Abstract
Background and Aims:
Pre-analytical errors in sample collection affect the reliability of blood gas (BG) analysis. Amount of liquid heparin as anticoagulant in samples for BG can affect results by its dilutional direct binding and compositional effects. The aim of this study was to examine the effect of varying amounts of heparin in blood samples on results.
Materials and Methods:
The prospective study was conducted on 15 children at a pediatric intensive care unit (PICU). Three different heparinized syringes were used containing minimal, 60 IU and 120 IU of heparin. A total volume of 1 ml blood in each syringe was taken and was analyzed by blood gas analyzer. Statistical analysis used related samples Friedman's test and Wilcoxon signed ranks test for paired comparisons. The observed bias was also compared with the desirable bias according to specifications by Ricos et al.
Results:
There was a significant difference (P < 0.05) in values of pH, pCO2, HCO3−, Hb and Na+ in the three syringes. The pCO2, HCO3− and Na+ levels decreased with the increasing amount of heparin. The observed percentage bias was more than desirable percentage bias specifications for pCO2, HCO3−, Hb, Na+, K+ and Cl− levels.
Conclusions:
Syringes with minimal liquid heparin are most appropriate for studying BG parameters as they have the least effect on these parameters. There is a need to standardize the procedure of syringe preparation for BG analysis. Further studies are needed to compare minimal amounts of heparin with commercially available dry balanced heparin syringes.
Keywords: Blood gas analysis, liquid heparin, pre-analytic error
Introduction
Blood gas analysis has pronounced value in detecting alteration in respiratory and metabolic parameters of the body. Reliability of these reports is of utmost importance in the management of critically sick children. Very often BG samples taken in the intensive care units and emergency units are not collected properly leading to erroneous BG results, which affects management.
There are many pre-analytical errors in the collection of samples for BG analysis.[1] One source of pre-analytical error is lack of a standardized protocol for collection of blood sample for BG analysis leading to use of different volume syringes, different sample volumes and use of varying quantities of liquid heparin by the residents leading to varying dilution of blood samples. Other modality for BG analysis is using pre-heparinized capillary tubes. The capillary blood is clinically acceptable to arterial blood only if acid-base parameters (pH and pCO2) are of interest; however, there is poor agreement between capillary and arterial pO2. The reliability of capillary BG analysis in clinically hypotensive states decreases further.[2]
Although guideline[3] recommends the use of commercially prepared dry-balanced heparin syringes for BG analysis, liquid heparin is still being used in various intensive care units due to cost factors. Liquid heparin as anticoagulant in samples for BG can affect BG parameters by its dilutional, direct binding and compositional effects.[3] It is recommended that the amounts of liquid heparin should preferably be 5% or less and certainly below l0% to prevent dilutional effects.[4] Hence, the aim of this study was to examine the effects on various BG parameters of increasing amounts of heparin in blood samples and titration of the amount of liquid heparin suitable for the purpose of BG analysis in pediatric intensive care unit (PICU) settings in resource poor countries.
Materials and Methods
The prospective study was conducted at 20 bedded PICU of Kalawati Saran Children's hospital in Delhi. PICU of our hospital admits patients with respiratory failure, shock, post-diphtheritic neuropathy, Gullian Barre syndrome etc., The BG samples were collected over 5 days in October 2012 and a convenient sample size of 15 children was taken as BG analysis includes multiple parameters and it is not possible to calculate sample size on the basis of a single parameter. Samples were collected only in children in whom BG analysis was clinically indicated. Full parental consent was taken before sample collection.
Three blood samples were collected from each child in self-prepared heparinized syringes. Samples were collected with 1 ml tuberculin (DispoVan plastic, Ballabgarh, India) syringe with needle (Needle; 26 G; 0.5 inch) containing the smallest measurable division of 0.02 ml. Average dead space volume of a tuberculin syringe is 0.06 ml. We measured the dead space of the syringe by first filling the syringe followed by fully emptying it and then again withdrawing the piston. The volume of heparin in the dead space was thus drawn into the syringe barrel and was measured. Sodium heparin solution derived from gut mucosa, containing 1,000 IU/ml strength of heparin was used for anticoagulation (Gland pharma limited, Hyderabad, India). The heparin in vial and syringe gets in equilibrium with the air, so analysis of heparin solution by blood gas analyzer (BGA) revealed pH of 6.85, Na+ of 15 mmol/l and pO2 of 150 mmHg.
