Abstract
Rasamanikya a familiar drug, frequently used by Ayurvedic physicians. It also has a high demand in current pharmaceutical industry. Rasamanikya possesses different pharmaceutical methods with many a proved clinical studies. But it is of utmost importance to understand the safety profile of drug based on assurance which could be done by carrying out animal experimentation. In the present study, Rasamanikya was prepared with three methods. The toxicological study was carried out on acute and sub-acute toxicity of the drug. The three samples when compared together showed that Rasamanikya prepared out of classical Abhraka Patra method and modified Sharava Samputa method showed minimal histopathological changes proving its non-toxicity, whereas Rasamanikya prepared out of electric bulb method showed mild toxicity, but with chances of recovery. Acute toxicity study showed no immediate and evident toxic signs and mortality in histopathology reports and liver function test. However, sub-acute toxicity study showed mild to moderate fatty changes in liver.
Keywords: Histopathology, liver function test, Rasamanikya, toxicity study
Introduction
Rasamanikya, is a type of preparation made out of Shuddha Haratala (Orpiment). This name leads to some confusion as it neither contains Rasa nor the Manikya. It is named so as its final product colour resembles like Manikya (Ruby). It has been commonly used in various Kustha Roga (skin diseases), Shwasa (Bronchial Asthama), Vicharchika (Eczema), Bhaganadara (Fistula), Vatarakta (Gout) and Phirana Roga (Syphillis).[1]
These days there are lot of works and discussions going on globally about heavy metals and toxicity of heavy metal poisoning such as mercury, lead, arsenic etc., Hence, nowadays in the present era it has become very important to understand a drug by carrying out certain animal experimental studies as such studies provides a clear judgment and revalidate the efficacy of Ayurvedic drugs in living organisms.
Rasamanikya formulation is a very popular preparation used in clinical practice, but this particular preparation possesses different pharmaceutical methods described in classics showing vide variations in the therapeutic side. By keeping above facts in view the present study was taken up where in the preparation of Rasamanikya is carried out as per the classical method and adopted method. The results obtained after histopathological findings and liver function test (LFT) are tabulated after doing standard statistical analysis.
Aims and Objectives
-
Acute toxicity study:
- To find maximum tolerated dose (MTD) of Rasamanikya prepared with three methods
- To assess the significant toxicity/non toxicity of all the three samples.
-
Sub-acute toxicity study:
- To uncover a response that might not be evident after a single dose
- To form a basis for providing further long term sub-acute study by providing guidance on likely tolerated doses
- To assure the safety of Rasamanikya prepared with three methods.
Materials and Methods
Materials
Test drug
Raw Haratala (Orpiment) was collected from Shri Dharmsthala Manjunatheshwar Pharmacy, Udupi, Karnataka by considering Grahya Lakshanas.
Preparation of Rasamanikya was as per:
Test animals and housing
The Institutional Ethical Committee (IAEC) approved the experimental protocol SDMCAU IAEC558/C/2/2010 and registered it SDMCAU/ACA-15/IAEC/2009-2010. The present study was conducted on Wister strain albino rats considering the inclusion and exclusion criteria. The rats were maintained under strict laboratory conditions, controlled with environmental, temperature, humidity and light dark cycles. Rats were fed with balanced pelleted diet as prescribed by CFTRI and water.
Chemicals used during experimentation
Chloroform
Spirit
Formalin
Gum acacia (G.C).
Route of administration
The powder form of drug Rasamanikya was mixed with 1 ml of 2% solution of G.C and then oral administration was carried out with the help of infant feeding tube No-8. Tube was then fixed to the 2 ml syringe.
Methods
Design of acute toxicity test
Following the “up and down method” or popularly known as staircase method, determination of LD50 was carried out for the purpose of finding out the maximum non-lethal and the minimum lethal doses by using about 10 rats.[5]
Experimental evaluation of acute toxicity study of Rasamanikya (sample - R.M-I)
Two healthy male albino rats were taken, weighing about 200 g, kept in separate cages, which were fasted overnight. The rats were administered with 1 ml of 2% G.C solution +200 mg of (sample R.M-1), both rats had sedation effect after 10 min of feeding, later on after 24 h of observation no death was recorded.
