Abstract
Triphaladi Kwatha, a polyherbal Ayurvedic formulation, is recommended by Chakradatta and Yogaratnakara in the management of Prameha which has resemblance with type 2 diabetes mellitus. The present study deals with development of pharmacognostical and preliminary pharmaceutical profile of Triphaladi granules. The pH (5% aqueous extract) was 6.0, water-soluble extract 48.66% w/w, alcohol-soluble extract 33.91% w/w, ash value 5.97% w/w, and loss on drying at 105°C was 6.53% w/w. High performance thin layer chromatography were carried out after organizing appropriate solvent system in which maximum nine spots were distinguished and few of the Rf values were identical in the alcoholic extract.
Keywords: High performance thin layer chromatography, Pharmacognosy, Prameha, Triphaladi granules
Introduction
Recent years have witnessed a gradual decline of medicinal plant availability as well as the number of tribal traditional healers and their medicinal knowledge. Herbal medicines have a long history for their therapeutic application and are still serving many of the health needs of a global population. However, the quality control and quality assurance still remains a challenge because of the high variability of chemical components involved. Herbal drugs, singularly or in combination, contain numerous active principles in complex matrices in which no single active constituent is responsible for the overall efficacy. This creates a challenge in establishing specific quality control standards of finished products.
Administration of drug in various dosage forms provides an opportunity to the physician to choose better options. Various dosage forms have been described in Ayurvedic texts. One among them is Kwatha,[1] which is highly effective when used freshly prepared, but they are often overlooked due to preparation method and palatability. In the present study, Triphaladi Kwatha,[2] a known formulation used in Prameha (type 2 diabetes mellitus), was converted into granules by Rasakriya method to make it palatable, easy to dispense and for dose fixation, etc. As no standard finger print is available for this compound formulation, an attempt has been made to evolve preliminary physico-chemical profile of Triphaladi granules (TG).
Materials and Methods
Collection and authentication of raw drugs
The formulation composition of TG was obtained from Pharmacy of Gujarat Ayurved University, Jamnagar. The API standards were used for pharmacognostical authentication of Amalaki,[3] Asana,[4] Bibhitaki,[5] Haritaki,[6] Kutaja,[7] Daruharidra,[8] and Musta[9] based on the morphological features, organoleptic characters and powder microscopy of individual drugs.
Method of preparation of Triphaladi granules
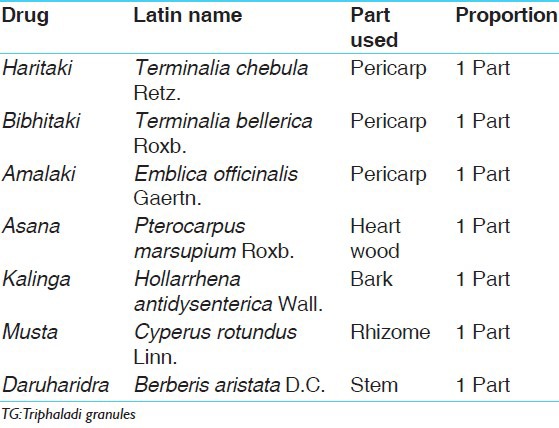
Dried raw materials in equal proportions [Table 1] were crushed to prepare coarse powder separately and mixed with 8 parts of water in a stainless steel container. Continuous mild heat was applied until it was reduced to one-fourth of its initial quantity. During heating process, continuous stirring was done to facilitate the evaporation and avoid any deterioration due to burning of materials. After a desirable reduction in volume was achieved, Kwatha was filtered through single folded cotton cloth and collected into a separate vessel.
Table 1.
Ingredients of TG
Subsequently, the Kwatha was boiled again over slow fire on a gas stove, maintaining the temperature between 90°C and 95°C till a semisolid consistency was obtained. As the water evaporated, the viscosity of the extract increased, resulting in solid mass (Ghana).[10] Ghana was mixed with 10% of the fine powder of Triphaladi Kwatha.
