Abstract
Lepidium sativum Linn. (Chandrashura) of Family Cruciferae (Brassicaceae) is being used by the people of Gujarat for treating inflammatory condition like arthritis. To evaluate its anti-inflammatory activity, Charles Foster albino rats were selected and experiments were carried out in three groups, therapeutic dose group, twice of therapeutic dose group and control group. In Carrageenan-induced paw oedema, the test drug produced moderate anti-inflammatory activity; however, the effect did not show statistically significant activity due to variation in the data of the control group. In formaldehyde-induced paw oedema in rats, the test drug produced moderate to significant suppression. This indicates that Chandrashura has a strong inhibitory effect on proliferation of fibroblasts and also probably has connective tissue modulation effect.
Keywords: Anti-Inflammatory, carrageenan and formaldehyde-induced paw oedema, Chandrashura (Lepidium sativum Linn.)
Introduction
The seed powder of Lepidium sativum Linn. has strong folklore claim to be effective in the treatment of arthritis[1] hence; it was explored for its anti-inflammatory activity by employing different experimental models. Inflammation is a dynamic multifactorial complex phenomenon important for the survival of the animal. A number of cellular and humoral factors take part in its initiation and maintenance. It is considered to occur in three distinct phases, each apparently mediated by a different set of mechanisms.[2] They are: (i) acute transient phase characterized by local vasodilatation and increased capillary permeability, (ii) a delayed sub-acute phase characterized by cell infiltration, mainly of leukocytes and phagocytic cells, and (iii) a chronic proliferative phase characterized by tissue degeneration and fibrosis formation.
Anti-inflammatory activity was evaluated by noting the effect of drug administration on carrageenan-induced hind paw edema and formaldehyde-induced edema. The test drug was evaluated in a battery of tests representing different phases of inflammatory process.
Materials and Methods
A suspension of Lepidium seed powder was made with sufficient quantity of distilled water according to the required dose. Charles Foster albino rats were selected for the animal study. Drug dose was calculated with the help of reference table[3] and the calculated dose was 550 mg/kg body weight of the rat. Drug was administered through oral route with the help of gastric catheter sleeved to a syringe. Animals were randomly divided into low-dose group and high-dose groups, and compared with a control group which was administered distilled water of the same volume. Institutional animal ethics committee had approved the experimental protocol (approval number IAEC 04-05/01/PhD.03) and the care of animals was undertaken as per the CPCSEA guidelines.
Carrageenan-Induced Paw Edema
The method of Winter et al.[4] was adopted to screen the anti-inflammatory activity of test drug seed against carrageenan-induced paw edema in rats. Rats of either sex, weighing between 150 and 240 g, were used. They were provided with food and tap water up to the start of the experiment. Initially, left hind paw volumes to the tibio-tarsal articulation were recorded by using a plethysmograph.
Procedure
One hour after the drug administration, edema was produced by injecting 0.1 ml freshly prepared 1% carrageenan in sterile saline solution to the sub-plantar aponeurosis of the left hind limb. The rats were administered tap water at a dose of 2 ml/100 g body weight to ensure uniform hydration. This is supposed to minimize the variation in edema formation. The paw volume was recorded at intervals of 1 h, 2 h, and 3 h. Results were expressed as percentage increase in paw volume at various intervals of time in comparison to the initial values.
Formaldehyde-Induced Paw Edema
Procedure
The procedure of Brownlee[5] was employed to screen the anti-inflammatory activity of the test drug against formaldehyde-induced paw edema in rats. Initial left hind paw volumes of all animals were noted before the drug administration, with the help of the plethysmograph. One hour after the drug administration, 0.05 ml of formalin was injected subcutaneously into left hind paw of each animal. Three hours after formalin injection, the paw volumes were noted again, and they were again noted after intervals of 24 h and 48 h. Results were expressed as percentage increase in paw volume at various intervals of time in comparison to the initial values. Percentage increase in paw volumes was calculated by subtracting the initial paw volumes from the paw volumes obtained after the injection of the phlogistic agent. The figure was divided by the initial paw volume and multiplied by hundred.
Statistical analysis
Student's “t” test for unpaired data was used for analyzing the data generated during the study. However, in case of comparing more than two samples, the analysis of variance (ANOVA) test was applied by using Dunnet's multiple “t” test.
Results and Observation
The data regarding the effect of test drug on carrageenan-induced paw edema in therapeutic and double dose levels have been shown in Table 1. At therapeutic dose level, a weak to moderate suppression of edema was observed at all the time intervals at which paw edema was measured. At higher dose level, the magnitude of suppression was much higher. However, the observed decrease at both the dose levels was found to be statistically nonsignificant in comparison to the control group.
Table 1.
Effect of test drug on carrageenan-induced paw edema in albino rats
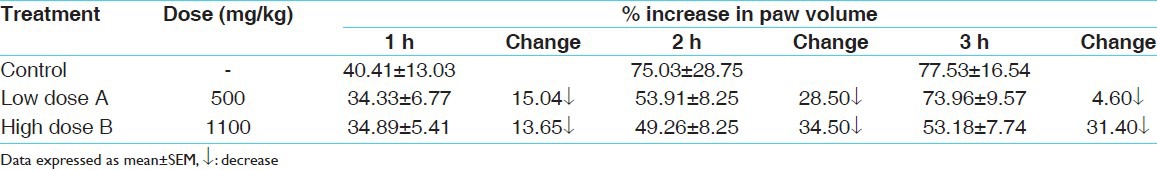
The data regarding the effect of test drug on formaldehyde-induced paw edema in therapeutic and double dose levels have been tabulated in Table 2. At both the dose levels, an apparent moderate suppression of formaldehyde-induced paw edema was observed at both the time intervals. However, only the suppression observed at the higher dose level at 24 h was found to be statistically significant in comparison to the control group.