The subsequent doses were increased by factor 1.5 as the dose was tolerated. The doses were given as 300 mg, 450 mg and 675 mg for 6 rats, in all the 6 rats very minimal changes were seen in their physiological activities and no death was recorded.
As the given dose was tolerable therefore further increase in the dose was done up to 1012.5 mg, 1518.75 mg and 2278.12 mg, in all the 6 rats no signs of toxicity and mortality were noticed even after 24 h of observation. Dose of 3417.18 mg/200 g body weight of the drug was administered as the highest one and again no sign of toxicity or mortality was observed in rats within 24 h, rats were kept under observation for 1 week to observe any mortality or abnormality in their activities.
Following the same procedure, the experimentation was conducted for sample R.M-II and sample R.M-III and it was observed that till 3417.18 mg/200 g no sign of toxicity and mortality was seen.
Design of sub-acute toxicity test of three sample of Rasamanikya
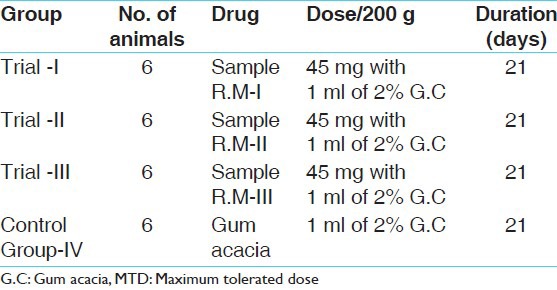
In this instance as the MTD was not traced out, so for designing the sub-acute study 10 times that of the therapeutic dose was used in the three groups and in the fourth group equal numbers of rats were fed with normal food and water [Table 1].[6]
Table 1.
The drug schedule (MTD) for sub-acute toxicity study
Procedure
Randomly either sex of rats falling under the inclusion criteria was taken for each group and they were starved for 12 h before the administration of medicine. The dose was administered by syringe and infant feeding tube. 25 g of pellet food and 20 ml of water was supplied for each rat daily.
Calculation of the dose
The normal human adult dose is 125-250 mg. Hence the suitable dose for rats was fixed as 45 mg/200 g of body weight of rat.
Study groups
Histopathological study
Histopathological study of liver-kidney and brain was carried out for all the study groups. The results are highlighted in Figures 1–4 and Tables 2-7.
Figure 1.
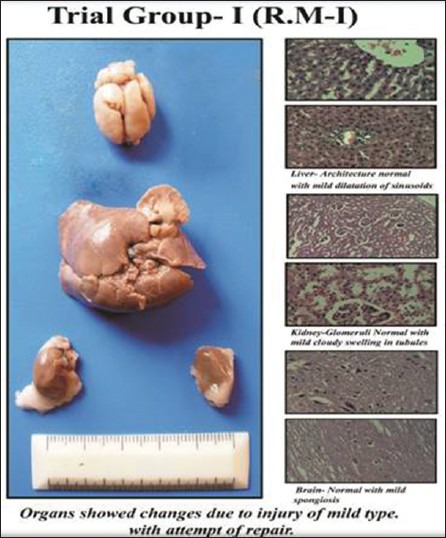
Trial Group-1
Figure 4.
