Abstract
Hepatoprotective activity of methanolic extract of Syzygium jambos (Alston) (Linn.) leaves against Paracetamol-induced hepatic damage in Wistar albino rats was observed at two different doses, 100 and 200 mg/kg body weight. The healthy control, disease control, and standard drug Silymarin-treated groups were also maintained for the comparison. The liver marker enzymes SGOT, SGPT, ALKP, Serum Bilirubin and other metabolic parameters like total cholesterol, HDL-cholesterol were evaluated in all the experimental groups. The changes in liver function parameters were significant in comparison to disease control group and the observed efficacy was comparable to standard drug. The efficacy of the extract was found to be dose dependent. The histopathology study of liver also supports the presence of hepatoprotective activity in S. jambos by showing improved cytoarchitecture of liver cells in the treated groups. The results obtained in this study indicate necessity for further research on isolation and characterization of functional molecules from the extract.
Keywords: Hepatoprotection, liver marker enzymes, paracetamol, Syzygium jambos
Introduction
Liver diseases resulting from liver damage is a global problem. A major causative factor is the increasing alcohol consumption in developed countries.[1] Malnutrition, anemia, infection, and availability of over-the-counter hepatotoxic drugs are the most frequent causes of liver damage in developing countries.[2] It is well recognized that free radicals are critically involved in various pathological conditions such as cancer, cardiovascular disorders, arthritis, inflammation and liver diseases.[3] Chemicals and drugs such as CCl4 and Paracetamol promote formation of free radicals and consequent lipid peroxidation damages the membranes of liver cells and organelles and causing the swelling and necrosis of hepatocytes. This becomes responsible for the release of cytosolic enzymes such as SGOT, SGPT, and ALKP into the circulating blood.[1,4] Indian medicinal plants and many herbal formulations belonging to the traditional systems of medicine like Ayurveda have been investigated as hepato protective drugs.[5] Less side effects and cost effectiveness are the added advantages of these drugs.
Syzygium jambos popularly known as Jambu is a small tree with spreading branches, leaves, simple, opposite, lanceolate, narrowed into short petioles, secondary nerves joined by a prominent looping intramarginal vein. Flowers greenish white in short terminal racemose cymes, stamens many, yellowish white, fruits pale yellow to pinkish white, globose, seeds 1-2, grey in large cavity of the succulent pulp. The bark is astringent, bitter, hemostatic, depurative, vulnerary, antidiarrheal and anthelmintic. S. jambos is used in traditional system of medicine for various clinical conditions like gout, hemorrhages, syphilis, leprosy, colic helminthiasis, wounds, and ulcer.[6]
The present study has been designed to evaluate the hepatoprotective activity of methanol extract of S. jambos leaves in the experimental animal models of Paracetamol-intoxicated Wistar albino rats.
Materials and Methods
Animals
Adult Wistar Albino Rats of either sex weighing between 150 to 200 g were used for the present study. The animals were housed in polypropylene cages and maintained under standard laboratory conditions (temperature 25 ± 2°C) with dark and light cycle (14/10 h). They were allowed free access to standard dry pellet feed (Amrut Feeds, Pune) and water ad libitum. The rats were acclimatized to laboratory condition for 30 days before commencement of experiment. All procedure described were reviewed and approved by the Institute Animal Ethics Committee (No. 612/02/A/CPCSEA/IAEC/CRIA/2006-07/02 dated 22-12-2006).
Chemicals
Methanol (Qualigens Fine chemicals limited, Mumbai), Paracetamol (GSK), Silymarin (Serum Institute of India, Pune), Sodium chloride (Merck Specialties Private limited, Mumbai), 40% Formaldehyde (Nice chemicals Private limited, Cochin), Serum Glutamate Pyruvate Transaminase (Transasia laboratories, Daman), Serum Glutamate Oxaloacetate Transaminase (Transasia Lab), Alkaline phosphatase and Total protein kits were obtained from Transasia Lab.
Experimental set up
The following experimental protocol was followed:[7]
Healthy Wistar albino rats were divided into five groups consisting of six animals each.