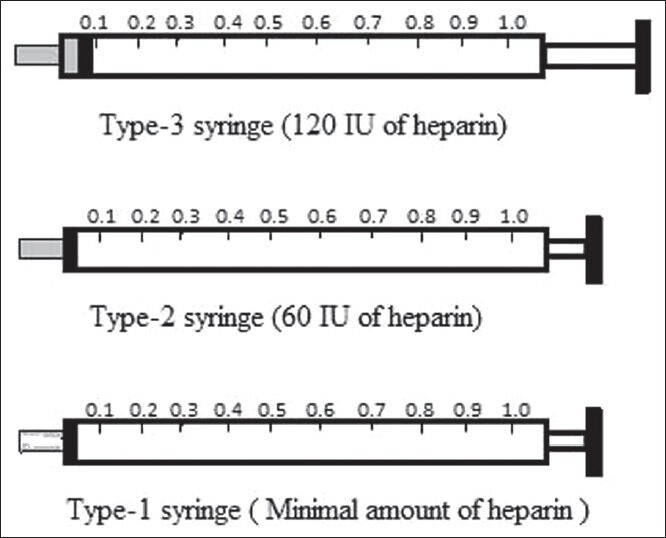
Three different heparinized syringes were prepared with a different amounts of liquid heparin. Type-1 syringe was prepared by first filling the barrel of syringe until 1 ml marking and then flushing out all the heparin solution and air 4 times so that no visible heparin solution was left in the syringe barrel or hub. The Type-2 syringe was prepared with heparin solution equal to dead space (0.06 ml) of tuberculin syringe (60 IU, 60 IU/ml concentration and 6% blood dilution). The Type-3 syringe was prepared with 0.12 ml of liquid heparin solution (120 IU, 120 IU/ml concentration and 12% blood dilution). Syringes were prepared immediately before the sampling [Figure 1].
Figure 1.
Procedure for syringe preparation
After preparation of syringes, the venous blood samples were collected during the single sampling procedure to make a total volume of 1 ml in each of three syringes. Samples were collected at the bedside with free flow of blood by a single trained resident of PICU and immediately tested. Before analysis, the heparinized samples were mixed thoroughly by gently rubbing of syringes between palms of two hands to ensure that a homogenous sample was submitted for analysis. Bubbles in the samples were avoided as much as possible and bubbles if formed were removed immediately after sampling. The sequence of sampling and analysis among the syringes was rotated. All analyses were performed using the BGA (ABL 800 basic, Radiometer, Copenhagen, Denmark) installed in PICU. BGA calibrated automatically every 4 hourly with weekly quality check with control solutions. A review of the earlier literature revealed that excess of liquid heparin affects various estimates of BG by its dilution and direct binding effects;[3] therefore, we considered Type-1 syringe as the reference for all comparisons because it had a minimal amount of liquid heparin and hence least chances of error.
Before conducting main study, we conducted a pilot study including 10 samples to calculate test-retest precision of BGA for various parameters. Coefficient of variation (%) for pH, pCO2, pO2, SO2, HCO3−, lactate, Hb, Na+, K+, Cl− was 0.07, 4, 4.9, 2.8, 4.4, 4.2, 13.5, 1.2, 4.9 and 0.9% respectively.
A database of definitive analytical quality specifications for a large number of clinical laboratory tests was prepared by Ricos et al.[5] We compared the observed bias with the desirable bias specifications given by Ricos et al.[5]
Statistical analysis
BG parameters were expressed as mean and standard deviation (SD). The results were analyzed using related samples Friedman's two-way analysis of variance. For paired comparisons, we used Wilcoxon signed ranks test. Mean percentage bias (calculated as the difference between means of a parameter divided by the average of two means and multiplied by 100) was determined and P < 0.05 was considered significant. Statistical analysis were performed with Microsoft excel and IBM Statistical Package for Social Sciences version 20.