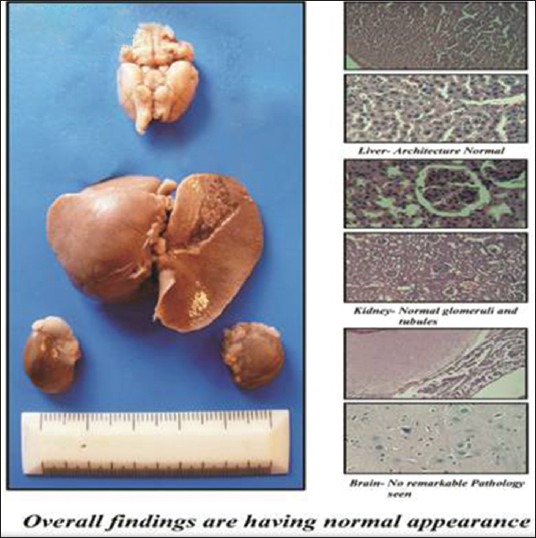
Control Group
Table 2.
Effect of Rasamanikya prepared with Abhraka Patra method-R.M-I in acute toxicity study
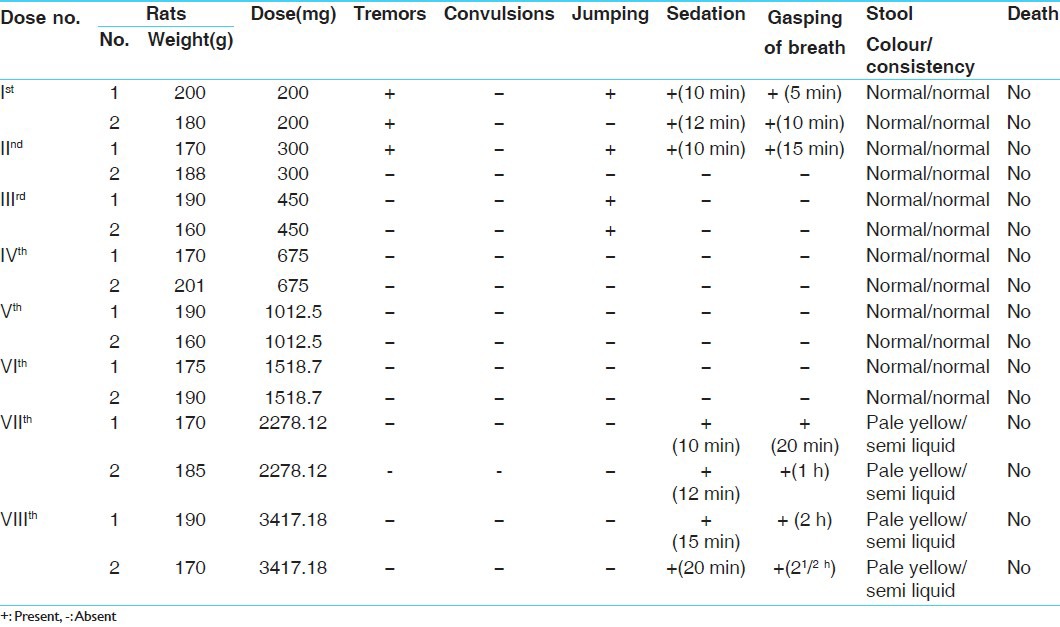
Table 7.
Master chart showing (one-way analysis of variance) in all groups
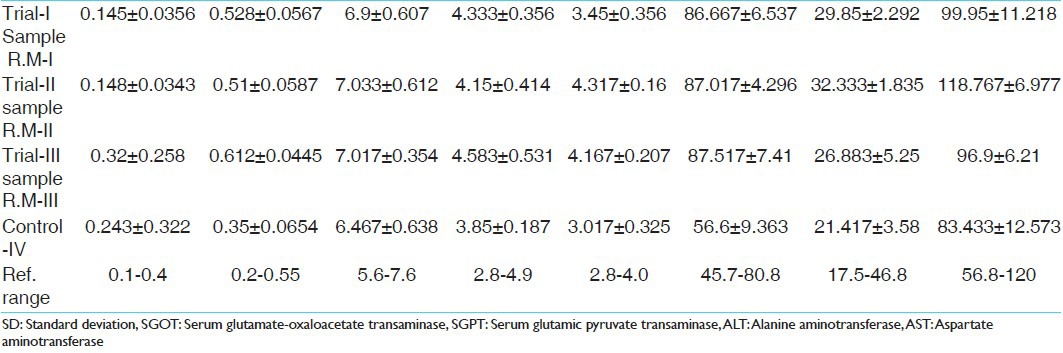
Figure 2.