The first group (I) consisted of normal control rats which received single daily dose of distilled water throughout the experiment. The Paracetamol group (II) received single daily dose of distilled water for nine days and single dose of Paracetamol on day 8 (2.5 g/kg). The third group (III) was treated with standard drug Silymarin (100 mg/kg) on all nine days and Paracetamol (2.5 g/kg) on day 8, two hours after administration of Silymarin. The fourth group (IV) was treated with lower dose of methanol extract of S. jambos (Alston) (Linn.) (100 mg/kg) throughout the experiment and single dose of Paracetamol (2.5 g/kg) on day 8, two hours after administration of test extract. The fifth group (V) was treated with higher dose methanol extract of S. jambos (200 mg/kg) on throughout the end and Paracetamol (2.5 g/kg) on day 8, two hours after administration of test extract.
On tenth day of the experiment, rats were anesthetized by light chloroform anesthesia and the blood was withdrawn from retro-orbital plexus. The animals were fasted 12 hours before the collection of blood. After blood collection, the rats were sacrificed by cervical dislocation and their liver, kidney, and heart were excised, rinsed in ice-cold normal saline, and stored in refrigerator.
Biochemical studies
Serum was separated by centrifugation at 2500 rpm at 30°C for 15 minutes and utilized for the estimation of various biochemical parameters including Serum Glutamate Pyruvate Transaminase (SGPT),[8] Serum Glutamate Oxaloacetate Transaminase (SGOT),[9] Alkaline Phosphatase (ALKP),[10] Total bilirubin,[11] and Lipid profile.[12]
Histopathological investigation
Small pieces of liver tissues of each group of animals were stored in solution of commercial formaldehyde for histopathological studies.
Statistical analysis
Each experimental value is expressed as the Mean ± SEM. Statistical calculations of the data were performed using Student's t-test and ANOVA analysis. A probability of P < 0.05 and P < 0.01 was considered as significant.
Results
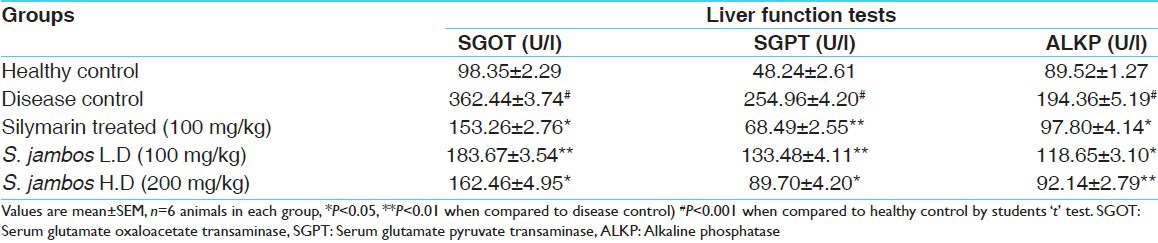
Administration of Paracetamol (2.5 g/kg, p.o.) resulted in a marked increase in the serum hepatic enzyme levels, SGOT, SGPT, ALKP, and serum Bilirubin as compared to the normal controls, which indicates liver damage. Pretreatment of the rats with methanol extract of S. jambos prior to Paracetamol administration caused a significant reduction in the values of SGOT, SGPT, ALKP activity and serum Bilirubin in dose-dependent manner almost comparable to the Silymarin-treated group [Table 1].
Table 1.
Efficacy of test extracts on liver function tests in Paracetamol intoxicated Wistar albino rats
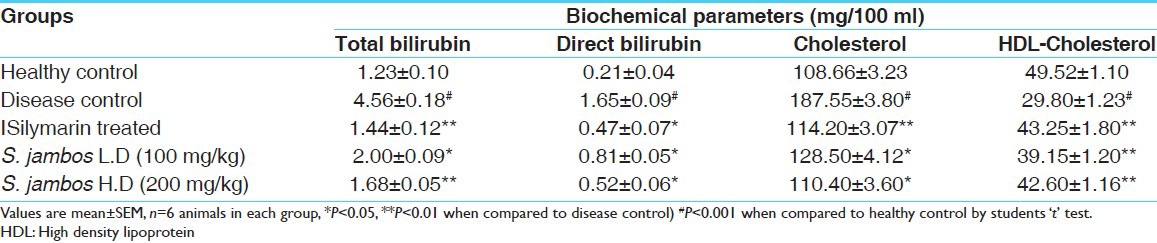
The other biochemical parameters like Cholesterol and HDL-Cholesterol were also analyzed for the understanding of extract's influence on fatty acid metabolism [Table 2] and the parameters were found to be influenced to significant extent. The Paracetamol control group showed increase in Cholesterol level and decreased HDL-Cholesterol level in comparison to the normal control. S. jambos -treated groups showed reduced level of total cholesterol (110.40 ± 3.60 mg/deciliter) and the elevated level of HDL-Cholesterol in serum. These effects were found to be statistically significant in comparison to paracetamol control group. The efficacy of the extracts was comparable with that of the standard drug Silymarin and activity was found to be dose dependent.