Results
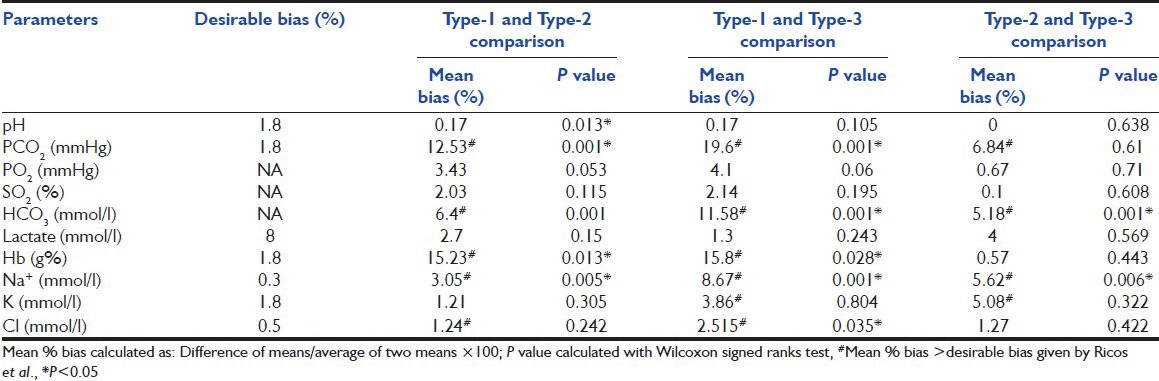
Mean, SD and P values of pH, pCO2, pO2, SO2, HCO3−, lactate, Hb, Na+, K+ and Cl− are as shown in Table 1. The P values were significant for pH, pCO2, HCO3−, Hb and Na+ levels. The pair wise comparison between Type-1 and Type-2, Type-1 and Type-3, Type-2 and Type-3 syringes are as shown in Table 2. Comparison of observed mean % bias with desirable % bias specifications as given by Ricos et al.[5] is presented in Table 2. The observed bias was more than desirable % bias for pCO2, HCO3−, Hb, Na+, K+ and Cl− levels. The precision of analyzer was within acceptable limits for all parameters except for Hb and K+.
Table 1.
Comparison the res ults (mean±SD) of blood gas parameters by three syringes
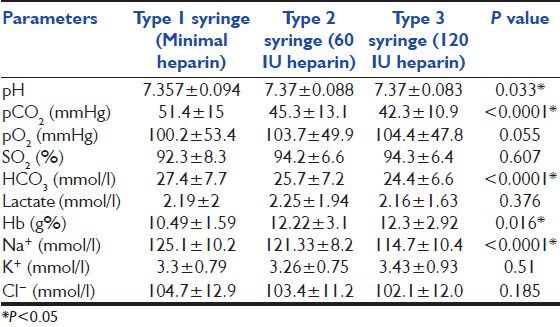
Table 2.