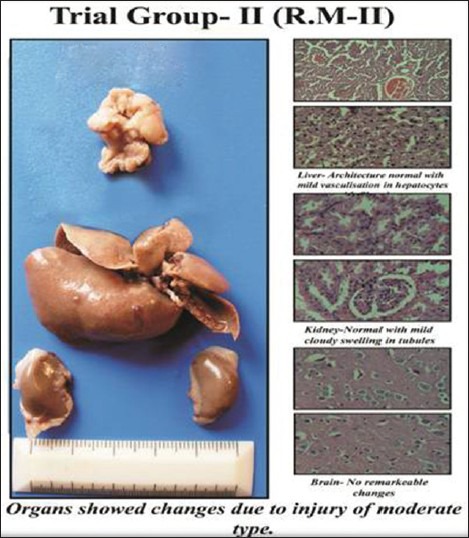
Trial Group-2
Figure 3.
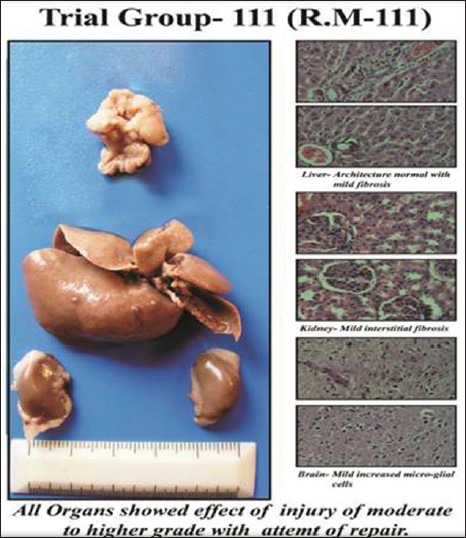
Trial Group-3
Table 3.
Effect of Rasamanikya prepared with modified Sharava Samputa method-R.M-II in acute toxicity study
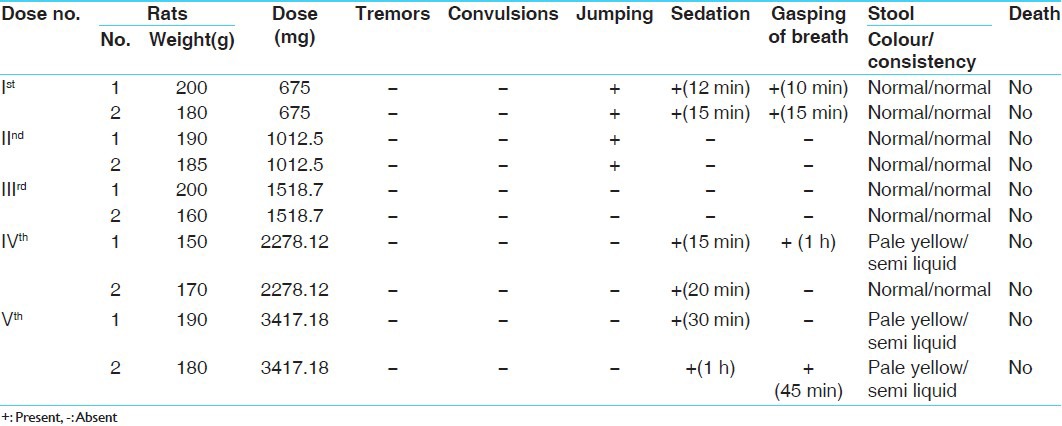
Table 4.