Table 2.
Effect of test extracts on biochemical parameters in experimental rats
The hepatoprotective effect of the S. jambos was confirmed by histopathological examination of the liver tissue. The histological architecture of paracetamol-treated liver sections showed extensive hemorrhage and necrosis in the liver parenchyma; hepatocytes showed vacuolated cytoplasm; and collections of inflammatory cells and siderophages [Figures 1 and 2]. Pretreatment with higher dose of the S. jambos extract prevented the toxicant-induced changes to significant extent -the sections were almost normal similar to the effect observed in the Silymarin-treated groups [Figures 3 and 4]. This shows presence of significant hepatoprotective effect in the test extracts. Paracetamol is a well-known antipyretic and analgesic agent, which is safe in therapeutic doses, but can produce fatal hepatic necrosis in man, rats, and mice in toxic doses.[13,14] It is used as an experimental hepatotoxic agent.[15,16] It is metabolized in the liver to extractable glucuronide and sulfide conjugates.[17] An obvious sign of hepatic injury is the leaking of cellular enzymes into the plasma due to disturbance caused in the transport functions of hepatocytes.[18,19] When liver cell cytoplasm is damaged, a variety of enzymes located normally in cytosol are released into the blood, thereby causing increased enzyme levels in the serum. The estimation of enzymes in the serum is a useful quantitative marker of the extent and type of hepatocellular damage.
Figure 1.
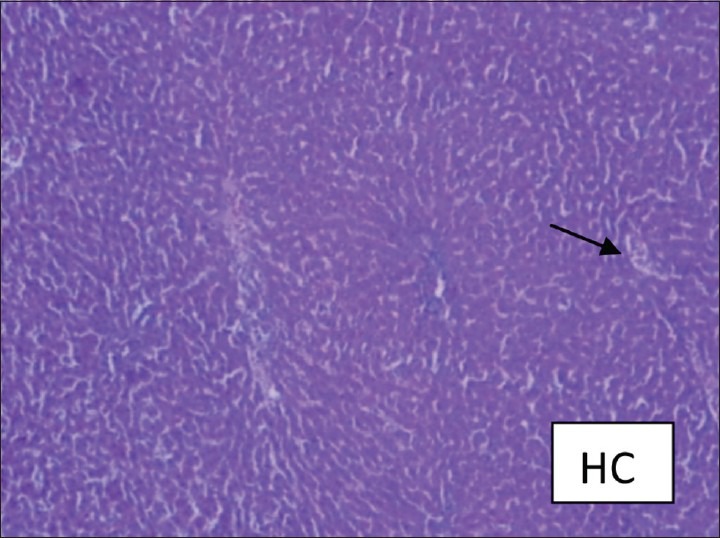
Healthy control rat showing normal hepatic nuclei and cytoplasm. Central venous system and sinusoidal spaces are normal
Figure 2.
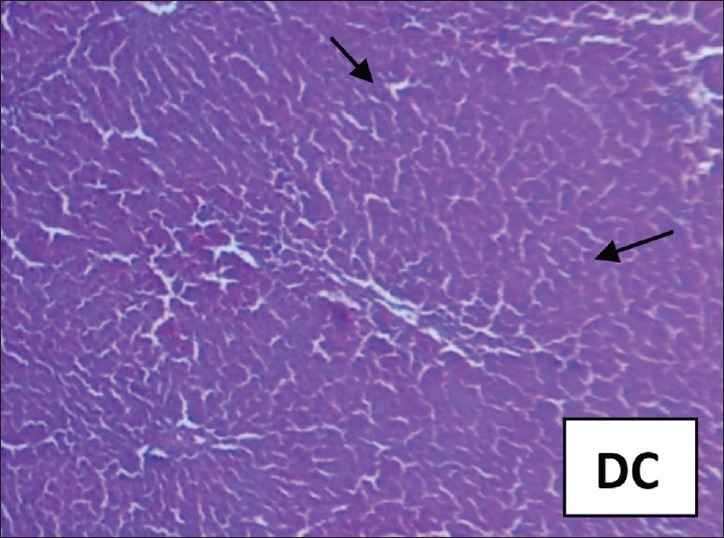
PCM treated rats: Liver showing extensive area of hemorrhage and necrosis in the liver parenchyma, hepatocytes show vacuolated cytoplasm. Collection of inflammatory cells and siderophages are observed
Figure 3.
