Abstract
Nizam's Institute of Medical Science is a premier institute of Hyderabad, established in 1980. The Medical Oncology Unit is the 1st comprehensive cancer center established for the state of Andhra Pradesh. The department has presented a data of total 201 patients with the median age of 32 at diagnosis. Among these 66 (33%) patients belonged to low Hasford risk group. Complete hematologic response was seen in 195 (97%) of patients. The progression free survival (PFS) was 77% for all patients while those who achieved complete cytogenetic response, PFS was 88% at 29 months.
Keywords: Chronic myeloid leukemia, chronic phase, imatinib
INTRODUCTION
Chronic myeloid leukemia (CML) is the most common adult leukemia in India. Optimizing imatinib (IM) therapy for early disease is clearly relevant to the majority of patients with CML.[1,2] In the International Randomized study of Interferon versus ST1571 study, complete cytogenetic response (CCR) was observed in 69% and major molecular responses in 40% at 1 year. Compared with western data, responses of patients from Indian Subcontinent with chronic phase (CP)-CML are sub optimal.
PATIENTS AND METHODS
Retrospective analysis of the newly diagnosed cases was done from year 2002 to 2009. The primary objective of this analysis is to evaluate hematologic and cytogenetic response rates of patients with early CP-CML to IM.[2,3]
RESULTS
There were 201 treatment naive patients who presented within 6 months of diagnosis. The median follow-up for all patients was 29.5 months (range: 3-58 months). The median age at diagnosis was 32, with a range of 18-72 years. There were 139 males and 62 females. Of these, 66 (33%), 81 (40%) and 54 (27%) belonged to the low, intermediate and high Hasford risk groups, respectively. Among the various Hasford groups, 52 (79%) of patients in the low risk category achieved CCR compared with 46 (57%) and 15 (27%) of patients in the intermediate and high-risk category, respectively. The difference in CCR between the risk groups was significant (P = 0.0018).
At 29 months, the estimated progression free survival (PFS) for all patients was 77% [Figure 1]. The response rates of chronic phase CML are shown in Table 1. The PFS events were as follows: Hematologic progression in 13 (6%), cytogenetic progression in 24 (12%) and accelerated or blast phase in 6 (3%) patients. For patients who achieved CCR, the PFS at 29 months was 88%, compared with 64% for all the other cytogenetic response categories (P = 0.0118) [Figure 2]. The estimated annual rate of treatment failure after the start of IM was 5% in the 1st year, 9% in the 2nd year, 7% and 1% in the 3rd and 4th year, respectively.
Figure 1.
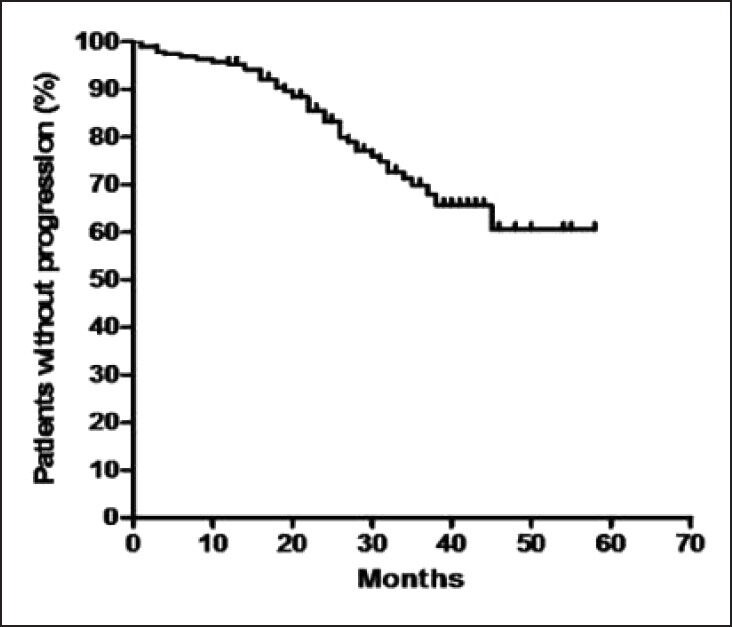
Kaplan-Meier curve of progression free survival for all patients
Table 1.
Response rates of chronic phase chronic myeloid leukemia to imatinib mesylate (n = 201)

Figure 2.
