Abstract
Purpose:
CD163 is a monocyte/macrophage-associated antigen which has recently been identified as a hemoglobin scavenger receptor and has also anti-inflammatory properties and an immunoregulatory role. This surface receptor undergoes ectodomain shedding upon an inflammatory stimulus, leading to increased fraction of soluble CD163 (sCD163). Hence, we hypothesized that the mechanical ventilation (MV) which is known to elicit inflammatory response may cause increased serum levels of sCD163 which can predict the outcome of patients from MV.
Subjects and Methods:
Thirty patients with acute respiratory distress aged >18 years who required MV were enrolled for the study. Serum levels of sCD163 were estimated using quantitative immunometric sandwich enzyme immunoassay technique from 3 mL of the venous blood sample which was collected immediately and at 24 h after the patient was connected to MV. On the basis of the outcome of the patient from MV, they were divided into two groups; survivors and nonsurvivors.
Results:
Out of the 30 patients, 18 patients survived and 12 patients expired. Serum levels of sCD163 were significantly increased in nonsurvivors when compared with survivors (P < 0.01) at 24 h after connecting to MV. sCD163 > 1020 ng/mL at 24 h of MV increases the probability of mortality by factor 6. An increase of sCD163 by 1 ng/mL significantly increases the relative probability of mortality by a factor of 1.0017 (95% confidence interval, 1.0004-1.0030, P = 0.0005).
Conclusions:
Elevated levels of sCD163 at 24 h of MV help in predicting the outcome of patients with acute respiratory failure from MV.
Keywords: Acute respiratory distress, mechanical ventilation, soluble CD163
Introduction
Patients requiring intensive care frequently have some degree of systemic inflammatory response syndrome (SIRS), which, when severe, places them at risk of multiple organ failure. A very early feature of inflammation is release of acute phase reactant proteins. Several authors have reported that C-reactive protein, an acute-phase protein, is a marker of inflammation and infection[1] and may be associated with an increased risk of organ failure and death.[2] Though mechanical ventilation (MV) is a life-saving intervention for patients with acute respiratory distress syndrome, it can aggravate or cause lung injury, known as ventilator-induced lung injury. CD163 is a member of the cysteine-rich scavenger receptor superfamily, which is expressed exclusively on human monocytes and macrophages.[3] It was shown to be a receptor for the haptoglobin–hemoglobin complex.[4] Apart from clearance of hemoglobin, it has also anti-inflammatory properties and an immunoregulatory role.[5] The extracellular portion of CD163 undergoes ectodomain shedding upon an inflammatory stimulus, and CD163 expression on a monocyte surface is inversely related to the concentration of its soluble CD163 (sCD163).[6] These observations clearly indicate that elevated levels of sCD163 are found in plasma of patients with inflammation. Hence, we hypothesized that the MV, which is known to elicit inflammatory response, may cause increased serum levels of sCD163 which can predict the outcome of patients from MV.
Subjects and Methods
Type of study
Prospective follow-up study
Inclusion criteria
Thirty patients (aged >18 years) admitted to the intensive care unit (ICU) of tertiary care hospital with acute respiratory distress and who required volume controlled continuous MV for at least 24 h were enrolled for the study. Causes for acute respiratory distress included chronic obstructive pulmonary disease without any exacerbation in the past 3 month, severe asthma and pneumonia. Approval from the institutional ethical committee was taken before commencement of the study. Written consent was taken from the patient's relatives before collection of blood.
Exclusion criteria
Patients with sepsis, neuromuscular diseases, left ventricular dysfunction, chest or abdominal trauma, injury due to burns, and history of exacerbation of chronic respiratory failure during the last 3 months were excluded from the study.
Sample size
All the occurrences in 6 months between July 2012 and December 2012 were included in the study. There were 176 admissions to the ICU during the study period. Out of these, 35 had acute respiratory distress and were intubated. Five patients were excluded from the study as two of them had pulmonary edema due to left ventricular dysfunction; two of them had exacerbation of chronic respiratory failure and one was weaned from MV before 24 h. Hence, thirty patients were included in the study.
Data collected from the patients record included age, sex, underlying disease, sequential organ failure assessment score (SOFA) score within 24 h after the patient was connected to MV, documentation of the need for MV, and duration of MV. Strict input − output chart was maintained for all the patients to estimate their fluid balance. A total of 3 mL of the venous blood was collected immediately and at 24 h after the patient was put on MV and centrifuged at 3000 rpm for 10 min and serum was stored at −80°C until further analysis. Levels of sCD163 were estimated in duplicate (with the technician blinded to the patient outcome from MV) using a solid-phase enzyme-linked immunosorbent assay method based on the quantitative immunometric sandwich enzyme immunoassay technique, following the manufacturer's instructions (R and D systems).
On ICU admission, the following standard ventilation protocol was applied: Patients were continuously sedated (benzodiazepines and/or opioids), remained supine, and were ventilated with volume controlled continuous MV. Respiratory rate and fraction of inspired oxygen (FIO2) were adjusted to maintain arterial oxygen saturation >90%, PaCO2 of 35-45 mm Hg and pH >7.25. Tidal volumes of all the patients were between 5 and 7 mL/kg of the predicted body weight. Positive end expiratory pressure (PEEP) was kept at 10 cm H2O. The inspiratory:expiratory (I:E) ratio was 1:2.
