Abstract
The data of 156 patients was presented from Hematology clinic, Ahmedabad. This hematology clinic caters large number of the population from Gujarat as well as from neighboring states such as Rajasthan and Madhya Pradesh. Out of 156 patients, 146 (94%) patients were in chronic phase. Complete hematological response was seen in 90% of patients and overall survival was 82% at 5 years.
Keywords: Chronic myeloid leukemia, Imatinib, Sterling Hospitals
INTRODUCTION
The state, hospital and patient profile: The city of Ahmedabad is the largest growing city of India and the populist city of vibrant Gujarat. The ever increasing population and expanding horizons, has a number of people with increasing awareness of diseases, with earlier diagnosis and subsequent earlier treatments.
The city of Ahmedabad drains a number of people in and around Gujarat, as well as Madhya Pradesh, Rajasthan and borders of Maharashtra. Patients of chronic myeloid leukemia (CML) are followed-up by three prominent groups, Hematology Clinic (we as a part of the team), Vedant Clinics and Gujarat Cancer and Research Institute (part of government setup). All the three centers fortunately have Gleevec® International Poor patient Assistance Program (GIPAP), through which the Imatinib (Gleevec®) is provided free of cost or at a minimal amount, after adequate financial assessment by third party is carried out. This is run by Max foundation, on behalf of Novartis Company, to provide expensive drugs free of cost.
PATIENTS AND METHODS
This is retrospective analysis and study period was from October 2000 to July 2010. A total of 156 patients were evaluated during this period. The prospective data of these patients was obtained from the Max foundation and well-kept Hematology follow-up sheets and cards, the procedure of which is operational from the inception of the clinic. Diagnosis of the patient was made on the basis of clinical examination, routine and special laboratory tests. Patients were examined for palpable liver and spleen and the presence or absence of lymph nodes. A through systemic examination was also carried out.
The routine tests were complete blood counts, reticulocyte count and liver function tests including lactate dehydrogenase, serum creatinine and uric acid. The special tests included a Bone marrow aspirate and trephine biopsy along with Cytogenetics to look for Philadelphia chromosome. The fluorescent in situ hybridization (FISH) test was carried out on peripheral blood once this test was made available in the last 3 years. The stage of CML was assigned based on standard World Health Organization criteria after evaluation.
TREATMENT CHARACTERISTICS
After diagnosis was made, patients were explained about the nature of the disease and various treatment options including their side-effects.[1,2] The goals of therapy in patient with CML were to achieve hematologic and cytogenetic control of the disease. The current treatment options for patients with CML include Gleevec, hydroxyurea, cytosine-arabinoside, interferon-alpha, Dasatinib, Nilotinib and allogeneic stem cell transplantation. The quantitative polymerase chain reaction (PCR) was performed at 6 months of interval to monitor new progress.
The procedure to enroll to get Glivec capsules (through Novartis Oncology Access Program) from Max foundation was been initiated, at diagnosis. Meanwhile, patient was initiated on cytoreductive therapy with hydroxyurea. Patients who did not qualify for GIPAP were given generic imatinib. The dose of Imatinib at the start of treatment or after disease progression was given as follows, with CML in chronic phase (CP) given 400 mg OD, CML in accelerated phase (AP) given 600 mg OD and CML in blast crisis (BC) given 800 mg OD. The doses were modified in children and were given as 260 mg/m2.
Response criteria
Patients were followed-up every 3 monthly and at each visit, general examination and systemic examination was done. Special note was made of liver and spleen size and any presence of lymph nodes. Furthermore, questions related to drug side-effects and toxicity was asked such as rash, urticaria, recent onset hypopigmentation-generalized and localized etc.
The investigations included a complete blood count, as well as serum glutamic-pyruvic transaminase and creatinine, as part of tests for toxicity. A FISH test was conducted every 6 monthly to assess response with Imatinib. In the absence of FISH test, a reverse transcription PCR (RT-PCR)-qualitative was carried out every 6 monthly and if negative, then RQ-PCR was tested every yearly. The RT-PCR has an in house sensitivity of 104. Patients who progressed or were resistant to Imatinib were not tested for BCR/ABL mutations, as this facility was not available in house.
The response criteria were based on FISH and RQ-PCR tests and are defined as follows:
Complete hematological response: Normal peripheral blood count, total leukocyte count <10,000/mm3, Myelocytes + Metamyelocytes <5%, No blasts in peripheral blood, platelet Count < 4.5 lakhs/mm3, no extra-medullary disease
Cytogenetic response (based on FISH/cytogenetics):[1] Complete cytogenetic response (CCyR) – 0% Ph positive, partial cytogenetic response – 1-34% Ph positive, major cytogenetic response – 0-34% Ph positive, minor cytogenetic response – 35-90% Ph positive
Molecular response[2]-undetectable BCR/ABL transcripts or BCR-ABL/ABL ratio <0.045%.