Desirable % bias specifications of blood gas parameters and pair wise comparison of the results of three syringes
Discussion
Most common sources of error in BG analysis are pre-analytic errors[1] that includes hemolysis, effect of temperature, use of plastic syringes, presence of air bubble, delay in sample transport, dilution effect of liquid heparin, effect of different volume of blood in syringes, manufacturing differences among syringes with dry balanced heparin, micro-clotting in the sample, errors in analyzer machines. We used syringes with minimal heparin (Type-1) and heparin in syringe hub (Type-2) because they represented two commonly observed practices of syringe preparation at our center. Although taking 120 IU of heparin in a syringe (Type-3) was not a commonly observed practice, but during internal audit we observed that blood was rarely collected up to 1 ml mark so high heparin to blood ratio may lead to higher final heparin concentration and blood dilution. High heparin to blood ratio may be especially important factor for error in neonates where blood collection is difficult. Minimal volume required by the analyzer for the analysis is 0.3 ml, so it is a common mistake to subject a lesser volume for analysis, which has relatively high concentration of heparin. In a previous study, Hamilton et al.[6] reported a similar problem where inexperienced residents sampled with large volume heparin and small volume of blood and then small volume samples were analyzed by the technicians if the volume was greater than the minimum amount required for the analyzer. Hedberg et al.[7] compared four different sample volumes in plastic heparin balanced syringes and demonstrated that low sample volumes were associated with significant bias in BG, electrolyte and lactate measurements. Our study was similar in the sense that we varied the volume of liquid heparin and kept the volume of blood constant. Knowles et al.[8] suggested immediate analysis of samples taken in plastic syringes and no need of ice storage and storage of delayed samples in glass syringe. Lima-Oliveira et al.[9] even demonstrated that pre-heparinized syringes prepared by different manufacturers may also be a source of variability in results. We used same batch of plastic syringes and liquid heparin preparation and all three samples were analyzed immediately after collection so both these factors were not important in our study. Biswas et al.[10] suggested that for accurate estimation of pO2 and pCO2, it was necessary to avoid frothing and removal of all air bubbles within 2 min of sample collection. We took care of the problem of bubble formation by collecting a free flow venous sample and by removing any formed bubbles immediately. Collection of free flow sample also reduced the possibility of significant hemolysis. Further we did not notice any micro-clotting in any of samples and BGA was calibrated regularly so these were not the possible reasons for observed findings. So the only possible reason for significant bias could be the presence of increasing quantities of heparin. Results of our study revealed significant P values for mean pH, pCO2, HCO3−, Hb and Na+ levels. The pCO2, HCO3− and Na+ levels showed progressively increasing bias with the increasing amount of liquid heparin whereas pO2, lactate and SO2 did not reveal any significant bias. Findings of our study agreed with an earlier study,[11] which showed that pCO2 and HCO3− were inversely related to the volume of heparin used and >10% dilution was associated with a significant reduction in pCO2 and HCO3−. Alterations in pCO2 and HCO3− affect the metabolic and respiratory components of acid-base measurements leading to unexplained simultaneous respiratory alkalosis and metabolic acidosis.[12] Although, difference in pH between Type-1 and Type-2 syringes (0.013) was lower than desirable bias specifications [Table 2]; however, it was statistically significant. This is different from an earlier study, which demonstrated that despite heparin being an acidic solution, blood pH is not affected until 40% dilution because of the buffering capacity of blood.[11] The pO2 measurement is also relatively resistant to the dilution effect with an increase in pO2 only observed at high (>35%) dilutions.[4] Several studies[13,14] comparing point of care testing using heparinized syringes with central laboratory non-heparinized testing has demonstrated negative bias in Na+ and K+ estimations, possibly due to dilution effects of heparin. Yip et al.[15] similarly demonstrated negative bias in sodium estimations associated with incomplete filling of syringes in a pediatric setting.
We compared the observed bias with the desirable bias specifications given by Ricos et al.[5] In our study, the observed bias exceeded the desirable bias for pCO2, Na+, K+, Cl− and Hb [Table 2]. Desirable bias specifications for HCO3− and pO2 are not available. We considered test retest precision performance of the analyzer before attributing the observed bias to effects of liquid heparin. The precision of analyzer was within acceptable limits for all parameters except for Hb and K+. So observed significant bias in values of pCO2, HCO3−, Na+, Cl− were most likely due to the effect of liquid heparin only. The bias more than desirable limits for K+ and Hb in our study could be explained by imprecision of BGA at the time of study. The four affected parameters are all important for calculation of base excess and anion gap (AG). So alteration in these parameters can have a serious effect on the validity of AG calculations.