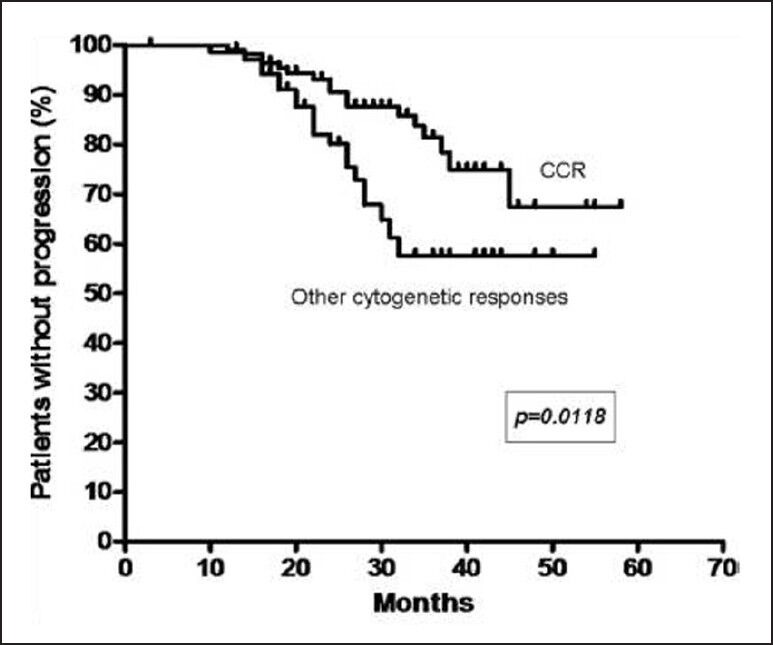
Kaplan-Meier curve of progression free survival based on cytogenetic response (CCR – Complete cytogenetic response)
At a median of 29 months, 94% patients are alive and on follow-up. 9 (4%) have died and 4 (2%) are lost for follow-up. The estimated survival at 29 months for patients with CCR is 100% and 94% for the other cytogenetic response groups (P = 0.0088). The adverse events are shown in Table 2.
Table 2.
Adverse events
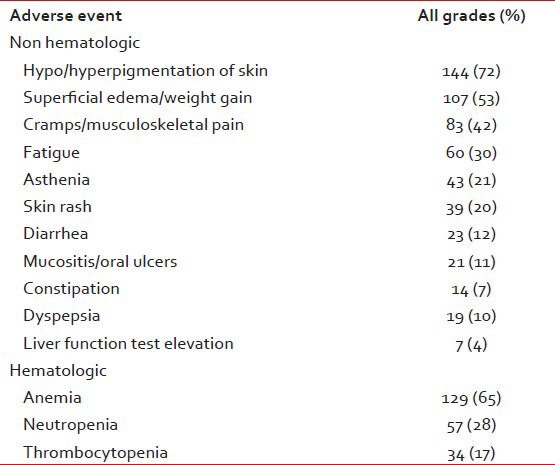
Among all patients, 43 (21%) needed temporary discontinuations in IM therapy due to adverse events. Reasons for treatment discontinuations included myelosuppression in 26 (13%), 11 (5%) for skin reactions and unknown in 6 (3%). The mean daily dose was 346 mg or 86% of scheduled. No patient needed permanent discontinuation of IM therapy.
DISCUSSION
Most patients with CP-CML are expected to attain CCR with IM.[4] The major cytogenetic responses in our study are higher than that previously reported for early CP patients from India. The CCR rates achieved in our patients with early CP were comparable to the CCR rates ranging from 41% to 64% reported in western studies for patients with late CP. Patients in late CP have inferior results with IM. Even patients who achieved CCR had poorer PFS with more patients losing responses every year than in western studies.
Most of the patients in this study had sub optimal monitoring with annual cytogenetics only. In fact, as cytogenetic progression was the most common form of loss of response (12%), annual cytogenetic evaluations may have overestimated the PFS. More patients in our series belonged to intermediate and high Hasford risk groups, which indicate late presentation and predict for poorer responses in chronic phase. Intrinsic differences in the biology of the disease and pharmacogenomics in our population may also be reasons for these poorer outcomes. However, until now, we do not have any laboratory evidence to support our hypothesis.
Considering the sub optimal outcomes with IM and the definite positive correlation between CCR and survival, allogeneic transplantation, higher doses of IM or alternative newer drugs may be important options for some patients at diagnosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bhutani M, Vora A, Kumar L, Kochupillai V. Lympho-hemopoietic malignancies in India. Med Oncol. 2002;19:141–50. doi: 10.1385/MO:19:3:141. [DOI] [PubMed] [Google Scholar]
- 2.Rajappa S, Varadpande L, Paul T, Jacob R, Digumarti R. Imatinib mesylate in early chronic phase chronic myeloid leukemia: Experience from a developing country. Leuk Lymphoma. 2008;49:554–8. doi: 10.1080/10428190701824585. [DOI] [PubMed] [Google Scholar]
- 3.Redaelli A, Bell C, Casagrande J, Stephens J, Botteman M, Laskin B, et al. Clinical and epidemiologic burden of chronic myelogenous leu kemia. Expert Rev Anticancer Ther. 2004;4:85–96. doi: 10.1586/14737140.4.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ. Chronic myelogenous leukaemia. Br J Haematol. 2000;111:993–1009. doi: 10.1046/j.1365-2141.2000.02216.x. [DOI] [PubMed] [Google Scholar]


