Abstract
This is a retrospective analysis of patients of chronic myeloid leukemia (CML) registered and under treatment at the Leukemia Lymphoma Clinic at the Birla Cancer Center, SMS Medical College Hospital, Jaipur. Approximately, two-thirds of the patients are getting imatinib mesylate (IM) through the Glivec International Patient Assistance Program while the rest are on generic IM. In addition to comparison of hematological and molecular responses in the Glivec versus the genetic group, in this analysis, an attempt is also made to assess the socio-economic (SE) status of the patients and its effect on the response rates. Of the 213 patients studied, most (28.6%) are in the age group between 30 years and 40 years and the mean age of the patients in 39 years, a good decade younger that in the west. There is a suggestion that patients in lower SE class present with higher Sokal scores and with more disease burden. Possibly hematological responses are similar with both Glivec and generic IM. No comment can be made with regards to molecular response between the two groups as a significant number of patients in the Glivec arm (42%) do not have molecular assessment because of economic reasons. CML is a common and challenging disease in the developing world with patients presenting at an earlier age with more advanced disease. SE factors play a significant role in therapy and disease monitoring decision making and may impact on response rates and prognosis.
Keywords: Chronic myeloid leukemia, chronic phase, Jaipur
INTRODUCTION
Various hematological cancers in developing countries, chronic myeloid leukemia (CML) included, have significant differences in incidence, age of onset, stage at presentation, phenotype and stage-for-stage response rates and prognosis as compared to their western counterparts. Several reasons have been postulated for this and include socio-economic (SE) status, genetic differences, environmental factors (infections, particularly, viral infections, exposure to pesticides, etc.) and nutritional factors.
CML is the most common adult leukemia in India being much more common that chronic lymphatic leukemia and acute myeloid leukemia. Figures from various Indian cancer registries show a CML incidence of 0.8-2.2/100,000 population for men and 0.6-1.6/100,000 population for women.[1] As cancer is not a reportable disease in India, most practicing medical oncologists and hematologists believe that this is a gross underestimation of the true incidence of the disease and the actual figure is at least three times this number. The disease is seen in a younger population, the median age at onset being between 30 and 40 years.[1] In most Indian studies patients diagnosed on routine testing form 5% to 8% of CML cases as compared to 20% in Western studies. A majority of CML patients, approximately 80-90% present in the chronic phase at diagnosis. Majority of Indian patients present with anemia, massive organomegaly and very high counts (more than 50% have total leukocyte count at presentation of more than 200.0 K/uL). Complete hematological responses (CHR) to imatinib are seen in more than 90% patients but complete cytogenetic and molecular responses are documented in less than 60% patients after 1 year of treatment.
In addition to Glivec (which is available to a significant number of poor Indian patients without charge under the Glivec International Patient Assistance Program [GIPAP]), there are several companies in India which manufacture and market the generic version of imatinib. The cost of the generic imatinib is approximately 10-15 times less than Glivec.
The present paper will attempt to compare and contrast the efficacy, in terms of hematological and molecular response, of the generic imatinib versus Glivec. An attempt, possibility for the first time in the world, will also be made to correlate responses to the SE status of patients.
MATERIALS AND METHODS
Case records of patients of CML on imatinib mesylate (IM) at the Birla Cancer Center, SMS Medical College and Jaipur were retrospectively analyzed and a data base created. An attempt was made to track the original hematology reports of all patients to determine the Sokal score at diagnosis. Questionnaire was designed to assess the Kuppuswamy's SE status and administered to patients. SE status was assessed by the method described by Kuppuswamy. Charts were scanned to assess hematological and molecular responses. As is the policy at the center, cytogenetics, other than at diagnosis, was not done for monitoring of the disease. Molecular responses were assessed by real-time quantitative polymerase chain reaction (RQ-PCR). Definition of molecular response (complete, major response and partial response) was as published by the European Leukemia Net guidelines.[2,3]
OBSERVATIONS AND RESULTS
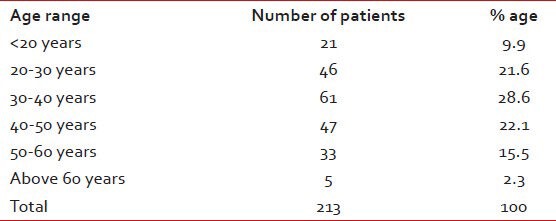
There were a total of 213 patients of CML on IM, 141 (66%) males and 72 (34%) females. 137 (64%) are on GIPAP Glivec while 76 (36%) are on generic IM. Mean age at presentation is 39 years with the maximum number of cases (28.6%) between ages 30 and 40 years. Age distribution is shown in the Table 1.
Table 1.
Showing the age distribution of the CML patients
Majority of the patients belong to the lower (39%) or lower middle (29%) SE class, with only 7% being in the upper class. Understandably, in the Glivec group, there are more patients in lower and lower middle class (72%) as compared with the generic group (42%) as shown in Table 2.
Table 2.
Socio Economic status: Glivec versus generic group
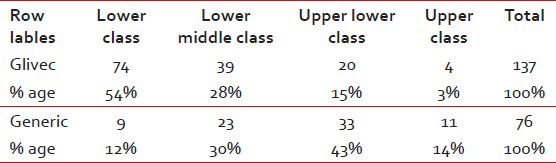
SE status: Glivec versus generic group
Baseline information to be able to calculate Sokal Score at diagnosis was not available in 64.5% patients in the generic group compared to 24.1% in the Glivec group suggesting better record keeping in the Glivec group.