Effect of Rasamanikya prepared with electric bulb method-R.M-III in acute toxicity study
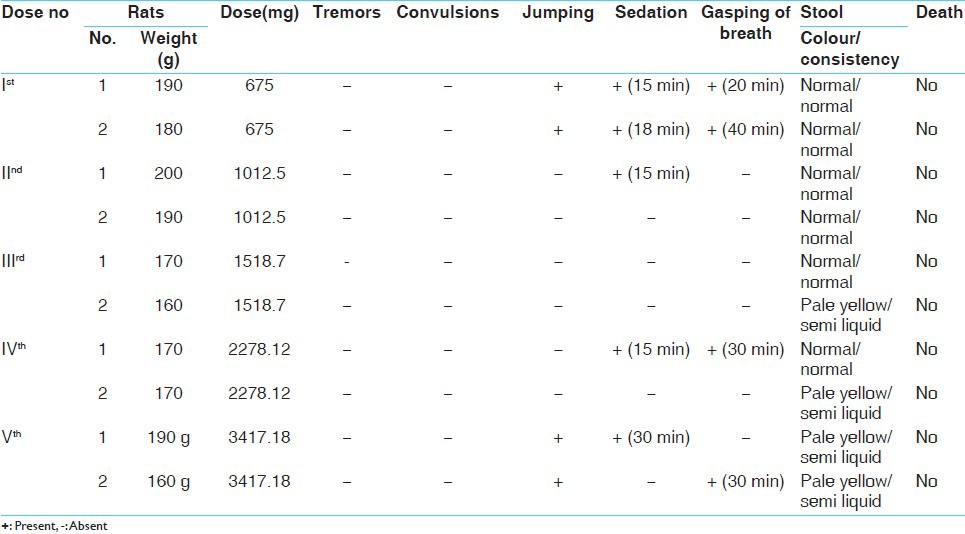
Table 5.
Changes observed in albino rats after administration of 1 ml of 2% G.C and R.M I-II and III for 21 days
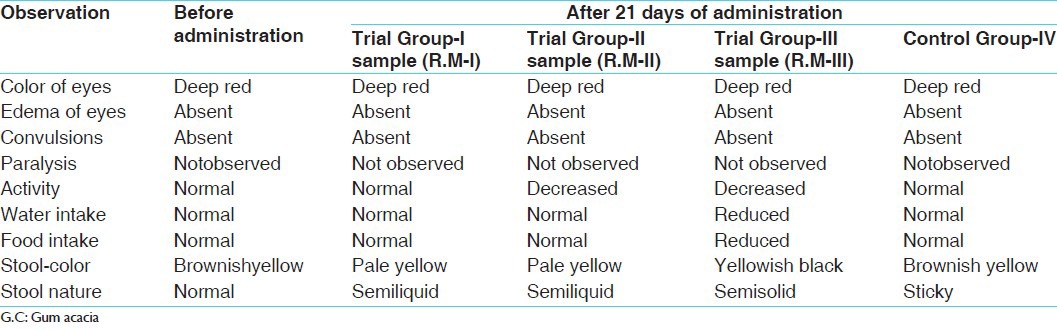
Table 6.
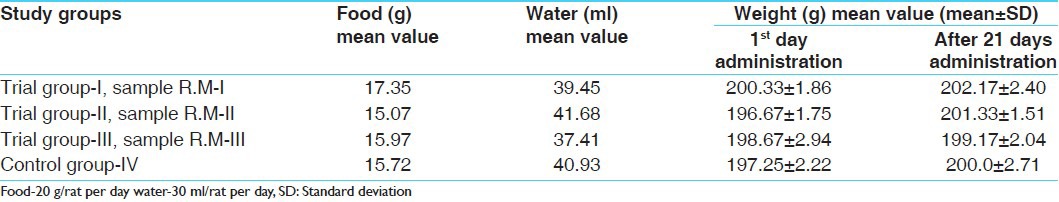
Master chart showing food and water intake and weight variation in sub-acute toxicity study (n=6)
Liver function test
Blood samples were collected prior administration of drug and after 21 days of trial drug administration. The results are highlighted in Tables 2-7.