Duration of mechanical ventilation
Duration of MV was calculated from the time the patient was intubated and connected to ventilator, till the time he was disconnected from the ventilator. A combination of subjective and objective parameters was used to wean the patient from MV. The objective parameters used were (a) PaO2/FiO2 > 150-200; (b) level of PEEP between 5 and 8 cm H2O; (c) FiO2 level <50%; (d) pH >7.25; and (e) ability to initiate spontaneous breaths. The subjective parameters used were (a) hemodynamic stability; (b) absence of active myocardial ischemia; (c) absence of clinically significant vasopressor requiring hypotension; and (d) adequate muscular strength allowing the capability to initiate/sustain the respiratory effort. On the basis of their outcome from MV, the patients were divided into two groups; survivors and nonsurvivors.
Statistical analysis
Arithmetic mean and standard deviation were estimated to assess the level of various parameters in the study. Differences in the levels of sCD163 between the two groups were assessed using the Mann-Whitney nonparametric test. Receiver operating characteristics (ROC) curves were constructed to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive or negative likelihood ratios (± LR) for sCD163 in predicting the outcome of patients from MV. The analyses were facilitated with the use of the MedCalc 11.5.1.0 software packages. Logistic regression analysis was used to determine if sCD163 was an independent risk factor for predicting the outcome of patients from MV. Differences were considered significant if the P < 0.05.
Results
Out of the 30 patients (21 males and 9 females) enrolled for the study, 18 patients successfully improved after mechanical ventilatory support was given to them and 12 patients expired. The mean age of the patients who improved, when compared with those who expired, did not show any statistically significant difference (P value 0.56).
The mean and standard deviation of different parameters in the two groups is shown in Table 1. Serum levels of sCD163 were comparable between the two groups immediately after the patient was connected to the ventilator. However, the levels increased significantly in nonsurvivors when compared with survivors (P < 0.01) at 24 h after MV. sCD163 > 1020 ng/mL at 24 h of MV increases the probability of mortality by factor 6. The cut off level of 1020 ng/mL had a sensitivity of 69.2, specificity of 88.2, PPV and NPV of 80 to predict the outcome of the patient from MV. The area under the curve of ROC of sCD163 was 0.842 and of SOFA score was 0.839 [Figure 1]. Also, by performing logistic regression analysis, it was shown that an increase of sCD163 by 1 ng/mL significantly increases the relative probability of mortality by a factor of 1.0017 [95% confidence interval (CI), 1.0004-1.0030, P = 0.0005]. When sCD163 was considered as a single predictor, the odds ratio was found to be 4.251. When SOFA score was brought into the equation the odds ratio which indicates predictive ability of parameter was: sCD163 − 1.712; SOFA score − 0. This implies that sCD163 is an independent predictor of mortality even though the SOFA score is brought in to the equation.
Table 1.
Mean and standard deviation of different parameters in the two groups based on their outcome from mechanical ventilator
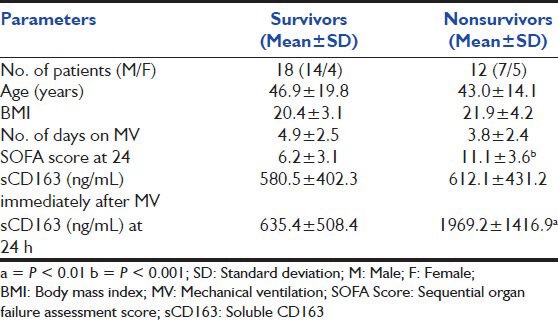
Figure 1.
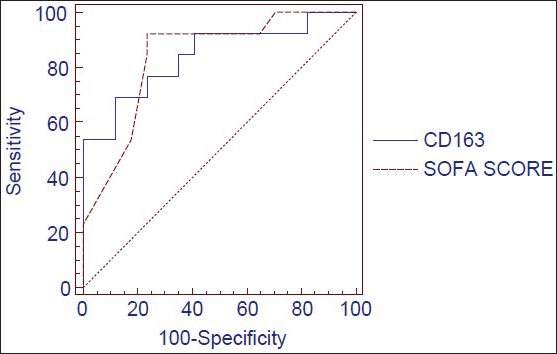
Receiver operating characteristics curve for soluble CD163 and sequential organ failure assessment score score
Discussion
As a high-cost technology, MV will likely be increasingly scrutinized due to the increased focus on improving cost efficiency and documenting patient's outcomes. Unfortunately, our current ability to accurately assess patient outcomes from assisted ventilation is hindered by several factors. In this study, the serum concentration of sCD163 was significantly increased in patients who had poor prognosis and could not survive.