The solid mass (Ghana) was passed through a sieve no. 8 to prepare granules and were then dried at 50°C in a hot air oven for 5 h.[11]
Pharmacognostical evaluation
Pharmacognostical analysis of TG comprises of organoleptic characters [i.e., color, odor, taste, and texture, was recorded] and microscopic studies. Small Quantity of TG was dissolved in distilled water, filtered through filter paper. The precipitate was treated with and without stain to find out the lignified material along with other cellular components. These findings were compared with the characters of individual components of TG. The microphotographs were taken under Carl Zeiss Binocular microscope attached with camera.[12,13]
Physicochemical evaluation
TG were analyzed through relevant physicochemical parameters such as loss on drying, ash value, water-soluble extract, alcohol-soluble extract, and pH value.[14,15,16] In qualitative analysis, presence of glycosides, tannins, and flavonoids were assessed. High performance thin layer chromatography (HPTLC) is carried out with methanolic extract of TG.[17,18]
High performance thin layer chromatography
Methanolic extract of TG was spotted on pre-coated silica gel GF 60254 aluminum plate by means of Camang Linomat V sample applicator fitted with a 100-μL Hamilton syringe. Chloroform: MeOH (9:1) was used as the mobile phase. After development, densitometric scan was performed with a Camag TLC scanner III in reflectance absorbance mode at UV detection as 254 nm and 366 nm under the control of Win CATS Software (V 1.2.1. Camag). Then the plate was sprayed with vanillin sulfuric acid followed by heating and then visualized in daylight.
Observations and Results
Pharmacognostical analysis
Detailed pharmacognostical evaluation was carried out for all the ingredients of TG. Organoleptic characters of TG are placed in Table 2 powder microscopy of TG showed striking characters of all individual components of TG. Microscopy of Terminalia chebula Retz. showed features like brownish content [Figure 1a], fibers and sclereids [Figure 1b], pitted fibers [Figure 1c], rosette crystals [Figure 1d], sclereids and stone cells [Figure 1e], starch grains [Figure 1f], tannin contents, fibers, etc., Emblica officinalis Gaertn. showed group of fibers [Figure 1g], pitted stone cells [Figure 1h], prismatic crystals of calcium oxalate [Figure 1i], spiral vessels [Figure 1j], and yellowish colored parenchyma cells in surface view. Whereas Terminalia bellerica Roxb. presented with epidermal cells in surface view [Figure 2a], pitted sclereids [Figure 2b], starch grains [Figure 2c], pitted vessel and group of fibers [Figure 2d], rosette crystals and brownish colored matter [Figure 2e], stone cells and sclereids [Figure 2f], etc., Berberis aristata D.C. consisted of cork cells in surface view, group of fibers [Figure 2g], pitted sclereids and parenchymas [Figure 2h], pitted stone cells [Figure 2i], pitted vessel [Figure 2j], prismatic crystals [Figure 2k], starch grains, and yellowish and brownish colored content [Figure 2l]. Pterocarpus marsupium Roxb. Showed pitted vessel [Figure 2m], group of fibres [Figure 2n], parenchyma cells having brownish deposition [Figure 2o], and prismatic crystal [Figure 3a]. Hollarrhena antidysenterica Wall. consisted of cork cells in surface view, parenchyma cells embedded with starch, pitted sclereids and stone cells [Figure 3b], pitted vessel and stone cells [Figure 3c], prismatic crystals and brownish colored content [Figure 3d], and starch grains [Figure 3e]. Whereas Cyperus rotundus Linn. presented with brownish content, cork cells in surface view [Figure 3f], group of fibers [Figure 3g], parenchyma cells having brownish content [Figure 3h], reticulate vessel [Figure 3i], and starch grains [Figure 3j]. The diagnostic features obtained by powder microscopy were found to be complying with the standards mentioned at respective volumes of API.
Table 2.
Organoleptic characters of TG
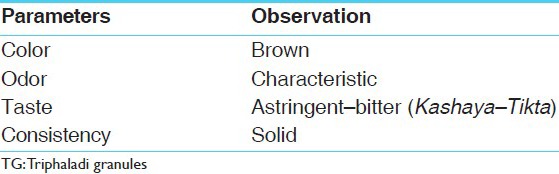
Figure 1.