Results and Observations of Acute Toxicity Study
The results and observations are highlighted below in a tabular manner:
Discussion
The main intention of conducting this study was to find out and understand various effects produced by the drug Rasamanikya in the present era when it is prepared with three different pharmaceutical procedures, though with the same drug Haratala (Orpiment).
The drug Rasamanikya prepared with three methods was subjected for both acute and sub-acute toxicity study on Wister strain albino rats.
In the acute toxicity study no immediate and evident signs of toxicity or mortality were observed. The maximum dose administered was 3417.18 mg/200 g body weight of rats, thereafter administration was stopped. It was observed that no signs of toxicity or mortality were there in 24 h of the time period and when the rats were kept under observation for 1 week. It therefore represents that the prepared Rasamanikya samples were practically non-toxic when administered for a short period of time, but the efficacy of the drug was better assessed when further sub-acute toxicity study was conducted.
During the acute study pale yellow and semi-liquid stools were observed in higher doses (1518.75 mg, 2278.12 mg and 3417.18 mg/200 g body weight) of all 3 samples suggestive of minor biliary clearance. After 1 week the mean value of food, water and body weight was calculated. Decrease in weight was noticed in all the three groups, which could be due to disproportionate food intake.
In this instance as the MTD was not traced out in the acute study, therefore for sub-acute toxicity ten times the therapeutic dose, i.e. 45 mg/200 g was given in all the three groups. After administration of drugs and during 21 days period, water intake was reduced to some extent, food intake was reduced in Trial Group-III, stool was pale yellow and semi-liquid in nature in Group II and IV may be due to minor to moderate biliary clearance.
The mean value of food, water and weight was calculated. The food intake and increase in weight were almost proportional in the groups. Significant increase in weight was noted in all the three samples, which may be due to the food consumption as even in the control group too increase in the weight was seen which was insignificant. All other rats showed a considerable increase in their weight including the control group, which might be due to the food intake. Water intake was not proportionate to its food intake and weight ratio, which may be because of some climatic changes.
After 21 days duration of drug schedule, from each group albino rats were anesthetized and scarified to obtain organs such as kidney, liver and brain. The tissues were subjected for histopathological study.
From the reports, it was evident that in the control group, no any remarkable damage was seen to brain, liver and kidney and overall findings were suggestive of normal appearance.
Trial Group-I (sample R.M-I) showed that Liver architecture was preserved with mild inflammation and dilatation of sinusoids, in kidney glomeruli appears normal with cloudy swelling of tubular cells and in brain mild neuronal cell enlargement. This group showed mild changes of injury with chances of healing and repairing with mild post-mortem changes. Altogether the study was within the normal limits.
Trial Group-II (sample R.M-II) showed that Liver lobular architecture was well-maintained and hepatocytes showed micro vesicular fatty droplet deposition indicating fatty liver (mild injury), in kidney cloudy swelling of tubular cells, some vessels showed sclerotic changes and in brain no remarkable changes were observed. This group showed changes due to injury of moderate type. Altogether the study was within the normal limits.
Trial Group-III (sample R.M-III) showed that liver architecture was normal with moderate inflammation and hepatocytes showed enlargement with duct proliferation and fibrosis, in kidney tubules showed granular material in cells and lumen with mild fibrosis and in brain increased microglial cells and increase in cell size. In this group, all organs showed effect of injury of moderate to higher grade with an attempt of repairing. Altogether, it showed some minor toxic effects.
Bio-chemical investigations (LFT) of sub-acute toxicity study
The LFT report results in relation with corresponding histopathological changes revealed that mild increased values of serum glutamate-oxaloacetate transaminase, alkaline phosphatase and total bilirubin indicative of mild liver dysfunction and bile duct injury may be correlated with the mild fatty changes of liver cells, which were noted in doses given in all the trial groups. These changes may be non-specific and reversible and may not be actually due to drug toxicity.
