Abstract
Shweta Musali (Chlorophytum borivilianum (CB)) is a traditionally used herb for its benefits in male sexual and general health. In the recent past, the herb has attained much commercial significance, both in domestic and international markets. However, limited clinical data is available to establish its traditional claims. The present study was aimed at evaluating the effect of the water soluble extract of CB root tubers on semen and testosterone in healthy adult males. The research was designed as a randomized, double-blind, placebo-controlled, trial upon the volunteers registered from the outpatient department (OPD) with age ranging from 20 to 40 years. Water extracts of CB and placebo was administered in the patients of groups A and B, for 12 weeks, in two divided doses of 500 mg. Assessment was done based upon Semen (Volume, Liquefaction Time, Sperm Count, Sperm motility) and Serum Testosterone levels parameters. Highly significant improvement was noted in the above parameters after administration of CB extract in comparison to Placebo. Hence it was concluded that the trial drug was effective in improving male sexual health.
Keywords: Chlorophytum borivilianum, Shweta Musali, semen, testosterone
Introduction
Vajikarana has remained one of the major healthcare segments from yore, because this is said to enhance not only sexual performance, but also improves the quality of the off-springs.[1] The segment has become more important in the recent past, because the changing lifestyle is taking a toll on the status of male sexual health in the global population. The incidence of infertility, erectile dysfunction, subnormal desire and performance in sexual intercourse are increasing alarmingly.
Approximately 15-20 percent of all cohabiting couples are infertile. Up to 50 percent of these cases belong to male infertility, which means that nearly 7.5 to 10 percent of all men in the reproductive age group are infertile. Erectile dysfunction is said to afflict as much as 10 percent of the male population.[2]
Conventional medicine has discovered few chemicals in this segment, but these agents are associated with many unwanted and serious adverse effects. Therefore, traditional medicines like Ayurveda and Chinese medicines have been the mainstay in this segment. However, owing to the associated hype and mystic, the activities of genuine herbs are subjected to criticism due to lack of scientific validation. The answer to this pessimism lies in the appropriate scientific evaluation of the genuine herbs improving male sexual health.
Chlorophytum borivilianum (CB) or Shweta Mushalee, popularly known as Safed Musli is an important herb in the Ayurvedic Materia Medica. The root tuber of this small, annual herb is used for its versatile Shukrala (beneficial effect on male sexual health), Rasayana (adaptogenic activity), and Balya (general health tonic) properties.[3] Documented first in Rajanighantu, this herb has been in wide clinical and cultural use for its benefits in male sexual health. Owing to its marked clinical results, CB is being promoted as herbal Viagra. Experimental studies reaffirm its role in sexual behavior, spermatogenic activity,[4,5] immunomodulatory activity,[6] anti-stress and anti-oxidant activities.[7] Clinical trials also confirm its positive impact on sexual behavior, sperm count, and so on.[8,9]
The present study was designed to assess the effect of CB on the semen and testosterone of healthy male adult volunteers in the age group of 20 to 40 years.
Objective
To assess the effect of the water extract of Chlorophytum borivilianum root tubers on semen and testosterone in healthy adult males.
Materials and Methods
Study design
A randomized, double blind, placebo-controlled trial.
Study population
The study population was made up of apparently healthy and consenting male volunteers between 20 to 40 years of age, registered at the OPD of NIA, Jaipur. The study followed the concept of Vajikarana in Ayurveda, which stated that Vajikarana should be administered on a daily basis (Nitya)[10] to disease-free individuals (Kalya) during a young age (Udagra/Tarunavaya).[11] Thus, the age of the study population was stratified at 20-40 years. The total number of 30 volunteers were registered after taking due consent and were randomly assigned to two groups A and B.
Trial drug
Botanically authenticated dried root tubers of C. borivilianum were sourced from the cultivated fields at Shivagangai Farm of Century Agrotech Limited, Chennai. Water extract of the quality-assured samples was prepared and capsulated in soft-gelatin capsules of 500 mg each. Barley powder was encapsulated in similar capsules as placebo in the study dose, because it had practically no effect on semen and testosterone.
