Abstract
We have analyzed our experience regarding use of tyrosine kinase inhibitors especially imatinib mesylate in patients of chronic myeloid leukemia-chronic phase over last 7 years at our center (2002-2009). The object was to report long-term efficacy (hematological, cytogenetic and molecular) and toxicity. Overall, 775 patients were treated. Out of these, 576 were analyzable with a median follow-up of 3.6 years. The median age was 42 years. Complete cytogenetic response (CCyR) was achieved in 351/576 patients, i.e., 62.1%. Grade 3/4 adverse effects were observed in 36 patients, i.e., 6.25%. Age under 40 years, low Sokal score, complete hematological response and CCyR were significant predictive factors for event free survival (EFS) on univariate analysis while low Sokal score and early chronic phase were significant predictive factors for EFS on multivariate analysis. Our results are almost similar to those reported from various studies from western population.
Keywords: Chronic myeloid leukemia, cytogenetic response, imatinib mesylate, India, molecular response, toxicity
INTRODUCTION
Chronic myeloid leukemia (CML) is the most common leukemia in India as against chronic lymphocytic leukemia, which forms the most common leukemia in the western world. The age distribution of CML in India and in general, in Asian countries shows a shift to left with disease occurring in relatively younger subjects.
Although curable by allogeneic bone marrow transplantation (BMT),[1] cost constrain and lack of matched donor availability and treatment related morbidity and mortality are major obstacles. In addition, the procedure is less widely available in the developing world including India. Treatment with interferon has become obsolete due to its inconvenience and toxicity.[2] Older treatment like hydroxylurea and busulfan are no more preferred as these do not produce cytogenetic responses (CyR).
Imatinib Mesylate (IM) is a potent and selective, competitive inhibitor of BCR-ABL protein. It is, at present, the front-line treatment for patients of CML-CP. The responses are high and durable.[3] The quality-of-life is normal and there is substantial prolongation of survival. Its superiority over interferon has been established in IRIS study, which is now more than 8 years old.
We are reporting our long-term follow-up data from 848 consecutive patients of CML-CP seen at our center over last 7 years, i.e., 2002-2009, out of which 576 were analyzable with a median follow-up of 41 months, i.e., 3.4 years.
MATERIALS AND METHODS
This is a retrospective observational study. Patients were diagnosed to have CML-CP when the clinical and hematological picture was consistent and either Ph chromosome was documented by cytogenetic studies (Karyotyping) on marrow or BCR-ABL translocation was shown in peripheral blood by fluorescence in situ hybridization or polymerase chain reaction (FISH or PCR). A baseline history and physical examination was performed on all patients. Laboratory data included complete peripheral blood counts, hepatic and renal profile, lactic dehydrogenase and uric acid. Patients were divided into two groups: Early-chronic phase (ECP) and late-chronic phase (LCP) where LCP included patients who had received some or other modality of non-transplant-treatment of CML for a period of more than 6 months. All the responses (hematological, cytogenetic and molecular) were analyzed as defined in the literature.
IM was invariably obtained from Max Foundation through Glivec International Patients’ Assistance Program (GIPAP), unless patient was not eligible or opted for a generic brand due to some other reason. The starting dose was invariably 400 mg once a day orally, 1 h after dinner with a glass of water except for pediatric patients or those under the weight of 30 kg, where 260 mg/m2 was the dose used (invariably rounded up to 200, 300 or 400 mg whichever closer to the calculated dose). The dose was reduced to 300 mg/day in case of intolerance and it was escalated to 600 or 800 mg in a step-wise fashion in those showing inadequate hematological, cytogenetic or molecular response (MR), i.e., failure to achieve a complete hematological response (CHR) by 12 weeks, complete cytogenetic response (CCyR) by 12 months and major molecular response (MMR) by 18 months. Patients were followed-up at monthly intervals to begin with and this included physical examination, complete blood counts and liver function tests. Cytogenetic analysis, i.e., karyotyping from marrow was ordered every 6 months until CCyR was achieved. Those unwilling to undergo marrow examination were followed-up by molecular testing (FISH in the early days and PCR during last 4 years) on peripheral blood at 3-6 monthly intervals. Adverse effects were documented at each visit and were graded.
Events were defined by the initial occurrence of any of the following:
Disease progression to accelerated phase (AP) or blast crises (BC)
Death due to any cause during treatment
Loss of CHR or major cytogenetic response
Intolerance to imatinib
Patients were censored at the time of imatinib discontinuation or if they were still receiving imatinib but had lost CyR.
