Abstract
Chronic myeloid leukemia (CML) has paradigm shift in its treatment modality after the discovery of targeted therapy Imatinib. At our centre we collected data of 100 consecutive patients over a period of 8 years. The interesting finding in our study was difference in the survival of patients presenting in early chronic phase (81%) versus late chronic phase (16%). Also the patients with primary resistance did poorly compared to the patients who developed secondary resistance to Imatinib.
Keywords: Chronic myeloid leukemia, chronic phase, veenat, Glivec
INTRODUCTION
The treatment of chronic myelogenous leukemia (CML) has changed completely over the years with the introduction of targeted therapy, a tyrosine kinase inhibitor: The imatinib mesylate (IM).[1] IM has brought a revolutionary change in the overall out-come of the patients affected with CML.[2,3] In India, this molecule has been made available to thousands of masses through MAX foundation (Glivec® International Patient Assistance Program [GIPAP]).
The purpose of this study was to collect the data of the patient treated with IM molecule and to know the various factors involved in the ultimate out-come of the treatment.
PATIENTS AND METHODS
The data was collected retrospectively from year January 2002 to January 2010. The names of the patients were extracted from the data register. The files of the patients were referred for information such as their age, sex, date of diagnosis, address, phone no., blood counts, spleen size at diagnosis, brand and dose of imatinib, change in dose, any primary or secondary resistance; tolerability, the side effects of imatinib and its outcome. Patients whose files had incomplete data were contacted on phone. For patients who were not reachable on the phone and were registered with GIPAP; their information regarding the follow-up was collected from the MAX foundation.
Definitions
Standard definitions of chronic phase (CP), accelerated phase (AP), blast crisis (BC) and primary and secondary resistance are considered. For CP CML, treatment failure to IM 400 mg was defined as primary resistance i.e., failure to achieve complete hematologic response (CHR) after 3 months, failure to achieve cytogenetic response after 6 months, major cytogenetic response (MCR) after 12 months and complete cytogenetic response (CCR) after 18 months of therapy. Secondary resistance was defined as loss of CCR or rising white blood cell count to >10 × 109/L on two occasions more than 4 weeks apart, progression to AP or blast phases.
In our study, maximum patients have been followed with blood counts only, cytogenetic study on follow-up was done in few patients only, who could afford it.
We also divided the patients in the category of new (early CP) and old cases (late CP). New cases were those cases that were diagnosed with CML and did first consultation with us or got registered with us within 3 month of diagnosis.
Old cases were those cases who after diagnosis took at least more than 3 months of treatment elsewhere before registering with us. This distinction was done to find out the difference in the survival of the patients in these two categories of patients.
Statistical analysis
Event free survival (EFS) was defined as the patient who failed to achieve response and progress to AP or would end up in BC or a death due to any cause, whichever occurred first. The SPSS system was used to analyze the data. The EFS and overall survival (OS) probabilities were estimated using the Kaplan-Meier method and were compared using the log rank test. Differences among variables were evaluated using the ×2 test.
RESULTS
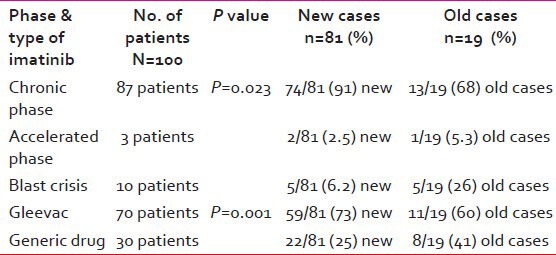
There were total of 100 patients of CML registered with the median age of 40 years (7-80 years) during the year January 2002-January 2010. The numbers of new cases were 81 and old cases were 19. The baseline characteristics of the patients and the type of imatinib taken are given in Tables 1 and 2.
Table 1.
Baseline characteristics of the patients
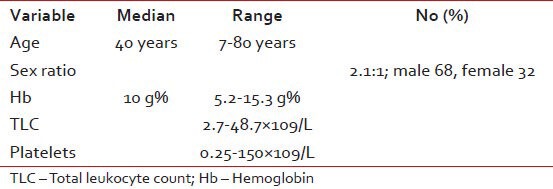
Table 2.
The disease characteristics at presentation and type of imatinib received by patients
Patients Sokal scores were calculated and following information was found:
42 patients in low risk group,
25 patients were intermediate risk group and
Nine patients were high-risk group.
In 24 patients, data was not complete (either spleen size or complete blood count report at baseline could not be retrieved).
Response and resistance pattern
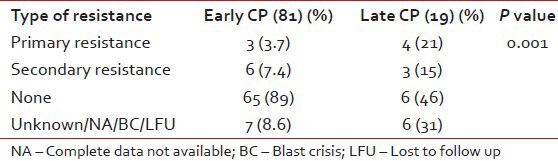
Documented CHR was seen in 69 patients while seven patients had no response, rest of the patients had incomplete data or their blood counts were not in the records. There was a significant difference in the resistance pattern depending on the baseline diagnosis as shown in Table 3. Furthermore, primary and secondary resistance was significantly high in the patients registered as old cases as shown in Table 4. However, as per risk group based on Sokal scores- the resistance patterns did not show any significant difference (P = 0.064). Many of old cases patients had received hydroxyurea for a median time of 12 months (range: 6 months-4 years), one patient had received interfereon therapy and or had other brands of imatinib compliance, which were not well-known.
