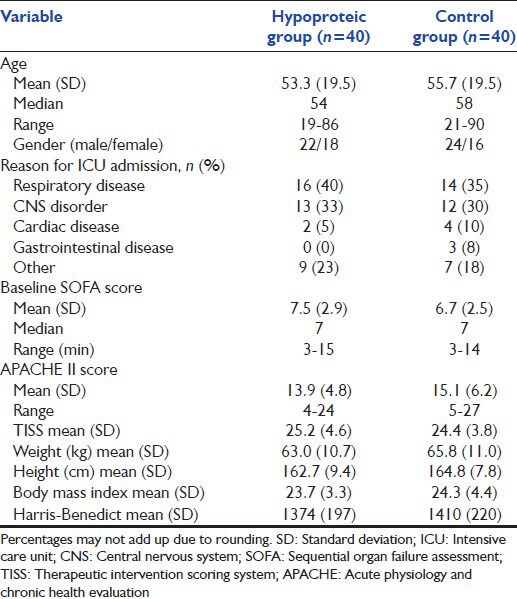
Our ICU receives both medical and surgical patients; there were, however, no surgical patients in our sample because they either recovered faster (before the 4 days of follow-up) and left the ICU, or required parenteral nutrition. Postoperative ileus and reintervention procedures with contraindication for enteral feeding were other reasons for not including surgical patients. Two-thirds of our patients had respiratory or central nervous system (CNS) indication for ICU admission. They were predominantly male and had, on average, a body mass index (BMI) in normal range.
Despite different prescriptions, the total amount of calories administered were similar in both groups (12 kcal/kg in the intervention group vs 14 kcal/kg in the control group). In the intervention group, however, 40% of total calories were from protein intake (30% from carbohydrates), while in the control group 20% of calories were from proteic origin (and 55% from carbohydrates). Both groups ended up with a caloric debt because the planned caloric goals (15 kcal/kg for the intervention group vs 25 kcal/kg for the control group) were not finally administered. We estimated the caloric debt in 17% in the intervention group and 40% in the control group. This difference is explained by variations in infusion speed as well as interruptions throughout the day. The intervention group was closer to the goal perhaps because the caloric intake was administered by exogenous protein boluses in addition to continuous enteral nutrition. Proteic delivery was significantly different in both groups (1.4 vs 0.76 g/kg P ≤ 0.0001). Table 4 shows the primary and secondary outcomes.
Table 4.
Primary outcome (delta SOFA) and all secondary outcomes of the study
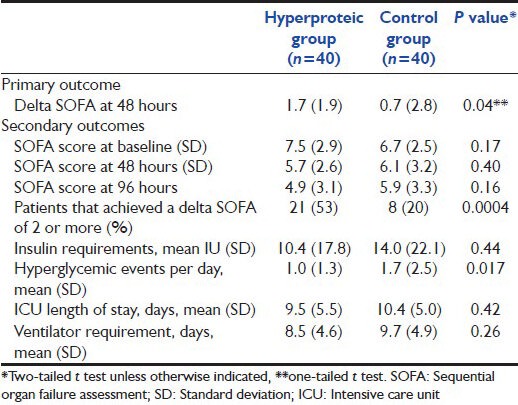
Both our primary outcome, the delta SOFA at 48 hours and the number of hyperglicemic events showed statistically significant differences. The intervention group had a higher baseline SOFA than the control group, but this difference was not statistically significant. In our study, there were no statistical significance difference in insulin requirements between groups, in contrast with the differences observed in the hyperglycemic events; there were more episodes of hyperglycemia in the isocaloric group than in the hypocaloric group. No hypoglycemic episodes, soy protein intolerance, or renal failure were reported in our sample.
Discussion
Our results suggest that hyperproteic hypocaloric enteral nutrition is associated with reduced risk of multiple organ failure. The loss of lean body mass during acute illness is well-known and has been associated with poor clinical outcomes;[21] the functional recovery after ICU discharge depends on the residual lean body mass. The objectives of nutritional support therapy are to overcome the acute catabolic phase, and to ameliorate the loss of body nitrogen, preserving rather than increasing the lean body mass. No precise method in clinical practice is available to determine the maximum acceptable loss of lean body mass, and to evaluate, in real time, the direct effect of nutritional therapy. In our usual practice, we estimate macronutrients using percentages of the total caloric amount, but in the critically ill patient population this approach is not appropriate. There is still controversy regarding the accurate nutritional therapy for critically ill patients, specially the amount of calories and the types of nutrients required.[11,22,23]
The characteristics of the patients included in our study were not much different in age and gender distribution to those patients included in the Arab study of Arabi et al.,[13] (51 years, 68% male), who studied underfeeding in 480 ICU patients receiving parenteral nutrition; or the Turkish study of Altintas et al.,[12] (58 years, 54% male), who analyzed prognostic characteristics of a sample of 71 ICU patients that required mechanical ventilation. The German study of Schneider et al.,[24] that studied low-volume enteral supplementation in ICU, had somewhat younger patients (47 years, 57% male). The APACHE II score of our patients, however, was substantially lower than in those three studies (our mean was 14.5, compared with 25.3 in the Arabi et al., study,[13] 23.8 in Altintas et al.,[12] or 21.6 in Schneider et al.,[24]). This difference can be explained because their patients required parenteral nutrition[13] or mechanical ventilation;[12] or were primarily polytrauma or sepsis patients.[24] In contrast, our sample was constituted by a wide variety of patients with mild to moderate severity in their critical illness state.