Dose, duration, and administration
The trial drug was given as capsules of 500 mg, twice daily, before food, with normal water, for 12 weeks. The volunteers were dispensed the required quantity of the trial drugs on every third week for better compliance.
Grouping of the volunteers
Registered and screened volunteers were randomly divided into two groups A (trial group) and B (placebo group). The trial drug and the placebo were sealed in plastic containers containing 180 capsules. These containers were coded by a person not related to the study. The coding documents were sealed and kept under safe custody. The envelope was opened after completion of the trial, to decode it for interpretation of the observations.
Volunteers Inclusion criteria
Apparently healthy male volunteers of age 20 to 40 years
Had not participated in a similar clinical investigation in the past 12 weeks
Was willing to give written informed consent and come for regular observation.
Volunteers exclusion criteria
Volunteers suffering from any organic disorder
Volunteers taking or likely to take steroid therapy
Volunteers taking other medicine for the same purpose.
Volunteers discontinuation/withdrawal criteria
Any adverse/serious adverse event is encountered, where continuation of the study is a serious risk to the volunteer
Volunteers not complying with the protocol
Volunteers not turning up for assessment in time
Volunteers expressing a desire to withdraw.
Criteria of Assessment
The enrolled volunteers were assessed at baseline (day 0 visit), and then after the end of the trial, that is, the twelfth week of medication, for the following parameters:
Serum Testosterone
Semen Analysis
Volume
Sperm Count
Sperm Motility
Liquefaction Time.
Statistical method
The values of the above parameters were recorded before and after the treatment for both the groups and were analyzed by using the Student's paired ‘t’ test.
Observation and Results
The values of various laboratory parameters of both the Groups A (CB) and B (Placebo) and their statistical analysis revealed the following observations [Table 1]
Table 1.
Effect of Shweta Musali (C. borivilianum) on various parameters after 12 weeks of administration and comparison with the Placebo Group (No. of volunteers - 15 in each group)
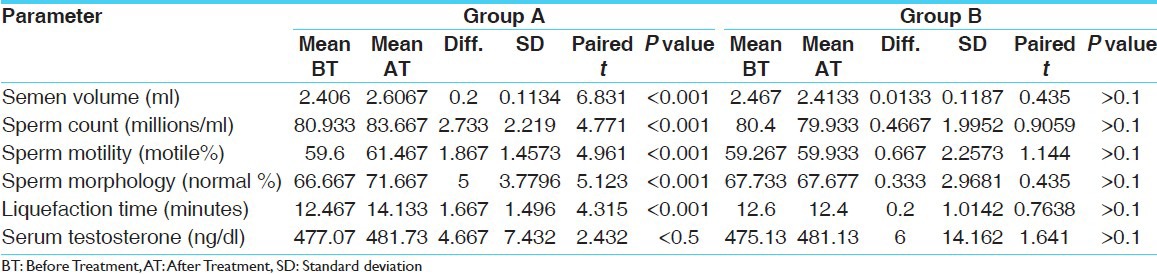
Statistically highly significant increase in semen volume, sperm count, sperm motility, and percent normal sperm morphology. Semen liquefaction time was observed in Group A in comparison to Group B
Statistically significant improvement was observed in serum testosterone in Group A when compared with Group B.
Discussion
C. borivilianum is a popular drug, and its root tubers are used for improving male sexual health. The water extract of the plant has shown similar activity and has been found to be safe. The study aims to reaffirm the traditional claim that the extract of this plant improves male sexual health, by assessing its effect on semen and testosterone, which are the nearest equivalents of Shukradhatu.
The treated group showed a statistically highly significant improvement in all the assessed parameters of semen, whereas, the placebo group showed no significant improvement in these parameters. The treated group showed statistically significant improvement in serum testosterone, but in a lesser degree in comparison to the semen parameters.
The most significant changes were seen in the semen volume and sperm count, in comparison to sperm motility, indicating the greater selective role of the trial drug in these two parameters. The trial drug contains saponin and stigmasterol, which are hypothesized to stimulate the process of spermatogenesis and the mucilage present in the trial drug is supposed to have a role in increasing the volume of semen.