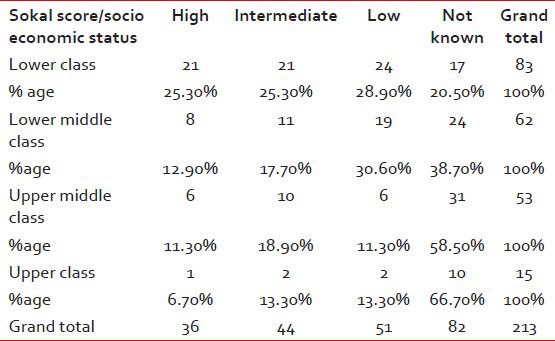
In patients in whom Sokal Score was calculable, 25.3% had a high score in the lower SE class as compared with 6.7% in the upper SE class as given in Table 3.
Table 3.
Showing patients as per socioeconomic status and the Sokal score
CHR were seen in 96% patients in the generic arm as compared to 88% in the Glivec arm. 47% of patients in the generic arm had complete molecular response as compared to 32% in the Glivec arm. It may be noted that molecular responses were not documents in 42% of patients in the Glivec arm because of economic reasons. As given inthe Tables 4a and b below:
Table 4a.
Showing Hematological response in generic vs Glivec group

Table 4b.
Showing Molecular response in generic vs Glivec group

With regards to SE class, the CHR rates in the lower SE class were 90.3% compared to 100% in the upper SE class. As given in Tables 5a and b below:
Table 5a.
Shows hematological response as per socioeconomic status
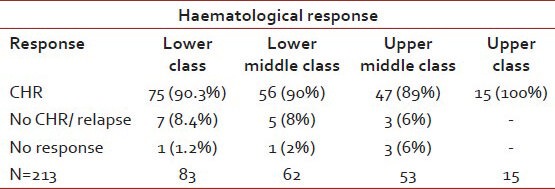
Table 5b.
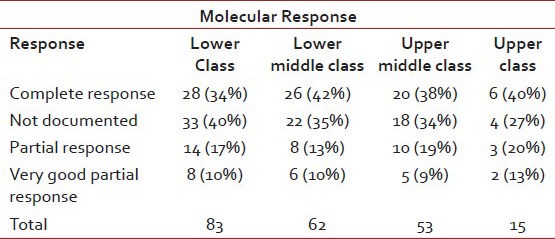
Shows molecular response as per socioeconomic status
DISCUSSION
Even though the present work suffers from some of the problems which all retrospective studies have missing data, some conclusions can be drawn from the same. A prospective analysis of CML patients have been initiated in our institution since the past couple of years and subsequent data should be much more accurate.
The majority of patients attending the oncology out-patient department of a government hospital are in the lower SE classes. These patients probably have more advanced disease at presentation because of late consultation and late diagnosis. As a consequence of this late diagnosis and more advanced disease at presentation, the response rates to treatment are also lower. Because of the availability of the GIPAP, these patients do have access to the IM, which may not have been possible if such a support program was not in place in the institution. However, disease monitoring as recommended in various national and international guidelines is not possible in the majority of these patients because of economic reasons. It should be the priority of each institution to have in-house, affordable and accurate testing (RQ-PCR) for the economically compromised set of patients.
The generic IM, in the present study, showed hematological response (HR) rates at par, if not better, that those with Glivec. However, it should be borne in mind that there were significantly more patients belonging to the lower SE class and more advanced disease in the GIPAP Glivec group than in the generic group and this could account for the difference in HR rates. As there were many patients in the Glivec group in whom molecular response assessment could not be done, it is difficult to compare molecular responses between the two groups. However, complete molecular responses in more than one-thirds of patients could be demonstrated.
The present study highlights some to the problems of treating patients of CML in India. SE class possibly adversely affects outcomes. Genetic IM possibly is as good as Glivec as far as HR is concerned.
SUMMARY AND CONCLUSIONS
Two-third of patients was males, maximum no. of patients in the age group 30-40 years and 2/3rd were on Glivec under GIPAP.
Overall 39% of patients were in poor SE class and 29% in lower middle class.
In the Glivec group, more than half of patients were in poor SE class, while in the generic group; only 12% were in this class.
In the lower SE class, more that 25% had high Sokal scores at presentation as compared to 6.7% in upper SE class.
In upper SE class, CHR rate was 100% as compared with 90.3% in lower SE class.
Rates of CHR in the generic group were similar to Glivec group.
Molecular response could not be assessed in 42% patients on the Glivec group b/o economic reasons.
Age at diagnosis lower by approximately one decade as compared with west.
Suggestion that patients in lower SE class present with high Sokal scores and more disease burden.
Suggestion of higher CHR in patients from upper SE class.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bhutani M, Vora A, Kumar L, Kochupillai V. Lympho-hemopoietic malignancies in India. Med Oncol. 2002;19:141–50. doi: 10.1385/MO:19:3:141. [DOI] [PubMed] [Google Scholar]
- 2.Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637–47. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 3.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]