All the surgical ICU patients initially randomized were excluded since they did not complete follow-up; therefore, our analysis was based on medical ICU patients alone. This leaves out an important population which might benefit from hyperproteic nutrition. Respiratory system failure, followed by neurologic diseases, was the most important reason for ICU admission. Hyperproteic nutrition in this population might improve respiratory coefficient and oxygenation pattern.[6] Interestingly, our study found a nonsignificant trend to fewer days on ventilator. Our results were consistent with several studies[11,25,26,27] that highlight the importance of high protein regimens, instead of a targeted caloric therapy.
Our hypothesis is that the hyperproteic hypocaloric regimen provides the energy requirements necessary for the cellular machinery to run during the acute phase of the illness, although it may lead to caloric debt. In recent years, therapeutic approaches in the ICU setting have changed towards more physiologic therapies, and the enhanced turnover and protein catabolism related to critical illness set the ground for an approach targeting hyperproteic nutritional support. In the literature there is not much evidence for the precise amount of nutrients required by the critically ill patient, but it has been suggested that hyperproteic nutrition regimens improve clinical outcomes.[28]
Our results support our hypothesis: We found a benefit from the hyperproteic hypocaloric regimen, expressed as a higher delta SOFA score at 48 h. This score has been suggested to be an indicator of therapy effectiveness, and has been positively correlated with good clinical outcomes and lower mortality.
In our study, we guaranteed the protein load with an exogenous protein, soy protein, complementing the nutritional formulas used in clinical practice. The average amount of caloric intake in both groups was similar, lower than the caloric goals recommended by current clinical guidelines (and the amounts actually prescribed); the real difference between the two groups was in protein intake (less than 1 vs 1.5-2 g of protein per kg). This finding supports the idea that a proteic-caloric debt scenario, instead of a caloric debt scenario, might be detrimental for the success of nutritional therapy.
None of our patients presented soy protein intolerance or renal function impairment. Although patients with previous renal failure were excluded from the study, the premise that high protein intake increases the risk of renal failure is still a matter of controversy, at least in a population without previous renal disease.[25]
Hyperglycemia in the critical care setting has been associated with complications and mortality,[29] and is crucial as a prognostic factor for survival. Therefore, close glycemic monitoring is highly desirable, but tight glycemic control with insulin infusion has been shown to increase the risk for hypoglycemic episodes.[30] The hyperproteic hypocaloric regimen we used in the intervention group produced less hyperglycemic events and may be associated with better outcomes in these patients. Although total caloric intake was similar, carbohydrate intake was much lower in the intervention group. We believe this shift from carbohydrates to protein as the main caloric source explains the difference in glycemic response. Nonetheless, there were no statistically significant differences in insulin requirements despite the differences in hyperglycemic events. Although insulin protocols do exit in our institution, insulin use depends on individual physician preferences.
One limitation of our study is the small sample size and low statistical power; this might be, at least in part, responsible for the lack of statistical significance observed in most of the secondary outcomes, which show a trend favoring the intervention group.
Another limitation is the per protocol analysis we used, instead of an intention to treat approach, which we discarded because we needed to guarantee at least 48 h of follow-up. If this approach leads to a biased analysis, it would be against our hypothesis, since more patients had an early discharge in the control group than in the intervention group, the latter would end up having patients with more severe disease.
Generalizability of this study in the clinical practice is difficult to predict because trained staff is needed to guarantee targeted protein delivery in order to achieve the goals of protein intake. While other studies replicate our findings we would suggest following the current caloric recommendations, but with a higher proportion of protein.
Conclusion
The enteral nutritional regimen of 15 kcal/kg with 1.7 g of protein per kg is safe and was associated with less organ failures, as measured with the SOFA score, and less hyperglycemic episodes.