Table 2.
Effect of test drug on formaldehyde-induced paw edema in albino rats
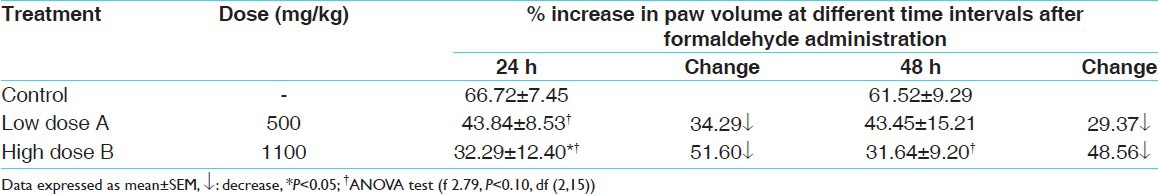
Discussion
Preliminary screening for anti-inflammatory activity was carried out with carrageenan-induced hind paw edema in rats. The test drug produced moderate anti-inflammatory activity; however, the effect did not reach statistically significant level due to variation in the data of the control group. The observed effect was also not dose dependent. This type of dose-independent activity is quite common with studies on medicinal plants.[6] It is believed to be due to the multicomponent nature of the plant-based medicinal preparations, which often contain active principles providing opposite effects. Hence, it is probable that the apparent activity observed might have been masked by the presence of opposite effect producing active principles that may be present in substantial amount, particularly at higher dose levels. Carrageenan-induced rat paw edema is considered to represent the first phase of the inflammatory reaction, which is characterized by fluid and cell exudation. A number of phlogistic mediators like histamine, serotonin, bradykinin, and prostaglandins have been implicated in the development of carrageenan-induced edema.[7] Since the test drug produced moderate edema suppression effect showing that it does possess anti-inflammatory activity, though a modest one at the dose level studied, it would be interesting to elucidate the probable mechanisms. Some of the possibilities are: (i) inhibition of formation and release of phlogistic mediators like prostaglandins, kinins, etc., (ii) modulation of reaction of mediators with their respective receptors, and (iii) blockade of receptor activity.[8]
Formaldehyde is reported to produce inflammation through proliferation and migration of fibroblasts, which are mainly concerned with the formation of connective tissue.[9] Hence, it is used as one of the models for assessing anti-proliferative effect. The test drug produced moderate to significant suppression of formaldehyde-induced paw edema in rats. This indicates that it has a strong inhibitory effect on proliferation of fibroblasts and also probably connective tissue modulation effect.
Conclusion
Anti-Inflammatory activity of Lepidium sativum seed powder was evaluated in two experimental models. In carrageenan-induced paw edema, the test drug produced moderate anti-inflammatory activity; however, the effect did not reach statistically significant level due to variation in the data of the control group. In formaldehyde-induced paw edema in rats, the test drug produced moderate to significant suppression.
The test drug at lower dose level was also found to have moderate anti-inflammatory activity in experimental models representative of both acute and chronic inflammation.
REFERENCES
- 1.Maharaj SK. Kaleda: Krusna Gopal Ayurved Bhavana; 1994. Gawo me Aushadha Ratna, Part 1. [Google Scholar]
- 2.Insel PA. The Pharmacological Bassis of Therapeutics. In: Gilman AG, Rall JW, Nies AS, Taylor P, editors. Vol. 1. New York: Peragaman Press; 1991. p. 639. [Google Scholar]
- 3.Paget GE, Barnes JM. Evaluation of drug activity: Pharmacometrics. In: Laurence DR, Bacharacha AL, editors. New York: Academic Press; 1969. [Google Scholar]
- 4.Winter CA, Risely EA, Nuss GW. Carrageenan induced edema in hind paw of the rat as assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee G. Effect of denycortone and ascorbic acid on formaldehyde-incuded arthritis normal and adrenalectomized. Lancet. 1950;1:157–9. doi: 10.1016/s0140-6736(50)90259-5. [DOI] [PubMed] [Google Scholar]
- 6.Ravishankar B. Ph.D Thesis. Gujarat, India: India. Institute for Post Graduation Teaching and Research in Ayurveda, Gujarat Ayurved University; 1992. Pharmacological and biochemical studies on strobilanthes species. [Google Scholar]
- 7.Hurley JV. Muir's Textbook of Pathology. In: Anderson JR, editor. London: ELBS Edward Arnold; 1985. p. 41. [Google Scholar]
- 8.Satoskar RS, Bhandarkar SD, Rege NN. 19th ed. Mumbai: Popular Prakashan Pvt. Ltd; 1995. Pharmacology and pharmacotherapeutics. [Google Scholar]
- 9.Parmar NS. Ph.D. Thesis. Madras, India: University of Madras; 1997. A pharmacological study on the effect of some bioflavonoids on experimentally induced inflammation, increated vascular permeability, gastric ulcer and galoctosemic cataracts. [Google Scholar]