CD163 was first identified in 1987[7] and received its CD number in 1996.[8] The two main biological role of CD163 is clearance of hemoglobin and a potential immunoregulatory function. There are several studies of CD163 regulation and function that link the receptor activity to an anti-inflammatory response. This association is based on the following observations. First, macrophages highly expressing CD163 constitute the predominant macrophage population during the late or resolution phase of inflammatory reactions.[9] Second, CD163 expression is strongly induced by anti-inflammatory mediators, such as glucocorticoids, interleukin (IL)-6, and IL-10,[10] resulting in a distinct population of cells called the “alternatively activated macrophages.” The plasma level of soluble CD163 reflects the total pool of membrane-bound CD163, which may be increased in case of proliferation of cells of myelomonocytic origin or in case of upregulation of CD163 expression by acute phase mediators.
The levels of sCD163 are significantly increased in patients with sepsis, myeloid leukemia, and in patients with Gaucher's disease,[11] which are all examples of diseases with proliferation of cells of myelomonocytic origin. In patients with rheumatoid arthritis and spondyloarthropathy synovitis, the level of soluble CD163 in synovial fluid is inversely correlated to the number of activated lymphocytes (CD69 positive). The concentration of sCD163 in the synovial fluid is several folds higher than in serum indicating a local production of sCD163. In serum, the concentration of sCD163 is slightly increased in both rheumatoid arthritis and spondylic arthritis.[12,13] However, no clear relation between clinical evaluation of the disease severity and the sCD163 levels has been observed. It has also been shown that CD163 can be used for discrimination of SIRS and sepsis in critically-ill neonates and children.[14]
However, we tried to correlate the serum levels of soluble CD163 with the outcome of the patients from MV. Our study clearly shows that the elevated levels of serum sCD163 at 24 h of MV indicates poor prognosis of the patients. sCD163 > 1020 ng/mL at 24 h of MV increases the probability of mortality by factor 6. An increase of sCD163 by 1 ng/mL significantly increases the relative probability of mortality by a factor of 1.0017 (95% CI, 1.0004-1.0030, P = 0.0005).
Limitation of the study
Small sample size is the limitation of the study. However, this study can be taken as a pilot study and large-scale, multicentric, prospective epidemiologic studies may be conducted to validate these findings. Also, serial levels of sCD163 during the course of MV may be estimated and correlated with the outcome of the patients from MV.
Conclusion
Elevated levels of sCD163 at 24 h of MV help in predicting outcome of patients with acute respiratory failure from MV. This needs to be validated using serial measurements of sCD163 during the course of MV with a larger sample size.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Póvoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragão A, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24:1052–6. doi: 10.1007/s001340050715. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya B, Prashant A, Vishwanath P, Suma MN, Nataraj B. Prediction of outcome and prognosis of patients on mechanical ventilation using body mass index, SOFA score, C-reactive proteins and serum albumin. Indian J Crit Care Med. 2011;15:82–7. doi: 10.4103/0972-5229.83011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Högger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–90. [PubMed] [Google Scholar]
- 4.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 5.Zuwa³a-Jagie³³o J. Haemoglobin scavenger receptor: Function in relation to disease. Acta Biochim Pol. 2006;53:257–68. [PubMed] [Google Scholar]
- 6.Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom. 2005;63:16–22. doi: 10.1002/cyto.b.20031. [DOI] [PubMed] [Google Scholar]
- 7.Graversen JH, Madsen M, Moestrup SK. CD163: A signal receptor scavenging haptoglobin-haemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34:309–14. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T, Goyert S, Kikutani H, Mason D, Miyasaka M, Moretta L, et al. CD antigens 1996. Blood. 1997;89:3502. [PubMed] [Google Scholar]
- 9.Zwadlo G, Voegeli R, Schulze Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 10.Schaer DJ, Boretti FS, Hongegger A, Poehler D, Linnscheid P, Staege H, et al. Molecular cloning and characterization of the mouse CD163 homologue a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics. 2001;53:170–7. doi: 10.1007/s002510100304. [DOI] [PubMed] [Google Scholar]
- 11.Møller HJ, de Fost M, Aerts H, Hollak C, Moestrup SK. Plasma level of the macrophage-derived soluble CD163 is increased and positively correlates with severity in Gaucher's disease. Eur J Haematol. 2004;72:135–9. doi: 10.1046/j.0902-4441.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita N, Kashiwagi M, Wait R, Nagayoshi R, Nakamura M, Matsuda T, et al. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin Exp Immunol. 2002;130:156–61. doi: 10.1046/j.1365-2249.2002.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeten D, Møller HJ, Delanghe J, Veys EM, Moestrup SK, De Keyser F. Association of CD163+macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheum. 2004;50:1611–23. doi: 10.1002/art.20174. [DOI] [PubMed] [Google Scholar]
- 14.Groselj-Grenc M, Ihan A, Derganc M. Neutrophil and monocyte CD64 and CD163 expression in critically ill neonates and children with sepsis: Comparison of fluorescence intensities and calculated indexes. Mediators Inflamm 2008. 2008 doi: 10.1155/2008/202646. 202646. [DOI] [PMC free article] [PubMed] [Google Scholar]