CHR was defined as total leukocyte count (TLC) <10,000/cmm with less than 5% myelocytes and metamyelocytes, no blasts or promyelocytes in peripheral blood together with platelet count <450,000/cmm, no extramedullary disease and no evidence of AP or BC.
CyR were assessed on marrow samples by karyotyping using G-banding technique on at least 20 metaphases per sample. The response was said to be complete if there was no Ph-positive metaphase, partial for 1-35% Ph-positive metaphases and major which included both complete and partial responses. MRs as stated earlier, were assessed by FISH in the early part of the study and by RQ PCR subsequently.
Event-free survival (EFS) was calculated from the date of the first IM dose to the first documentation of disease progression. Overall survival (OS) was calculated from the date of the first dose of IM to the date of last contact or death, whichever came first. Univariate and multivariate analysis was performed for factors predictive of a CCyR. Cox regression analysis was performed to evaluate following prognostic factors for survival: Age, gender, splenic enlargement, Hemoglobin (Hb) <10 g/dl, TLC >100,000/cmm, platelet count >450,000/cmm, blasts >5/cmm, basophils >7%, hematological and clinical response, CyR, Sokal score and previous treatment. A Sokal calculator was used to categorized patients into low, intermediate and high-risk groups.
Max Foundation has established GIPAP with the help of Novartis Pharmaceuticals globally including India. The primary objective of this program is to provide access to imatinib to as many patients as possible.
A total of 848 consecutive patients of CML-chronic phase were evaluated at our center between April 2002 and March 2009 (7 years). Out of these, 576 cases (68%) are on imatinib at the moment, 73 cases (9%) are on miscellaneous treatment while 199 cases (23%) have either not followed-up with us (130 i.e., 15%), expired (47 i.e., 5.5%) or discontinued imatinib due to adverse effects (22 i.e., 2.5%). Out of patients receiving imatinib, 536/576 (93.1%) are on Glivec through Max Foundation GIPAP program while the rest, i.e., 40 (6.9%) are on generics. We noticed no difference in the efficacy and toxicity between Glivec and generics and hence the whole group has been advised together. Majority of patients receiving miscellaneous treatment are either on dasatinib (33 cases) or nilotinib (10 cases). Patients on dasatinib (21/33) were receiving it as a part of an international trial while those on nilotinib received the drug from Novartis on compassionate grounds. Six patients went on upfront BMT and two received alpha interferon.
Evaluation included EFS, OS, CCyR, MR and adverse events. Event-free and OSs were calculated by Kaplan-Meier estimates. Cox regression analysis was performed to evaluate 12 prognostic factors for survival. Univariate and multivariate analysis were performed for factors predictive of CCyR and MR. These included age, gender, splenomegaly, Hb <10 g/dl, TLC >1,00,000/cmm, platelet count >4,50,000/cmm, blasts >5/cmm, basophils >7%, Sokal score, CHR, CyR and patients presenting in ECP versus LCP.
RESULTS
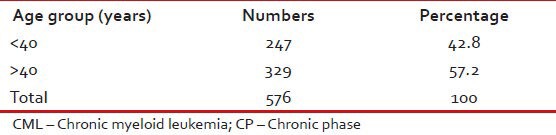
Tables 1–3 give the age and sex distribution of the patients. Median age was 42 years. This is approximately a decade earlier than what is reported from the western world. There were 378 (65.6%) males and 198 (34.4%) females with M:F ratio of 1.9. This difference in sex distribution was probably due to more importance given to the male patients for treatment.
Table 1.
Age distribution of patients with CML-CP (n = 576)
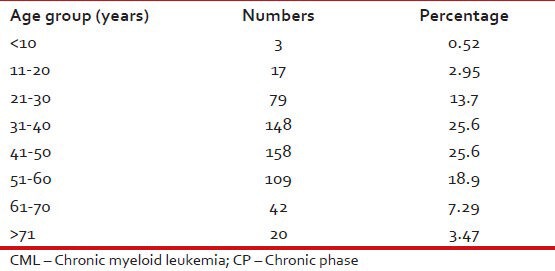
Table 3.
Sex distribution of patients with CML-CP (n = 576)
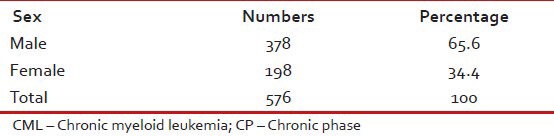
Table 2.
