Abstract
In an effort to collaborate the data of chronic myeloid leukemia (CML) patient from all over India,meeting was conceived by ICON (Indian Cooperative Oncology Network) in 2010. This article presents the summarized picture of the data presented in the meeting. In the meeting 8115 patients data was presented and 18 centres submitted their manuscripts comprising of 6677 patients. This data represents large series of patients from all over the country treated on day to day clinical practice and presents the actual outcomes of CML patients in India. The compilation of data confirms the younger age at presentation, increased incidence of resistance and poor outcomes in patients with late chronic phase. It also addresses the issues like Glivec versus Generic drug outcomes, safety of Imatinib during pregnancy and mutational analysis among resistant patients. It concludes that survival and quality of life of CML patients in India has improved over the years especially when treated in early chronic phase. The generic drug is a good option where original is unable to reach the patient due to various reasons. Hopefully, this effort will provide a platform to conduct systematic studies in learning the best treatment options among CML patients in Indian settings.
Keywords: Chronic myeloid leukemia, India, Indian cooperative oncology network, mylestone
INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder of the primitive hematopoietic stem cell and is characterized by the presence of unique translocation, i.e., BCR/ABL1 known as Philadelphia chromosome.[1] In Pre imatinib era, CML was treated primarily with radiotherapy , bulsulphan , hydroxyurea, interferon alpha, cytarabine or later with allogeneic hematopoietic stem cell transplantation. However, at that time also, it was a known fact that the BCR/ABL1, a fusion oncoprotein which is consitutively active tyrosine kinase , plays the central role in the pathogenesis of CML and its suppression can lead to halt in disease progression.[1,2] This fact led to the advent of imatinib, tyrosine kinase inhibitor (TKI), which inhibits the leukemogenic kinase activity of BCR-ABL1 oncoprotein. This small targeted molecule demonstrated high efficacy and was well tolerated. Imatinib was then evaluated by the famous international randomized study of interferon versus STI571 trial, initiated in the year 2000.[3] In this trial , newly diagnoised chronic phase CML patients were randomized to be either treated with Imatinib 400mg/day or Interferon plus cytarabine. The results of this study, as we know , completely changed the therapeutic landscape for Philadelphia chromosome positive CML patients (as shown in Table 1).[3]
Table 1.
Showing changing trends in survival of CML patients over the years[3]

A decade later , since the advent of Imatinib, the changing trends in the treatment and the introduction of new TKIs have shed light on various aspects of CML.[4] In this era of 2nd and 3rd generation TKI, it is important to understand the basic trends of CML disease, tolerability of the drug, drug resistance and need of transplant in our Indian patients.
The fact that there is scarcity of the data from India on CML and on the changing trends in the treatment of CML in India, a meeting was conceived to share the in-depth views and clinical data of CML patients from all over India as shown in Figure 1. This meeting “Indian evidence of CML-mylestone” was held on 24th and 25th July 2010.[5] It was organized by Dr. Kumar Prabhash, Associate Professor, TMH Mumbai in collaboration with Indian Cooperative Oncology Network. In this meeting, there was representation from 22 major cancers centers in India comprising 8115 patients. It was the first collaboration of such huge data on CML in Indian patients. 18 Oncology centers also submitted their manuscripts and in this article we are presenting the summary of the collaborated data from the submitted manuscripts. It is imperative to say that this data is retrospective and has its limitations; however, at the same time it gives useful insight to the presentation, treatment modalities and its outcome in an Indian patient with CML.
Figure 1.
Map of India showing location of 22 Oncology centers represented at myelstone chronic myeloid leukemia meeting July 2010
Another important fact that needs to be mentioned is that with the presence of various patient assisted programs such as GIPAP, Gleevec have been made available to hundreds of non-affording patients and has played a pertinent role in the improvement of the overall outcome of CML patients in India.
PATIENT CHARACTERISTICS
There were total 6677 patients from 18 centers and the total number of patients from each individual center is shown in Figure 2.
Figure 2.