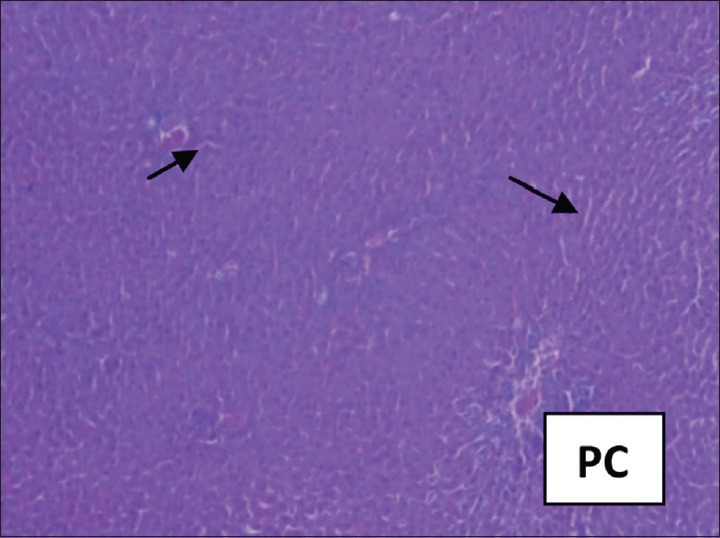
Silymarin-treated rat liver showing comparable normal architecture of liver. Some of the hepatocytes show vacuolated cytoplasm. Portal areas and central veins are normal
Figure 4.
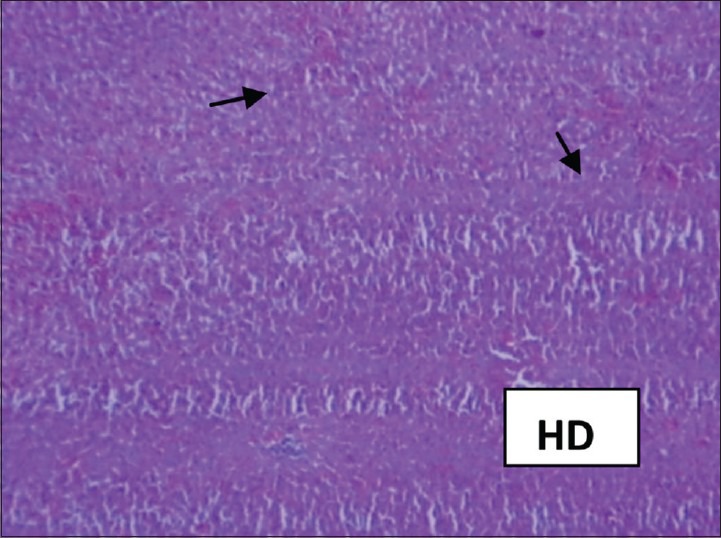
Syzygium jambos 200 mg/kg body weight treated group showing marked improvement over Paracetamol group. Hepatocytes show normal cytoplasm. Kupffer cells are normal
In the present investigation, the dose of Paracetamol administered has produced (2.5 g/kg) liver injury in rats. The rats treated with an overdose of Paracetamol developed significant hepatic damage, which was observed through a substantial increase in the concentration of serum parameters. The abnormal high levels of liver function test parameters like SGOT, SGPT, ALKP, bilirubin, and protein levels were observed in paracetamol control that indicate the damage to the hepatic cells. Pretreatment of the rats with methanol extract of S. jambos at 100 and 200 mg/kg, p.o., for 8 days before Paracetamol administration resulted in significant reversal of Paracetamol-induced elevation of serum marker enzymes.