Table 3.
Difference in the resistance patterns when compared with baseline diagnosis
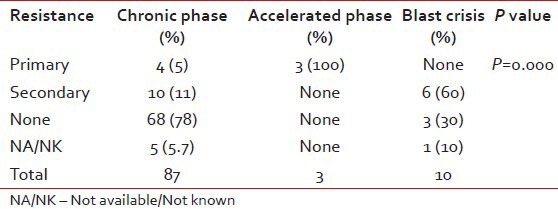
Table 4.
The difference in the resistance patterns based on the new and old cases
OS
At a median follow-up of 35 months (range: 1-111); around 71 patients were in CP, three patients were in AP, one in BC, 18 had died and seven were lost to follow-up. The survival of the new cases and old cases were taken out differently as there was marked difference in the two. The estimated 5 year OS was 81.5% in new cases and 16% in old cases as shown in Figure 1.
Figure 1.
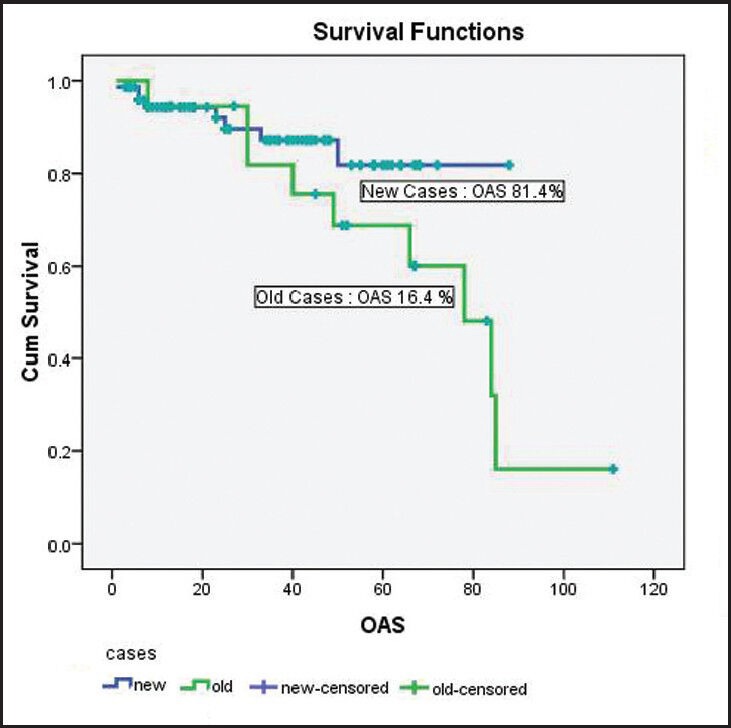
The OAS of patients diagnosed with chronic myelogenous leukemia
Change in the dose
The doses were changed in approximately 8 (8%) cases. In five cases, it was increased from 400 mg to 600 mg in view of poor response to the initial dose. Out of the five patients : Two patients went into CHR; however, cytogenetic response was not there in one patient. While two patients died, one progressed to BC. Last one patient continued to be in AP. In the rest of three patients dose was decreased due to intolerability to imatinib and all three were in CP phase till date.
Tolerability and side effects of imatinib
No side-effects were seen in 82 patients. Three patients had significant thrombocytopenia, in one patient it stopped for 3 months and then restarted, while in another patient-the dose of imatinib was reduced to 300 mg for almost 1 year. The third patient had thrombocytopenia followed by BC. Hyperpigmentation was seen in two patients, one had it in the skull area and another had it on the face (butterfly rash) as shown in Figure 2. Two patients had complained of fluid retention, swelling around the ankle joints. One patient had severe allergic reaction to imatinib, not able to tolerate even 100 mg dose and required frequent dose interruption. She was advised dasatinib; however this could not afford it. Patient has enrolled with us 6 months back only and at present continues to in CP with a thin layer chromatography in the range 20-30 × 109/L. Many of the patients had hypopigmentation; however exact numbers are not known.
Figure 2.