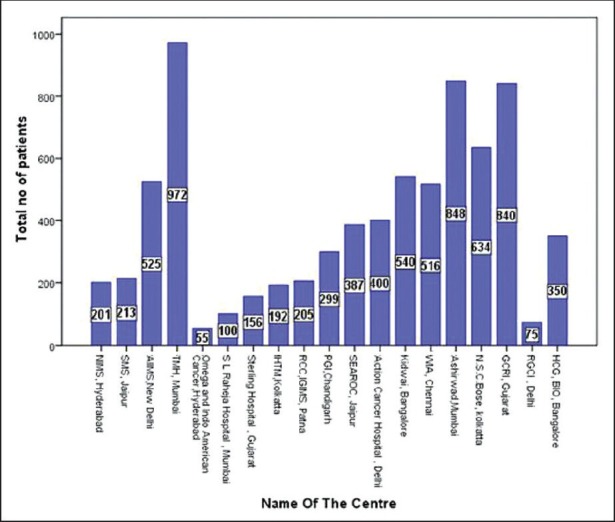
Number of patients from various oncology centers represented at myelstone – Chronic myeloid leukemia meeting July 2010
Incidence: As stated in various cancer registries , CML is one of the commonest adult leukemia in Indian population accounting for 30% to 60% of all adult leukemias.[6] The data presented at CML meeting showed that the incidence of CML cases varied from 70% of all leukemia cases at IGIMS, RCC, Patna to 16.6% GCRI, GujaratThis huge difference in incidence of CML cases at two different centres is difficult to explain, as these are not population based registries and may be because of different cancer populations they cater to.
Sex ratio: There is male preponderance. The male to female sex ratio varied from 1:08 (Sterlings, Gujarat) to 3:1 (TMH, Mumbai).
Median age: The median age of the population varied from minimum 32 years (Nizam Institute of medical sciences, Hyderabad, South India) to maximum 42 years (Ashirwad center, Mumbai, Southwest India). This decade younger population was the most consistent fact presented in almost all studies confirming that in India, median age at presentation is a decade younger compared with the age presented in European (median age 55years)as well as in American (median age 66 years) literature.[7,8]
Symptoms at presentation: The most common symptom was splenomegaly ranging from 100% (WIA, Chennai) to 81% (IGIMS, Patna) followed by hepatomegaly, fatigue, weakness, dragging pain, pallor or sometimes asymptomatic seen in 30% cases (HCG, BIO, Bangalore). No Organomegaly was seen in 5.4% patients (IHTM, Kolkata)In comparison to western data where approximately 40% of patients are asymptomatic and diagnosed on the basis of abnormal counts, majority of Indian patients are symptomatic and mostly present with dull aching pain in the left hypochondriac region secondary to splenomegaly.[7]
Blood counts at presentation: The median hemoglobin (hb) ranged from 9 g/dl (IGIMS, Patna) to 11 g/dl (AIIMS, India); the median white blood cell count ranged from 0.46 × 109/cumm (RGCI, Delhi) to 1.86 × 109/cumm (WIA, Chennai). CML is a myeloproliferative disease, but it can present with as low counts as 0.18 × 109/cumm (PGI, Chandigarh).
CML phase at presentation: The percentage of patients presenting in chronic phase varied from 85% (PGI, Chandigarh) to 97% (IGIMS, Patna) with a median of 89.5% as shown in Figure 3, while in European data, the presentation of CML in chronic phase has been reported to be as high as 96.8%.[7]
Sokal risk category: The Sokal risk category data being retrospective is not complete. However, few centers have presented their data and it seems the majority of patients are in Intermediate risk category ranging from 27% to 47%, while low risk category range from 25% to 55% and high risk category range from 12% to 28% of patients as shown in Figure 4.
Figure 3.
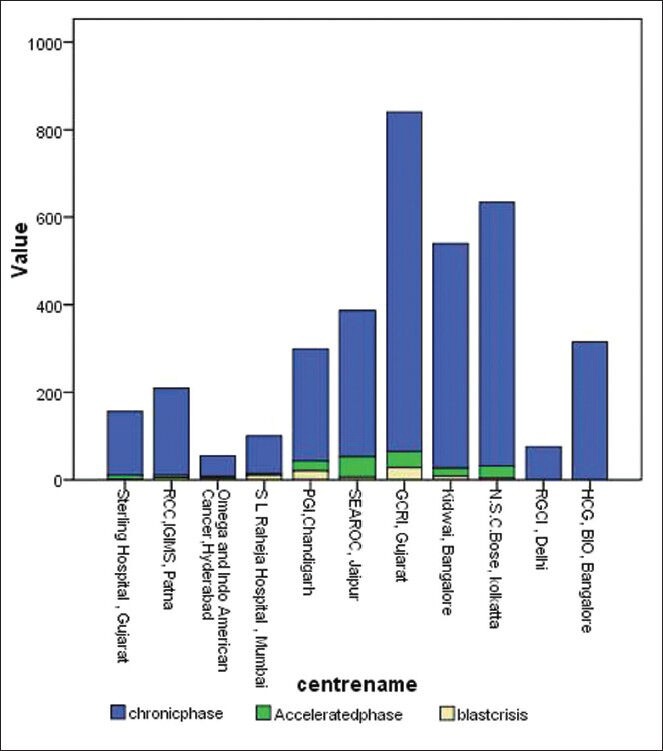
Chronic myeloid leukemia phase at the time of diagnosis
Figure 4.
