Abstract
Omega hospital and Indo-American Center from Hyderabad presented a data of 55 patients with chronic myeloid leukemia 87% (48) patients were in chronic phase. 5% (3) patients had primary resistance, while 64% (36) had shown good response to imatinib. At a median follow-up of 36 months 73% (40) of patients were in chronic phase.
Keywords: Chronic myeloid leukemia, chronic phase, Omega Hospitals
INTRODUCTION
The advent of imatinib mesylate (IM) has changed the management of chronic myeloid leukemia (CML) completely.[1] It would be prudent to say that a revolutionary change in the overall treatment outcome of patients affected with CML has emerged. In India, this molecule has been made available to hundreds of patients through the Glivec International Patient Assistance Program.
The purpose of this study was to collect the data of patient treated with IM molecule and to know the various factors involved in the ultimate outcome of the treatment.
PATIENTS AND METHODS
Data was collected retrospectively from year July 2004 to January 2010. The names of patients were extracted from the out-patient and in-patient data register. The files of patients were referred for information such as their age, sex, date of diagnosis, address, phone no., blood counts, spleen size at diagnosis, brand and dose of imatinib, change in dose, any primary or secondary resistance; tolerability, the side-effects of imatinib and its outcome. Patients whose files were incomplete data were contacted on phone and information accordingly gathered.
Definitions
Standard definitions of chronic phase, accelerated phase, blast crisis and primary and secondary resistance are considered.[2] For chronic phase CML, treatment failure to IM 400 mg was defined as primary resistance i.e., failure to achieve complete hematologic response (CHR) after 3 months, failure to achieve cytogenetic response after 6 months, major cytogenetic response after 12 months and CCR after 18 months of therapy. Secondary resistance was defined as loss of CCR or rising white blood cell count to >10 × 109/L on two occasions more than 4 weeks apart, progression to accelerated phase or blast phases.
RESULTS
There were total 55 patients of CML registered with median age of 45 years (12-65 years) during the year July 2004-January 2010. The baseline characteristics of patients and the type of imatinib taken are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of the patients
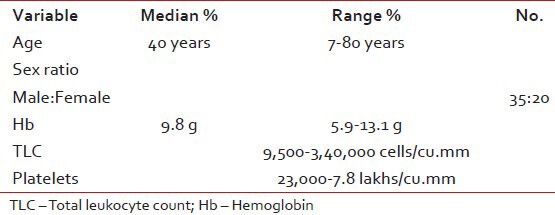
Table 2.
The disease characteristics at presentation and type of imatinib received by patients

Patients Sokal scores were calculated and following information was found:
35 patients in low risk group
Three patients were intermediate risk group and
Two patients were high risk group.
In 15 patients, data was not complete as in some patients either spleen size or complete blood count reports could not be retrieved.
Response and resistance pattern
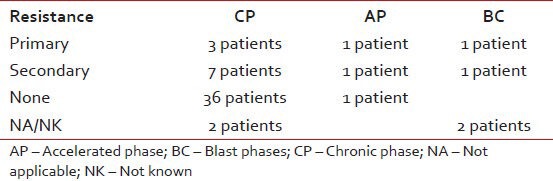
Documented complete hematological response was seen in 48 patients while four patients had no response, the resistance pattern to Imatinib in various phases of disease at presentation is shown in Table 3. Rest of the patients had incomplete data or their blood counts were not in the records.
Table 3.
Difference in the resistance patterns when compared with baseline diagnosis
Event free survival and overall survival
At a median follow-up of 36 months; 40 patients were in chronic phase, three patients were in accelerated phase, one in blast crisis, eight had died and three were lost to follow-up.
Change in the dose
The doses were increased in approximately three cases in CML-chronic phase (CP). In two cases, it was increased from 400 mg to 600 mg and in the third case it was further increased to 800 mg in view of poor response to initial dose. Two patients went into CHR; however, cytogenetic response was not there in all the three cases. Although two patients died, one has progressed to blast crisis.
Tolerability and side-effects of imatinib
No/minor side-effects were seen in 48 patients. Two patients had significant thrombocytopenia, in one patient it stopped for 6 weeks and then restarted, while in another patient-the dose of imatinib was reduced to 300 mg for almost 3 months and then escalated to 400 mg/day with fair tolerance. Two patients had fluid retention. Many of the patients had hypopigmentation, however exact numbers are not known.
CONCLUSION
Post imatinib era the treatment of CML has become easy, effective and affordable (due to availability of generic). In our study, the disease phase at diagnosis and the resistance experienced by patient were quite predictable. Patients in the (CML-CP) with good tolerability to imatinib have the best survival.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 2.Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–27. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]