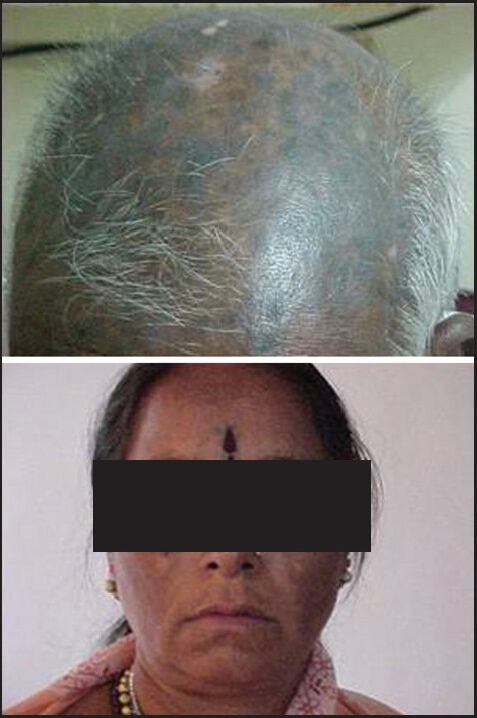
Hyperpigmentation on the skull and face (imatinib side effects)
Rare cases: Therapy related CML and secondary malignancy
A 58-year-old patient presented to us in 2003- he had white blood cells count of 29000/cumm and was Philadelphia positive (Ph+) chromosome. In the past, in 1987 he was treated for low grade non-Hodgkin's lymphoma with mechloroethamine, oncovin, procarbazine, Prednisolone (MOPP) ×6 cycles. In 1991 he had recurrence in radiotherapy (RT). Pre parotid nodes and received central venous pressure ×6. In 1996, had lesions recurrence in the region of breast, thigh, upper eyelid, he also received local RT with cyclophosphamide, etoposide, oncovin, Prednisolone (CEOP) chemotherapy and in 2001 again received fludarabine and endoxan for relapse. Post diagnosis of CML he was started on imatinib which he tolerated well and is at present alive and in CP.
Another patient diagnosed with CML in March 2008 and was on regular imatinib which he was tolerating well. In 2010 he complained of weakness, loss of appetite, nausea and periorbital puffiness, his BM revealed plasma cell dyscrasia and is presently receiving treatment for it.
One patient was taking methotrexate tablets since 1994 for psoriasis and was diagnosed in AP of CML in May 2007. He had primary resistance to imatinib and died in June 2007.
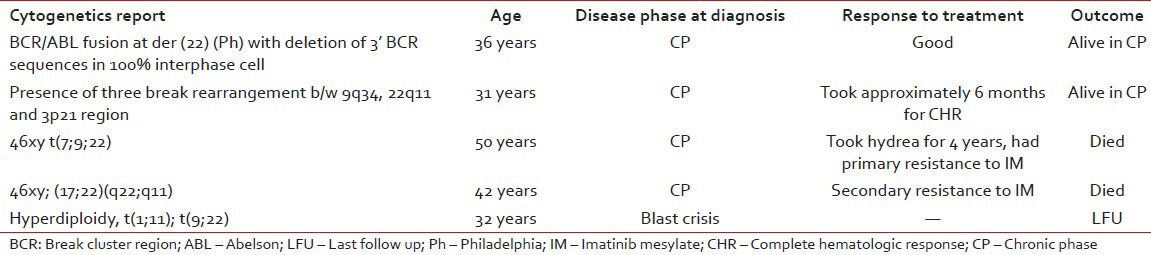
We also collected data of the patient who had additional abnormalities other than Ph+ chromosome. Their disease characteristics, response to treatment and out-come is given in the Table 5.
Table 5.
The data of the patient with additional abnormalities
DISCUSSION
Post imatinib era-the treatment of CML has become easy, effective and affordable (due to availability of generic). In our study, the disease phase at diagnosis and the resistance experienced by the patient were quite survival predictable.
We also found a significant difference in the outcome of the patients who were treated elsewhere, before getting registered with us. Most of these patients were either treated with hydrea, interferon or were presented in AP or BC with poor outcome. Hence, their outcome has been taken out separately.
Cytogenetics response was seen in very few patients as most of them refused the test and seeing their limited resources, we also did not force it. Moreover, even if the patient had poor cytogenetic response we did not have much to offer them and patients were not able to afford dasatinib or bone marrow transplantation. Two patients who took dasatinib post AP showed poor tolerability and ultimately succumbed to the disease.
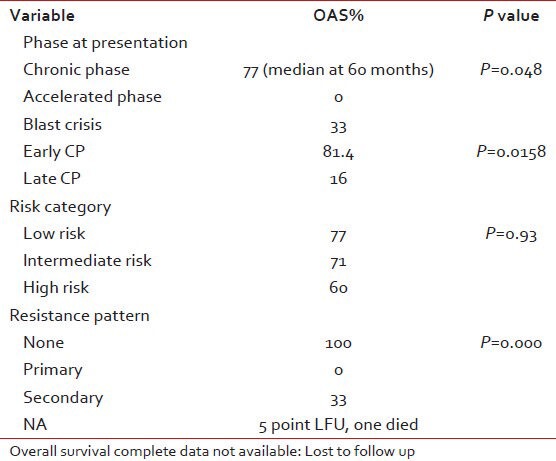
Our study concludes that patients in the CP with good tolerability to imatinib have the best survival as shown in Table 6.
Table 6.
Prognostic variables
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lee SJ. Chronic myelogenous leukaemia. Br J Haematol. 2000;111:993–1009. doi: 10.1046/j.1365-2141.2000.02216.x. [DOI] [PubMed] [Google Scholar]
- 2.Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:569–84. doi: 10.1016/j.hoc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Pinilla- Ibarz J, Bello C. Modern approaches to treating chronic myelogenous leukemia. Curr Oncol Rep. 2008;10:365–71. doi: 10.1007/s11912-008-0057-0. [DOI] [PubMed] [Google Scholar]