Age distribution of patients with CML-CP (n = 576)
At the time of the present analysis, majority, i.e., 417 (72.3%) were receiving the dose of 400 mg once a day while 27 (4.6%) were on 300 mg/day, 74 (12.8%) on 600 mg/day and 58 (10.3%) on 800 mg/day. The reason for 300 mg/day was usually intolerance to 400 mg/day while the same for 600 mg or 800 mg/day was inadequate cytogenetic and/or MR to 400 mg/day. In all, 132 i.e. 23.1% of subjects received 600 or 800 mg of IM daily. These details are shown in Table 4.
Table 4.
Imatinib mesylate: Dose

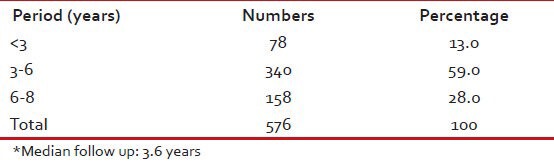
Median follow-up was 3.6 years with 158 (28%) having followed for 6-8 years and 340 (59%) for between 3 and 6 years [Table 5].
Table 5.
Follow-up duration*
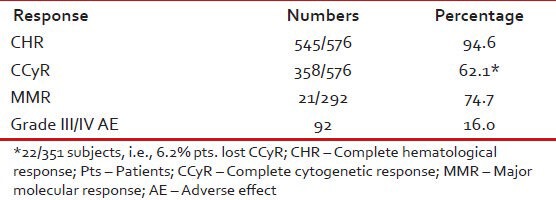
CCyR was achieved in 358 (62.1%) patients. On multivariate analysis, low Sokal score (P < 0.0001) and ECP (P < 0.001) emerged as the most significant predictors for achieving CCyR. An estimated 6.2% patients lost their CCyR. Grade III/IV toxicity was observed in 92 (16%) of patients [Table 6].
Table 6.
Results (n = 576)
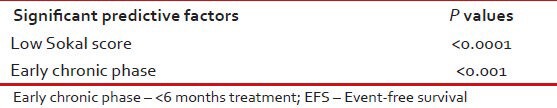
Estimated 5-year EFS and OSs of these 576 were 72% and 87% respectively [Table 7]. On Cox regression analysis significant predictive factors for EFS were age <40 years (P = 0.005), CCyR (P < 0.0001), low Sokal score (P = 0.005), complete clinical (P < 0.0001) and hematological responses (P < 0.0001) [Table 8]. Significant predictive factors for EFS on multivariate analysis were including low Sokal score and ECP [Table 9].
Table 7.
Estimated 5 years EFS and OS

Table 8.
Significant predictive factors for EFS (on Cox regression analysis)

Table 9.
Significant predictive factors for EFS (on multivariate analysis)
Tables 10 and 11 show patient characteristics according to CCyR.
Table 10.
Patient characteristics according to CCyR in CML: CP (n = 576)
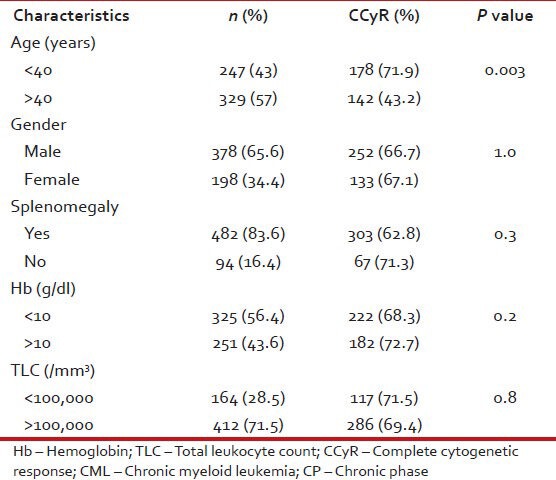
Table 11.
Patient characteristics according to CCyR in CML: CP (n = 576)
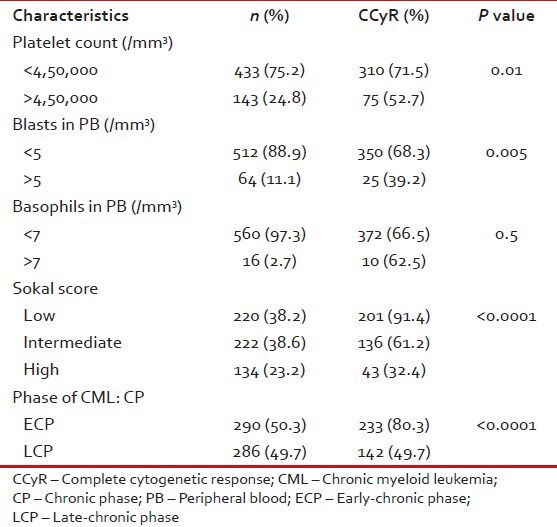
Table 12 shows response to imatinib with respect to ECP or LCP. MR was assessed in lesser number of patients and this may explain the discrepancy between patients achieving CCyR and MMR.