Although many works have been done in the past regarding the same drug[7,8] but this particular work gives a revalidation of safety and efficacy of the drug. Comparing all the above three Trial Groups of Rasamanikya, Trial Group-I (sample R.M-I) and Trial Group-II (sample R.M-II) are said to be as non-toxic whereas Trial Group-III (sample R.M-III) showed moderate to high grade injury changes with respect to the liver and kidney, which showed minor toxic effects, but the mild to moderate changes in rat cells with correspondence to human cells may be considered as non-specific and reversible.
Conclusion
Acute and sub-acute toxicity conducted among animals clearly indicate that no immediate and evident toxic signs and mortality were observed, but during histopathological and LFT study mild to moderate fatty changes in liver were seen, which was significant.
The three experimental groups when compared showed that Trial Group-I (sample R.M-I) prepared out of Abhraka Patra method and Trial Group-II (sample R.M-II) prepared out of modified Sharava Samputa method had a minimal histopathological changes proving its non-toxicity, whereas Trial Group-III (sample R.M-III) prepared out of Electric bulb method showed mild toxicity, but with a chance of healing and repairing. These toxic effects of mild to moderate changes in rat cells with correspondence to human cells may be considered as non-specific and reversible.
Acknowledgment
The author would like to acknowledge Dr. Mrs Pravina Santwani Professor and H.O.D, Department of Pathology, Shri M.P. Shah Medical College, Jamnagar, Dr. Laxmi Rao Professor and H.O.D, Department of Pathology, Kasturba Medical College, Manipal, Karnataka for helping in histopathological studies. Prof Ravishankar, Head/Chief Co-coordinator R and D Department, Shri Dharmasthala Manjunatheshwara College of Ayurveda, Udupi, Karnatala for his valuable guidance in the experimental study and last but not the least Mr. Naveen Chandra, Biochemist, Adarsh Hospital, Udupi, Karnataka for their co-operation in conducting bio-chemical tests.
REFERENCES
- 1.Acharya Agnivesha. Charak Samhita. In: Trikamji AY, editor. Ayurveda Deepika Commentary of Chakrapani Datta. 5th ed. Varanasi: Chaukhambha Sanskrita Samsthana; 2001. pp. 56–7. (84-5). 92-5. [Google Scholar]
- 2.Sadananda S. Talakadi Vignyanam. In: Haridatta S, editor. Rasa Tarangini. 11th ed. New Delhi: Motilal Banarasidas Publication; 2004. pp. 258–9. [Google Scholar]
- 3.Acharya Dhundhuknath. Hartala prakarana. In: Mishra SN, editor. Rasendra Chintamani, Siddhiprada Teeka. 1st ed. Varanasi: Chaukhambha Orientalia Publication; 2000. pp. 254–5. [Google Scholar]
- 4.Srimannarayana K, Patgiri BJ, Prajapati PK. Process standardization of rasamanikya. Ayu. 2010;31:7–11. doi: 10.4103/0974-8520.68195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosh MN. Fundamentals of Experimental Pharmacology. 2nd ed. Calcutta: Scientific Book Agency; 1984. Toxicity studies; p. 156. [Google Scholar]
- 6.Lawrence DR. 5th ed. I. U.S: Academic Press; 2003. Book of Drug Screening; p. 14. [Google Scholar]
- 7.Ramchandra G. M.D 4-5. Thesis. Karnataka: Department of Post Graduate Studies in Rasashastra, Taranath Govt. Ayurvedic Medical College, Bellary, Rajiv Gandhi University of Health Sciences; 2006. Preparation of Rasamanikya by Three Different Pharmaceutical Procedures and Its Analytical Study. [Google Scholar]
- 8.Srimannarayana K, Patgiri BJ, Prajapati PK. Process standardization of rasamanikya. Ayu. 2010;31:7–11. doi: 10.4103/0974-8520.68195. [DOI] [PMC free article] [PubMed] [Google Scholar]