The results are in agreement with the commonly accepted view that serum level of transaminase returns to normal with healing of hepatic parenchyma and the regeneration of hepatocytes.[17] Furthermore, the stimulation of hepatic regeneration was known to make the liver more resistant to damage by toxins.[20]
Conclusion
The present study revealed that the methanol extract of S. jambos leaves can be considered as a significant hepatoprotective agent in view of observed significant hepatoprotective activity in the model system. The results obtained suggest the necessity of carrying out further research on isolation and characterization of specific functional molecules to understand the exact mode of action.
REFERENCES
- 1.Nadeem M, Dangiya PC, Pasha KV, Imara M, Balani DK, Vohora SB. Hepatoprotectrive activity of Solanum nigrum fruits. Fitoterapia. 1997;58:245–54. [Google Scholar]
- 2.New Delhi: World Health House; 1992. WHO. Bulletin of Regional Health Information, 1980-1990: World Health Organization Regional Office for SE Asia. [Google Scholar]
- 3.Quambo X, Koji H, Yasuhiro T, Tadata T, Tsuneo N, Shigetoshi K. Hepatoprotective activity of Phenylehanoids from Cistanche deserticola. Planta Med. 1998;64:120–5. doi: 10.1055/s-2006-957387. [DOI] [PubMed] [Google Scholar]
- 4.Singh B, Saxena AK, Chandan BK, Anand KK, Suri OP, Suri KA, et al. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia. 1998;58:135–40. [Google Scholar]
- 5.Jose JK, Kuttan R. Hepatoprotective activity of Emblica officinalis and Chyavanprash. J Ethnopharmacol. 2000;72:135–40. doi: 10.1016/s0378-8741(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 6.Warrier PK, Nambiar VP, Ramankutty C. Vol. 5. Chennai: Orient Longman Ltd; 1996. Indian medicinal plants. A Compendium of 500 species; pp. 229–31. [Google Scholar]
- 7.Gupta AK, Misra N. Hepatoprotective activity of aqueous extract of Chamomile capitula in paracetamol intoxicated albino rats. Am J Pharmacol Toxicol. 2006;1:1–7. [Google Scholar]
- 8.Wolf PL, Williams D, Coplon N, Coulson AS. Low Aspartate transaminase activity in serum of patients undergoing chronic hemodialysis. Clin Chem. 1972;18:567–8. [PubMed] [Google Scholar]
- 9.Tietz NW. Philadelphia: W.B. Saunders; 1986. Text book of clinical chemistry; pp. 1388–91. [Google Scholar]
- 10.Wilkinsons JH, Winsten S. Evaluation of a new system for the kinetic measurement of serum alkaline phosphatase. Clin Chem. 1969;15:487–95. [PubMed] [Google Scholar]
- 11.Tietz NW. Philadelphia: W.B. Saunders; 1986. Text book of clinical chemistry; pp. 579–80. [Google Scholar]
- 12.Allain CC, Poon LS, Chan LS, Richmond W, Fu PC. Enzymatic determination of serum total cholesterol. ClinChem. 1974;20:470–5. [PubMed] [Google Scholar]
- 13.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol ExpTher. 1973;187:185–94. [PubMed] [Google Scholar]
- 14.Chen X, Sun CK, Han GZ, Peng JY, Li Y, Liu YX, et al. Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression. World J Gastroenterol. 2009;15:1829–35. doi: 10.3748/wjg.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torielli MV. Pathological aspects of liver injury produced by drugs. In: Slater TF, editor. Biochemical mechanism of liver injury. London, UK: Academic Press; 1978. p. 631. [Google Scholar]
- 16.Schmidt LE. Age and paracetamol self-poisoning. Gut. 2005;54:686–90. doi: 10.1136/gut.2004.054619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in an acetaminophen –induced liver injury. J Leukoc Biol. 2008;84:1410–21. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman HJ, Seeff LB. Enzymes in hepatic disease. In: Goodly EL, editor. Diagnostic Enzymology. Philadelphia, USA: Lea and Febiger; 1970. pp. 1–38. [Google Scholar]
- 19.Campion SN, Johnson R, Aleksunes LM, Goedken MJ, Rooijen NV, Scheffer LG, et al. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol. 2008;295:294–304. doi: 10.1152/ajpgi.00541.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satturwar PM, Fulzele, Joshi SB, Dorle AK. Hepatoprotective activityof Haridradighrita on carbon tetrachloride-induced liver damage in rats. Indian J Exp Biol. 2003;4:1447–51. [PubMed] [Google Scholar]