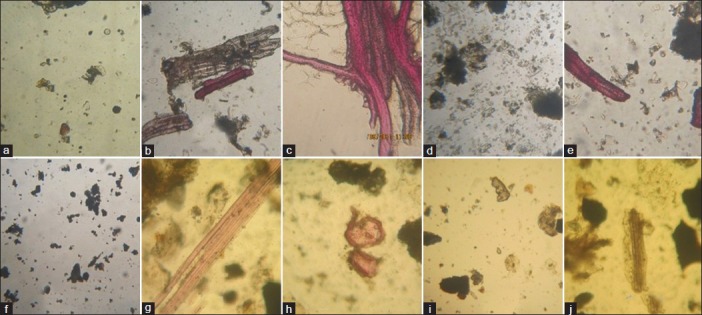
Microscopic characters: (a) brownish content; (b) fibers and sclereids; (c) pitted fibers; (d) rosette crystals; (e) sclereids and stone cells; (f) starch grains; (g) group of fibers; (h) pitted stone cells; (i) prismatic crystal of calcium oxalate; (j) spiral vessels
Figure 2.

Microscopic characters: (a) epidermal cells in surface view; (b) pitted sclereids; (c) starch grains; (d) pitted vessel and group of fibers; (e) rosette crystals and brownish colored matter; (f) stone cells and sclereids; (g) group of fibers; (h) pitted sclereids and parenchymas; (i) pitted stone cells; (j) pitted vessel; (k) prismatic crystals; (l) yellowish and brownish colored content; (m) bordered pitted vessel; (n) group of fibers; (o) parenchyma cells having brownish deposition
Figure 3.

Microscopic characters: (a) prismatic crystal; (b) pitted sclereids and stone cells; (c) pitted vessel and stone cells; (d) prismatic crystals and brownish colored content; (e) starch grains; (f) cork cells in surface view; (g) group of fibers; (h) parenchyma cells having brownish content parenchyma cells; (i) reticulate vessel; (j) starch grains
Physicochemical analysis
TG was analyzed using relevant physicochemical parameters at the pharmaceutical chemistry lab. The observations are presented in Table 3.
Table 3.
Physicochemical parameters

Preliminary phytochemical analysis and high performance thin layer chromatography
Qualitative tests
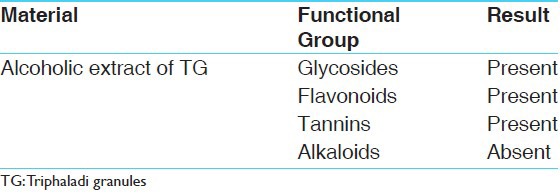
Presence of glycosides, flavonoids, and tannins was confirmed through the suitable tests while alkaloids could not be detected in TG [Table 4].
Table 4.
Functional groups of TG
HPTLC
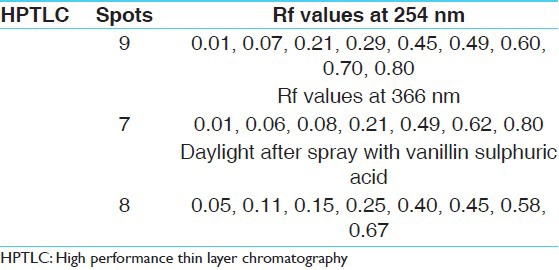
On analyzing under densitometer at 254 nm, the chromatogram showed nine peaks. While at 366 nm, the chromatogram showed seven peaks. When the plate was sprayed with vanillin sulphuric acid followed by heating and then visualized in daylight showed eight spots [Figures 4a and b, 5a-c, and Table 5].
Figure 4.
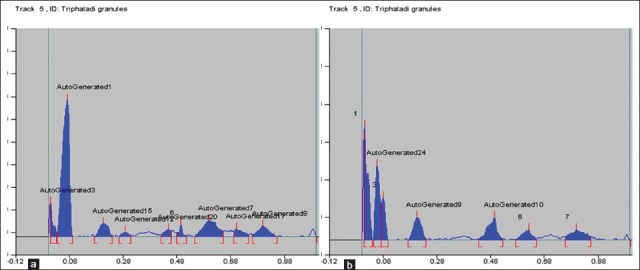
(a) Densitogram curve of methanol extract of Triphaladi granules at 254 nm; (b) densitogram curve of methanol extract of Triphaladi granules at 366 nm
Figure 5.