International Federation of Clinical Chemistry has given guidelines for preparing syringes for BG sampling and recommends blood collection up to 20 times the heparin volume.[4] Correct heparin concentrations in BG analysis are important for anticoagulation. Insufficient heparinization can lead to clotting and can cause incorrect test results, machine malfunction and unnecessary repeat sampling in patient. Conversely excess heparin may be a source of error due to dilution and direct binding effects of heparin. It is important to emphasize adequate amount of blood collection because smaller blood volumes are at a greater risk of error due to dilution, even when the amount of heparin taken in the syringe is small. The self-prepared syringes are still used at our center because of cost related reasons. To conclude syringes with minimal heparin had the least effect on BG parameters and no clotting was observed. Thus, syringes with minimal heparin are most appropriate for studying BG parameters. There is also a need to standardize the procedure of syringe preparation and taking the blood sample for BG analysis in hospitals. Further studies are needed in a similar setup comparing minimal amount of heparin with commercially available dry balanced heparin syringes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Baird G. Preanalytical considerations in blood gas analysis. Biochem Med (Zagreb) 2013;23:19–27. doi: 10.11613/BM.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins C. Capillary blood gases: To arterialize or not. MLO Med Lab Obs. 2008;40:44–7. [PubMed] [Google Scholar]
- 3.Higgins C. The use of heparin in preparing samples for blood-gas analysis. MLO Med Lab Obs. 2007;39:16–8. 20. [PubMed] [Google Scholar]
- 4.Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Maas AH, Müller-Plathe O, et al. International Federation of Clinical Chemistry (IFCC). Scientific Division. Committee on pH, Blood Gases and Electrolytes. Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH, blood gases and electrolytes. Eur J Clin Chem Clin Biochem. 1995;33:247–53. [PubMed] [Google Scholar]
- 5.Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: Pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton RD, Crockett RJ, Alpers JH. Arterial blood gas analysis: Potential errors due to the addition of heparin. Anaesth Intensive Care. 1978;6:251–5. doi: 10.1177/0310057X7800600314. [DOI] [PubMed] [Google Scholar]
- 7.Hedberg P, Majava A, Kiviluoma K, Ohtonen P. Potential preanalytical errors in whole-blood analysis: Effect of syringe sample volume on blood gas, electrolyte and lactate values. Scand J Clin Lab Invest. 2009;69:585–91. doi: 10.1080/00365510902878716. [DOI] [PubMed] [Google Scholar]
- 8.Knowles TP, Mullin RA, Hunter JA, Douce FH. Effects of syringe material, sample storage time, and temperature on blood gases and oxygen saturation in arterialized human blood samples. Respir Care. 2006;51:732–6. [PubMed] [Google Scholar]
- 9.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Different manufacturers of syringes: A new source of variability in blood gas, acid-base balance and related laboratory test? Clin Biochem. 2012;45:683–7. doi: 10.1016/j.clinbiochem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Biswas CK, Ramos JM, Agroyannis B, Kerr DN. Blood gas analysis: Effect of air bubbles in syringe and delay in estimation. Br Med J (Clin Res Ed) 1982;284:923–7. doi: 10.1136/bmj.284.6320.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchison AS, Ralston SH, Dryburgh FJ, Small M, Fogelman I. Too much heparin: Possible source of error in blood gas analysis. Br Med J (Clin Res Ed) 1983;287:1131–2. doi: 10.1136/bmj.287.6399.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons DH. The effect of heparin dilution on arterial blood gas analysis. West J Med. 1984;141:525–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Morimatsu H, Rocktäschel J, Bellomo R, Uchino S, Goldsmith D, Gutteridge G. Comparison of point-of-care versus central laboratory measurement of electrolyte concentrations on calculations of the anion gap and the strong ion difference. Anesthesiology. 2003;98:1077–84. doi: 10.1097/00000542-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Chhapola V, Kanwal SK, Sharma R, Kumar V. A Comparative study on reliability of point of care sodium and potassium estimation in a pediatric intensive care unit. Indian J Pediat. 2013. Feb, [cited 2013 Jun 17]. Available from: link.springer.com/content/pdf/10.1007%2Fs12098-013-0977-z . [DOI] [PubMed]
- 15.Yip PM, Chan MK, Zielinski N, Adeli K. Heparin interference in whole blood sodium measurements in a pediatric setting. Clin Biochem. 2006;39:391–5. doi: 10.1016/j.clinbiochem.2005.12.006. [DOI] [PubMed] [Google Scholar]