RESULTS
There were total 156 patients. The Median Age at diagnosis was 43 years (Range – 14-82 years). In terms of sex distribution, there were 81 males and 75 females, with sex ratio of male to female was 1.08 and is evenly spaced. The Median duration of follow-up for all patients was 2.3 years.
Baseline characteristics
At diagnosis 146 (98%) were in CP, 08 patients were in AP and 02 in BC phase. The symptoms were mainly dragging pain in the left side of the abdomen and occasional fever; however, symptomatology was not included in the analysis.
The side — effects have been standard such as hypopigmentation, transient suppression of blood counts, fluid retention etc.
Response
After completion of 3 months of treatment, complete hematological remission at 3 months was 90% (149/156 patients). At diagnosis, the FISH/cytogenetics positive for t (9; 22) was present in 98% of patients. Remaining 2% of patients were positive by RT-PCR for BCR/ABL, which was carried out in the absence of detection of Philadelphia chromosome.
Response to treatment
CCyR at 18 months was 55%. Additional 5 patients did not complete 1 year and 6 months of follow-up, 1 patient migrated and additional 3 patients expire. Thus, additional 9 patients were excluded in the analysis and total of 112 patients were included in the analysis at 18 months, Out of which 62 patients achieved CCyR giving % CCyR at 18 months –55%.
Molecular response at 2 years months was 56.3%. In addition, 7 patients did not complete 2 years of follow-up, 2 patients migrated and none expired. Thus, additional 9 patients were excluded in the analysis and total of 103 patients were included in the analysis at 18 months, Out of which 58 patients achieved molecular response –56.3%.
The 2 patients who did not responded were put on Dasatinib and are doing well. Both have no human leukocyte antigen matched siblings and are in progress of getting a matched unrelated transplant.
Event free survival (EFS)
EFS is defined as “molecular relapse” at any point in time and at 5 years is 42%.
Overall survival: Overall survival was 82% at 5 years, Figure 1.
Figure 1.
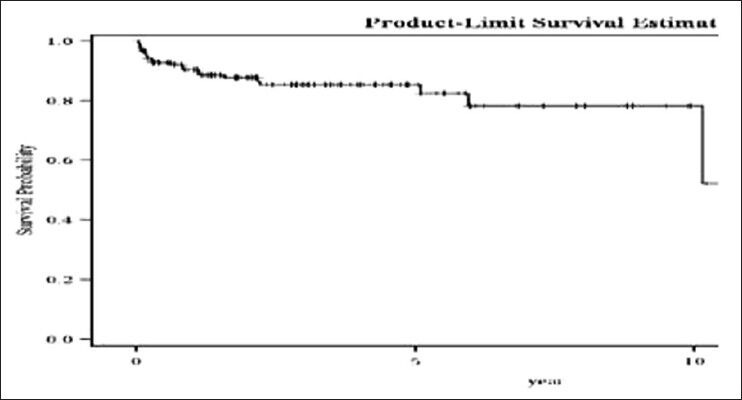
Overall survival of chronic myeloid leukemia patients
DISCUSSION
Imatinib and new generation tyrosine kinase inhibitors have improved the overall outcome of patients diagnosed with CML.[3] In our patient population, 90% of patients achieved complete hematological response, which is comparable with any published literature.[4] The tolerability of the drug was also acceptable and there was no grade IV toxicity related to the drug. The EFS was 42% and this was on the basis of loss of response to Imatinib at the molecular level. The EFS in our study is low compared with other reports.[5] Reasons for this needs to be evaluated. We could not do mutational analysis as this facility was not available. However, follow-up of patients will be continued.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kantarjian HM, Talpaz M, Cortes J, O’Brien S, Faderl S, Thomas D, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; Gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160–6. [PubMed] [Google Scholar]
- 2.Hochhaus A, Reiter A, Saussele S, Reichert A, Emig M, Kaeda J, et al. Molecular heterogeneity in complete cytogenetic responders after interferon-alpha therapy for chronic myelogenous leukemia: Low levels of minimal residual disease are associated with continuing remission. German CML Study Group and the UK MRC CML Study Group. Blood. 2000;95:62–6. [PubMed] [Google Scholar]
- 3.Lee SJ. Chronic myelogenous leukaemia. Br J Haematol. 2000;111:993–1009. doi: 10.1046/j.1365-2141.2000.02216.x. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Pearson K, Ferguson JE, Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120:990–9. doi: 10.1046/j.1365-2141.2003.04200.x. [DOI] [PubMed] [Google Scholar]