Footnotes
Source of Support: Major support for this study was provided by a grant from Lafrancol S.A.
Conflict of Interest: None declared.
References
- 1.Thibault R, Pichard C. Nutrition and clinical outcome in intensive care patients. Curr Opin Clin Nutr Metab Care. 2010;13:177–83. doi: 10.1097/MCO.0b013e32833574b9. [DOI] [PubMed] [Google Scholar]
- 2.Rugeles SJ. Nutrición y metabolismo en cirugía [Surgical nutrition and metabolism] Rev Colomb Cir. 2009;24:223–8. [Google Scholar]
- 3.Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–9. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 4.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: A systematic review and meta-analysis. Obesity. 2008;16:515–21. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiesmayr M. Nutritional risk assessment in the ICU. Curr Opin Clin Nutr Metab Care. 2012;15:174–80. doi: 10.1097/MCO.0b013e328350767e. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine E, Muller MJ. Adaptive alterations in metabolism: Practical consequences on energy requirements in the severely ill patient. Curr Opin Clin Nutr Metab Care. 2011;14:171–5. doi: 10.1097/MCO.0b013e328342bad4. [DOI] [PubMed] [Google Scholar]
- 8.Joseph B, Wynne JL, Dudrick SJ, Latifi R. Nutrition in trauma and critically ill patients. Eur J Trauma Emerg Surg. 2010;36:25–30. doi: 10.1007/s00068-010-9213-y. [DOI] [PubMed] [Google Scholar]
- 9.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 10.Kreymanna KG, Bergerb MM, Deutzc NE, Hiesmayrd M, Jolliete P, Kazandjievf G, et al. ESPEN Guidelines on enteral nutrition: Intensive care. Clin Nutr. 2006;25:210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 12.Altintas ND, Aydin K, Türkoğlu MA, Abbasoğlu O, Topeli A. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutr Clin Pract. 2011;26:322–9. doi: 10.1177/0884533611405790. [DOI] [PubMed] [Google Scholar]
- 13.Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: A randomized controlled trial. Am J Clin Nutr. 2011;93:569–77. doi: 10.3945/ajcn.110.005074. [DOI] [PubMed] [Google Scholar]
- 14.Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32:350–7. doi: 10.1097/01.CCM.0000089641.06306.68. [DOI] [PubMed] [Google Scholar]
- 15.Patiño JF, Echeverri S, Vergara A, Savino P, Rodriguez M, Escallon J. Hypocaloric support in the critically ill. World J Surg. 1999;23:553–9. doi: 10.1007/pl00012346. [DOI] [PubMed] [Google Scholar]
- 16.Allingstrup MJ, Esmailzadeh N, Knudsen AW, Espersen K, Jensen TM, Wiis J, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31:462–8. doi: 10.1016/j.clnu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25:686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, De Mendonça A, Cantraine F, Moreno R, Takala J, Suter P, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicentric, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluations of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 21.Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–8. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 22.Singer P, Pichard C. Reconciling divergent results of the latest parenteral nutrition studies in the ICU. Curr Opin Clin Nutr Metab Care. 2013;16:187–93. doi: 10.1097/MCO.0b013e32835c34be. [DOI] [PubMed] [Google Scholar]
- 23.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet. 2013;381:385–93. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 24.Schneider A, Markowski A, Momma M, Seipt C, Luettig B, Hadem J, et al. Tolerability and efficacy of a low-volume enteral supplement containing key nutrients in the critically ill. Clin Nutr. 2011;30:599–603. doi: 10.1016/j.clnu.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Lawson CM, Miller KR, Smith VL, McClave SA. Appropriate protein and specific amino acid delivery can improve patient outcome: Fact or fantasy? Curr Gastroenterol Rep. 2011;13:380–7. doi: 10.1007/s11894-011-0201-0. [DOI] [PubMed] [Google Scholar]
- 26.Strack van Schijndel RJ, Weijs PJ, Koopmans RH, Sauerwein HP, Beishuizen A, et al. Optimal nutrition during the period of mechanical ventilation decreases mortality in critically ill, longterm acute female patients: A prospective observational cohort study. Crit Care. 2009;13:R132. doi: 10.1186/cc7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CW, Sun SF, Lin SL, Kang SP, Chu KA, Lin CH, et al. Duodenal versus gastric feeding in medical intensive care unit patients: A prospective, randomized, clinical study. Crit Care Med. 2009;37:1866–72. doi: 10.1097/CCM.0b013e31819ffcda. [DOI] [PubMed] [Google Scholar]
- 28.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–37. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 29.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 30.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, et al. NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–18. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]