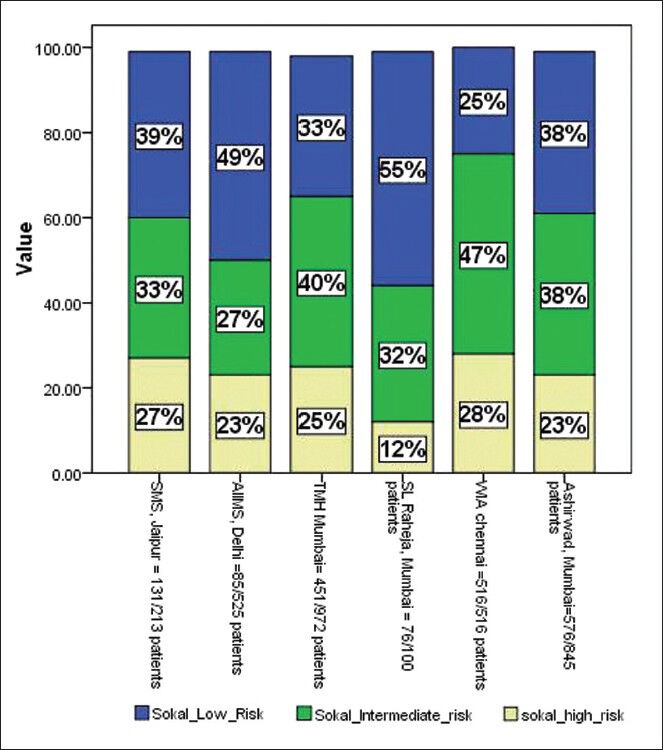
Sokal risk category at the time of chronic myeloid leukemia diagnosis
Monotoring and response
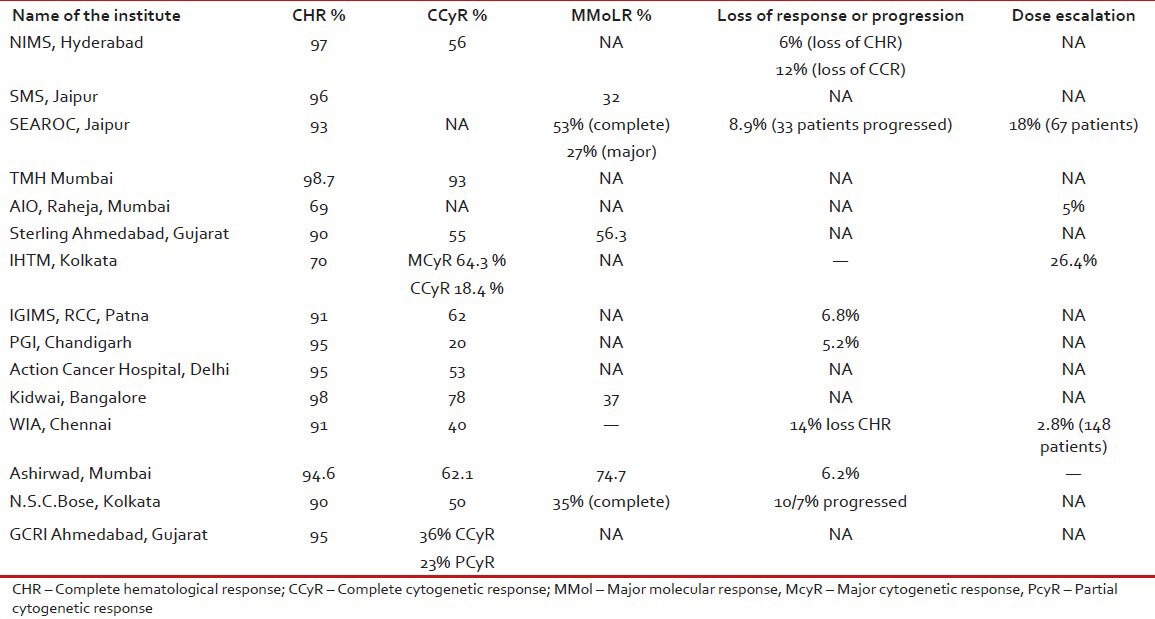
It is difficult to interpret the treatment response from various centers. As the time taken for response varies and also the treated population is quite heterogeneous. The most of the centers there is no mention of early chronic phase (ECP) or late chronic phase (LCP) at presentation. In many of the centers patients were treated with hydroxyurea, interferon at first and then shifted to imatinib. Details can be found in the individual chapters from various centers. The primary resistance to imatinib in newly diagnosed patients can vary from 0.1% (Omega, Hyderabad) to 3% (AIO, Raheja). AIIMS has reported 2 patients out 525 patients having primary resistance. The response from various centers is shown in Table 2.