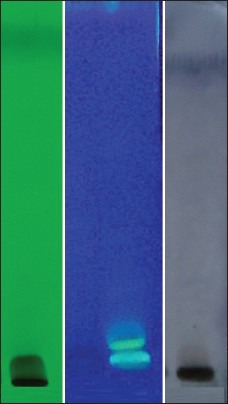
HPTLC: (a) 254 nm; (b) 366 nm; (c) after spray
Table 5.
HPTLC
Discussion
Powder microscopy of TG showed striking characters of all individual seven drugs of TG. This confirms the ingredients present in the finished product and there is no major change in the microscopic structure of the raw drugs during the pharmaceutical processes of preparation of granules. By preliminary qualitative analysis, presence of phytochemicals such as glycosides, flavonoids,[19] and tannins[20] was found.
Conclusion
Preliminary organoleptic features and results of powder microscopy reveal the presence of tannin contents, starch grains, pitted vessel, group of fibers, sclereids, stone cells. etc., This shows TG consist of all the ingredients. In preliminary physico-chemical analysis, water-soluble and alcohol-soluble extract, pH, ash value, and loss on drying were assessed. Preliminary qualitative analysis proves the presence of glycosides, flavonoids, and tannins. As no published information is available on physic-chemical profile of TG, this preliminary information can be used as standard in future.
Acknowledgments
Authors express their sincere gratitude to Prof. M. S. Bhagel, Hon’ble Director, IPGT and RA, and Dr. Ushanas Bhat, Pharmaceutical Laboratory, IPGT and RA, Gujarat Ayurved University, Jamnagar, for their valuable technical inputs and encouragement for this work.
REFERENCES
- 1.Charaka, Charaka Samhita. In: Ayurved Dipika, Commentary. Reprint ed. Acharya YT, editor. Varanasi: Chowkhamba Surabharati Prakashana; 2005. p. 31. [Google Scholar]
- 2.Tripathi I, Tripathi DS. Varanasi: Chowkhamba Krishnadas Academy; 2008. Yoga Ratnakar with vaidyaprabha Hindi commentary, Prameha chikitsa/73; p. 531. [Google Scholar]
- 3.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1; p. 5. [Google Scholar]
- 4.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1; p. 27. [Google Scholar]
- 5.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1; p. 45. [Google Scholar]
- 6.Anonymous . 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1; p. 74. [Google Scholar]
- 7.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1; p. 119. [Google Scholar]
- 8.Anonymous. 1st ed. Vol. 2. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1. [Google Scholar]
- 9.Anonymous. 1st ed. Vol. 3. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 1. [Google Scholar]
- 10.Shastri P. 4th ed. Varanasi: Chowkhamba Orientalia; 2000. Sharangadhara Samhita; p. 206. [Google Scholar]
- 11.Gupta AK. New Delhi: CBS Publishers and Distributors; 2002. Introduction to pharmaceutics – I. Reprint 2002; p. 254. [Google Scholar]
- 12.Khandelwal KR. Examination of powdered drugs. In: Khandelwal KR, editor. Practical Pharmacognosy techniques and experiments. 19th ed. Pune: Nirali Prakashan; 2008. pp. 162–6. [Google Scholar]
- 13.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 2. [Google Scholar]
- 14.Anonymous. 1st ed. Vol. 1. New Delhi: Ministry of Health and Family welfare, Department of AYUSH Government of India; 2001. The Ayurvedic Pharmacopoeia of India, Part 2. [Google Scholar]
- 15.Anonymous. Geneva: World Health Organisation; 1998. Quality control methods for medicinal plant materials. [Google Scholar]
- 16.Anonymous. New Delhi: CCRAS; 2005. Parameters for qualitative assessment of Ayurveda and Siddha drugs, Part A; p. 31. [Google Scholar]
- 17.Anonymous. Chennai: CSMDRI for Ayurveda and Siddha CCRAS, Govt. of India; 2008. Basics of Analytical and Phytochemical Analysis of Medicinal plants, analytical methods for ASU Single/Compound drugs sponsored by WHO; p. 11. [Google Scholar]
- 18.Stahl E. Berlin: Springer-Verlag; 1969. Thin layer chromatography a laboratory hand book; pp. 125–41. [Google Scholar]
- 19.Hii CS, Howell SL. Effects of flavonoids on insulin secretion and 45Ca2+handling in rat islets of Langerhans. J Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Kim JK, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr. 2005;135:165–71. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]