The trial drug did not exert such a significant effect on the serum testosterone level of the volunteers, although the improvements were better than in the placebo group. This could be due to the presence of normal levels of serum testosterone in almost all volunteers, leaving very little scope for improvement. Another trial involving subjects with diminished serum testosterone might clarify its activity, because C. borivilianum contains stigmasterol which hypothetically should have an effect on testosterone.
The absence of any adverse drug reactions (ADRs) suggests that C. borivilianum is a well-tolerated medicine in the trial dose and can be used for a longer duration.
Conclusion
The findings of the study prompt the authors to suggest that, Water extract of Chlorophytum borivilianum improves the quantity and quality of semen in a statistically significant manner in healthy male adults between 20 to 40 years of age, in comparison to the placebo, when used for 12 weeks, in a dose of 500 mg b.i.d. Water extract of Chlorophytum borivilianum does improve the serum testosterone level in a majority of volunteers in a trial dose, in comparison to the placebo, but a statistically significant effect was not observed. No volunteer developed any ADR, confirming its safety for human use. Therefore, the study corroborates the traditional claim that Chlorophytum borivilianum is a Shukraldravya, as it improves the semen and testosterone, the commonly accepted equivalents of Shukradhatu.
REFERENCES
- 1.Agnivesha, Charaka, Dridhabala . New Delhi: Rashtriya Samskrita Samsthan; 2006. Chikitsa sthana, 1st Chapter, 1st pada, 9-10th Verse with Ayurveda Dipika commentary of Chakrapani Dutta, Pt. Yadavji Trikamji Acharya editor; p. 377. [Google Scholar]
- 2. [Last accessed on 2011 Jun 01]. Available from: http://www.androgen.com .
- 3.Pandit N, Raja Nighantu, editors. 2nd ed. Varanasi: Krishna Das Academy; 1998. Indradeo Tripathi; p. 209. [Google Scholar]
- 4.Kenjale R, Shah R, Sathaye S. Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytother Res. 2008;22:796–801. doi: 10.1002/ptr.2369. [DOI] [PubMed] [Google Scholar]
- 5.Thakur M, Dixit VK. Effect of Chlorophytum borivilianum on androgenic and sexual behavior of male rats. Indian Drugs. 2006;43:300–6. [Google Scholar]
- 6.Kenjale RD, Shah RK, Sathay SS. Anti-stress and anti-oxidant effects of roots of Chlorophytum borivilianum (Santa Pau and Fernandes) Indian J Exp Biol. 2007;45:974–9. [PubMed] [Google Scholar]
- 7.Thakur M, Bhargava S, Dixit VK. Immunomodulatory activity of Chlorophytum borivilianum Sant. F. Evid Based Complement Alternat Med. 2007;4:419–23. doi: 10.1093/ecam/nel094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rath S, Sharma MC. Jaipur: National Institute of Ayurveda; 2002. Pharmacological Evaluation of Chlorophytum borivilianum, M.D. dissertation; pp. 134–5. [Google Scholar]
- 9.Venkatswarulu B. Andhra Pradesh: NTR Health University; 2001. Effect of Safed Musli and Krishna Musli in ksheen shukra (oligospermia), M.D. dissertation; pp. 168–9. [Google Scholar]
- 10.Agnivesha, Charaka, Dridhabala . New Delhi: Rashtriya Samskrita Samsthan; 2006. Charakasamhitha, Chikitsa sthana, 2nd Chapter, 1st pada, 3rd Verse with Ayurveda Dipika commentary of Chakrapani Dutta, Pt. Yadavji Trikamji Acharya editor; p. 390. [Google Scholar]
- 11.Sushruta, Sushruta Samhita, Chikitsasthan . 26th Chapter, 3rd Verse, with Nibandha Samgraha commentary of Dalhana. In: Pt. Yadavji Trikamji., editor. Varanasi: Chaukhamba Samskrit Pratisthan; 2006. p. 497. [Google Scholar]