Table 2.
The treatment response of patients at various centers
Other interesting issues
(ECP) versus LCP: TMH Mumbai data shows that there was 60% complete cytogenetic response (CCyR) in LCP compared with 80% in ECP patients. Data from AIO, Raheja, Mumbai shows that primary resistance was more common in LCP patients, i.e., 21% versus 3% in ECP patients. Ashirwad, Mumbai also reported similar findings with 80% CCyR in ECP and 43% in LCP patients.
Glivec versus Generic: In SMS Jaipur, 137 (64%) patients were on Glivec while 76 (36%) were on generic IM. The complete hematological response (CHR) was 88% in Glivec arm while it was 96% in generic arm. Another study from TMH Mumbai showed similar findings with 72% CCyR in Glivec arm and 75% CCyR in generic arm.
Safety of imatinib in pregnancy: From SEAROC, Jaipur, three patients conceived and all babies born did not have any congenital anomaly. AIIMS study showed that 10 female became pregnant while on imatinib, but only three stopped the drug as per instructions. However, there were uneventful outcomes except one baby had meningocele.
Mutation data: From SEAROC, Jaipur, imatinib mutation analysis was performed in five patients in accelerated Phase, but none showed any known mutation. In Kidwai Bangalore, mutation analysis was done in 101 patients showing poor response, in 73% there were no known mutation. The most common mutation seen was T315I (4 patients) and M351T (4 patients).
Toxicity profile
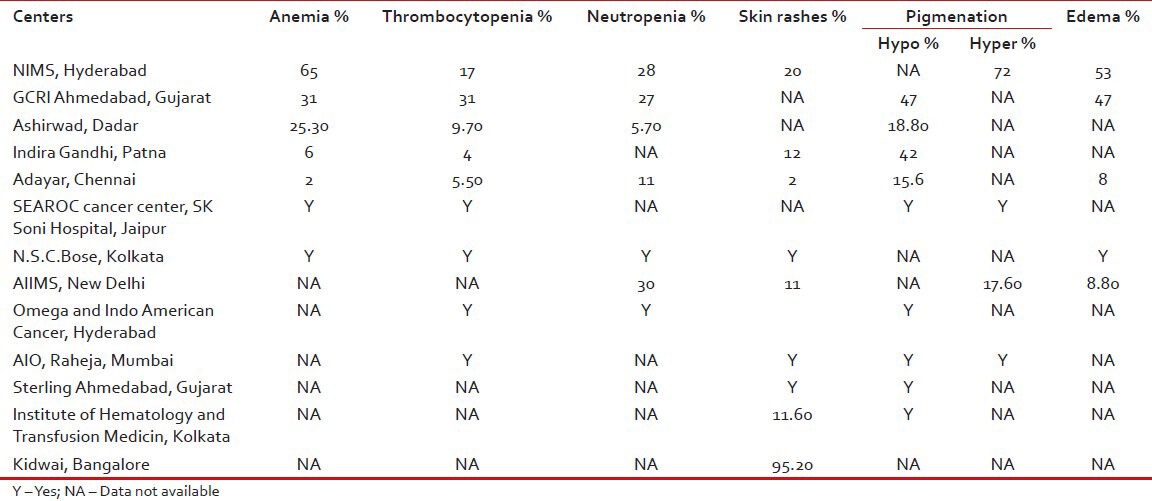
The most common non-hematological toxicity seen was changes in skin pigmentation followed by weight gain, edema, diarrhea, myalgias, arthralgias and transaminitis. Some have reported ototoxicity, decrease in vision also (RCC, Patna) and second malignancies (AIO, Raheja, Mumbai). Among non-hematological toxicity most common were anemia and thrombocytopenia. Grade III/IV toxicity requiring intervention was seen less than 1% (GCRI, Gujarat) and was reported up to 16% by Ashirwad, Mumbai. The various common toxicities as reported from various centers are shown in Table 3.