Table 12.
Response to IM in CML-CP (n = 286) as per nature of chronic phase, i.e., early or late
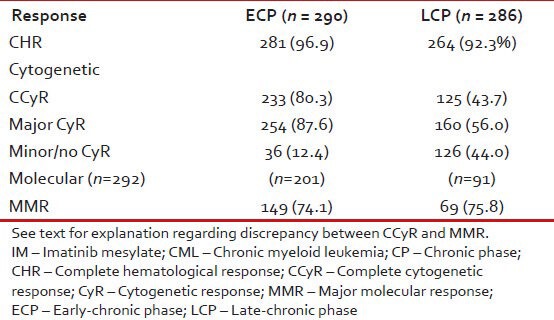
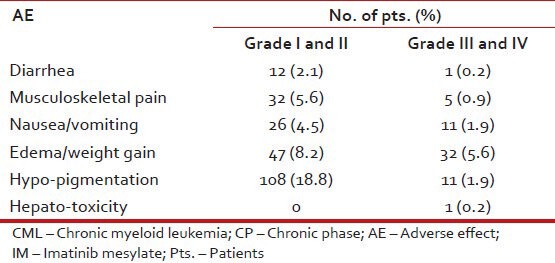
Table 13 shows the hematological adverse events while Table 14 shows non-hematological adverse events.
Table 13.
Hematological AE in pts. with CML: CP on IM (n = 576)
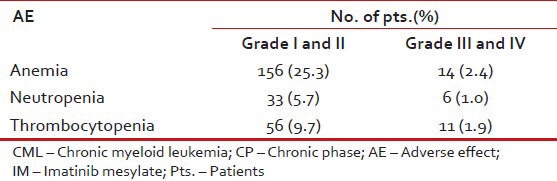
Table 14.
Non-hematological AE in pts. with CML: CP on IM (n = 576)
DISCUSSION
At our center, during the period of 7 years, i.e., April, 2002-March, 2009, 848 cases were seen with CML-CP. Out of these, 576 patients were analyzable with respect to efficacy and toxicity of IM. The median age of patients was 42 years and the median follow-up was 3.6 years. CCyR, i.e., CCyR was achieved in 358/376 patients, i.e., 62.1%. Grade 3/4 toxicity was observed in 36 subjects, i.e., 6.25%. Estimated 5-year EFS and OS were 72% and 87%respectively. On Cox regression analysis, age under 40 years, low Sokal score, CHR and CCyR were significant predictive factors for EFS while on multivariate analysis, low Sokal score and ECP were the significant predictive factors for CCyR.
We conclude that long-term management of CML-CP with IM produces excellent and durable responses with the minimal toxicity. Our survival outcome is nearly similar to that reported from various western populations and superior to those reported from certain institutions in India.[4,5,6,7,8] The difference is probably due to our center catering for higher socio-economic group (with higher education) coming from close vicinity permitting better follow-up.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gratwohl A, Brand R, Apperley J, Crawley C, Ruutu T, Corradini P, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: Transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91:513–21. [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Rosti G, Martinelli G, Bassi S, Amabile M, Trabacchi E, Giannini B, et al. Molecular response to imatinib in late chronic-phase chronic myeloid leukemia. Blood. 2004;103:2284–90. doi: 10.1182/blood-2003-07-2575. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Liu L, Wang Y, Wu G, Lai X, Cao W, et al. Efficacy and prognosis of chronic myeloid leukemia treated with imatinib mesylate in a Chinese population. Int J Hematol. 2009;89:445–51. doi: 10.1007/s12185-009-0292-7. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh C, Saikia T, Bakshi A, Amare-Kadam P, Baisane C, Parikh P. Imatinib mesylate in chronic myeloid leukemia: A prospective, single arm, non-randomized study. J Assoc Physicians India. 2005;53:291–5. [PubMed] [Google Scholar]
- 7.Hay J, Bapsy PP, Babu KG, Loknatha Imatinib mesylate in newly diagnosed patients with chronic myeloid leukaemia. ASCO annual meeting proceedings. J Clin Oncol. 2007;25:17251. [Google Scholar]
- 8.Rajappa S, Varadpande L, Paul T, Jacob R, Digumarti R. Imatinib mesylate in early chronic phase chronic myeloid leukemia: Experience from a developing country. Leuk Lymphoma. 2008;49:554–8. doi: 10.1080/10428190701824585. [DOI] [PubMed] [Google Scholar]