Table 3.
Toxicity profile of imatinib drug as reported from various centers
Survival
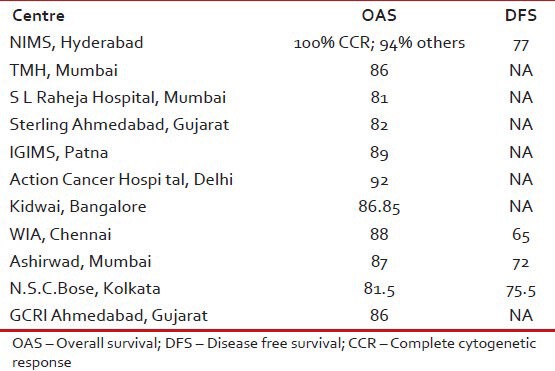
The survival varies from 81% to 100% in various studies as shown in Table 4.
Table 4.
Survival data from various studies
CONCLUSIONS
Various studies confirm that age at presentation is a decade younger and majority presents in chronic phase. Few reports have studied the factors responsible for response and survival of patients with CML. One such study from Ashirwad Mumbai shows that on Cox regression analysis, age under 40 years, low Sokal score, CHR and CCyR were significant predictive factors for event free survival event free survival while on multivariate analysis, low Sokal score and early chronic phase were the significant predictive factors for CCyR. Similarly from AIO, Raheja also reiterated that early chronic phase, better tolerability to the drug, no primary resistance are significant indicators of better survival of patients with CML.
Socio-economic (SE) status of patients also had an impact on the response to imatinib. Study by SMS, Jaipur shows that patients with upper SE status had 100% CHR while LE status had 90.3% CHR, also LE patients with increased disease burden with 25% having high Sokal scores compared with only 6% in Upper SE patients. Another important finding documented by data from TMH, Mumbai is regarding the compliance of the drug. Patients who had more than 4 weeks of gap had only 57% CCyR compared with 80% CCyR in patients taken less than 4 weeks of gap in their treatment.
Most important limitation of the retrospective data was uniformity and consistency in the response evaluation. However, most studies have regularly followed patients with blood counts, other modalities such as cytogenetic response; molecular response and mutation analysis have been done onlyat few centres. The main limitation is financial constraints and the second argument is non-availability of better options than imatinib again due to financial reasons. However, this collective data gives us some insight into patient presentation, tolerability, response and outcome of CML patients in Indian settings.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lee SJ. Chronic myelogenous leukaemia. Br J Haematol. 2000;111:993–1009. doi: 10.1046/j.1365-2141.2000.02216.x. [DOI] [PubMed] [Google Scholar]
- 2.Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637–47. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 3.Leitner AA, Hehlmann R. Modern therapy of chronic myeloid leukemia: an example for paradigma shift in Hemato-oncology. Internist (Berl) 2011;52:209–17. doi: 10.1007/s00108-010-2782-3. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, Radich JP, et al. International randomized study of interferon versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM) Blood. 2008;112:76. [Google Scholar]
- 5.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. [Last accessed on 2013 nov 6];J Clin Oncol. 2009 27:6041–51. doi: 10.1200/JCO.2009.25.0779. Available from: http://www.oncologyindia.org/myelstone . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutani M, Kochupillai V. Hematological malignancies in India. In: Kumar L, editor. New York: Progress in Hematologic Oncology.Pub. The Advanced Research Foundation New York; 2003. p. 10. [Google Scholar]
- 7.Tardieu S, Brun-Strang C, Berthaud P, Michallet M, Guilhot F, Rousselot P, et al. Management of chronic myeloid leukemia in France: a multi-centered cross-sectional study on 538 patients. Pharmacoepidemiol Drug Saf. 2005;14:545–53. doi: 10.1002/pds.1046. [DOI] [PubMed] [Google Scholar]
- 8.Cortes JE, Richard TS, Hagop K. Chronic myelogenous leukemia. In: Padzur R, Coia LR, Hoskins WJ, Wagman LD, editors. Cancer Management: A multidisciplinary approach. 10th ed. Lawrence: CMPMedica; 2007. p. 789. [Google Scholar]


