Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells with suppressive properties that preferentially expand in cancer. MDSC mainly suppress T cell proliferation and cytotoxicity, inhibit NK cell activation, and induce the differentiation and expansion of regulatory T cells (Tregs). The wide spectrum of MDSC suppressive activity in cancer and its role in tumor progression have rendered these cells a promising target for effective cancer immunotherapy. In this review we briefly discuss the origin of MDSC and their main mechanisms of suppression and focus more on the approaches developed up to date targeting of MDSC in tumor-bearing animals and cancer patients.
Introduction
Escape from immunosurveillance, is the last ‘E’ of the three ‘Es” in cancer immunoediting theory that consists of three main phases: Elimination, Equilibrium and Escape [1]. It has been well established that the immune system is a key player during tumor development, playing ambivalent roles in tumor elimination and tumor progression. Immune cells can recognize tumor-associated antigens [2] and eliminate tumor cells through numerous anti-tumor mechanisms. However, during tumor development, tumor factors in the tumor microenvironment modulate immune cells towards a protumorigenic phenotype leading to local and/or systemic immunosuppression, thus inducing tumor growth and establishing a suppressive niche in distant sites facilitating tumor metastasis. This unique interaction between tumor and immune cells in the tumor microenvironment has rendered immunotherapy a daunting task in our battle against cancer.
The tumor microenvironment exclusively promotes the induction and expansion of immune suppressors that ultimately inhibit effector T cell proliferation and the activation of cytotoxic T lymphocytes (CTLs) and anti-tumor NK cells. Earlier studies have described the presence of natural suppressors of lymphoproliferative responses with myeloid-cell characteristics in mice and humans [3–7]. The finding that suppressive monocytes expressing CD11b/Mac-1 accumulate in the spleens of tumor-bearing mice [8], has led to the identification of T cell suppressors that express CD11b/Mac-1 and Gr-1 antigens in the spleens of mice immunized with highly immunogenic recombinant anti-cancer vaccines and tumor-bearing mice [9–12]. A plethora of following studies in various cancer models, have identified these cells as a heterogeneous population of cells with myeloid origin that has the potential to differentiate into mature granulocytes, macrophages and dendritic cells, but under the influence of tumor factors are hampered in an immature state of differentiation with potent immune suppressive functions. This heterogeneous population of myeloid suppressive cells is collectively known as myeloid-derived suppressor cells (MDSC) [13].
Heterogeneity and T cell suppression are hallmarks of MDSC biology. In mice, MDSC are classified into two main subsets according to its morphology and markers: CD11b+Ly6ChighLy6G− cells resemble monocytes and are called monocytic MDSC (M-MDSC), and CD11b+Ly6G+Ly6C low/int cells with a polymorphonuclear morphology are called granulocytic or polymorphonuclear MDSC (G-MDSC/PMN-MDSC) [14, 15]. The absence of a Gr-1 homologue in humans led to the inclusion of a broader spectrum of markers in the description of suppressive myeloid cells in cancer patients. In general, M-MDSC express CD14−CD33+HLA−DRlowCD11b+, while G-MDSC express CD14−CD33+HLA-DRlowCD11b+CD15+ and/or CD66b+ (reviewed in ref [16]). M-MDSC expressing Lin−CD11b+CD14+ have also been described in patients with melanoma [17]. It is clearly noted that the expression of a unique marker of immunosuppression in MDSC in mice and humans has been one of the main challenges in the field.
The fact that MDSC immune suppression can be reversed in vitro and in vivo has led to the development of a multitude of strategies in cancer therapy. Herein, we briefly discuss MDSC origin and mechanisms of immune suppression, focusing more on the therapeutic strategies targeting MDSC in tumor-bearing mice and cancer patients.
MDSC origin
During differentiation hematopoietic stem cells (HSCs) diverge at a ‘decision-making point’ to common lymphoid progenitors (CLPs) to generate NK cells, T cells or B cells or common myeloid progenitors (CMPs) to generate monocytes, granulocytes, macrophages, dendritic cells (DC), megakaryocytes and erythrocytes in the presence of appropriate factors [18]. Since MDSC are a heterogeneous population of monocytic or granulocytic cells with a suppressive property, their origin and factors determining their fate, phenotype and function in cancer is controversial and need to be further elucidated. A common monocyte progenitor (cMoP) from monocyte-macrophage DC progenitor (MDP) that gives rise to monocytes or monocyte-derived macrophages was recently identified [19]. Under inflammatory conditions, cMoP differentiate into monocytes which can further differentiate into tissue-resident macrophages [19], consistent with the finding that MDSC in the tumor microenvironment differentiate toward tumor-associated macrophages (TAMs) via HIF-α induced by tumor hypoxic conditions [20]. MDSC can be induced from bone marrow (BM) precursors in the presence of the tumor-derived factors granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-6 [21]. Inhibition of paired immunoglobulin-like receptor-B (PIR-B) or C/EBPβ promotes MDSC differentiation to macrophages, granulocytes and DC with no suppressive function, suggesting that the suppressive function of MDSC induced by tumor factors is reversible [21, 22].
The origin and fate of Ly6Chigh and Ly6Clow monocytes are subject to further exploration, especially that Ly6Chigh monocytes with suppressive properties identified as M-MDSC have been widely studied in inflammation and cancer. It is still controversial whether Ly6Chigh monocytes differentiate to Ly6Clow monocytes or whether both monocyte subsets arise from independent precursors [23–27]. In EL4 mouse thymoma-bearing mice, splenic Ly6Chigh (Gr1int CD11b+) M-MDSC transferred to tumor-bearing mice are able to give rise to all CD11b+ subsets including Gr-1high granulocytes [28]. Similarly, Youn et al, recently demonstrated that M-MDSC (CD11b+Ly6Chigh) in tumor-bearing mice could differentiate into granulocytic MDSC expressing CD11b+ Ly6G+ Ly6C− [29]. This differentiation was mediated by epigenetic silencing of the retinoblastoma gene in M-MDSC mediated by histone deacytylase 2 (HDAC-2). These findings highlight M-MDSC plasticity and its potential to give rise to G-MDSC. It is still debatable whether G-MDSC consist solely of immature myeloid cells with suppressive function or whether mature neutrophils can acquire suppressive properties in the tumor milieu. A study by Fridlender et al identifies TGF-β as the inducer of suppressive neutrophils in the tumor (N2), since blockade of TGF-β enhances the recruitment and activation of neutrophils with anti-tumor functions (N1), suggesting that neutrophils can be polarized in tumor-bearing animals or potentially in cancer patients toward a suppressive phenotype [30]. Transcriptomic analysis revealed differences between tumor-associated neutrophils (TANs), splenic G-MDSC, and neutrophils from naïve mice [31]. Compared to neutrophils from naïve mice, TANs display lower expression of cell-cytotoxicity genes, a higher expression of MHC II complex genes and inflammatory cytokines (e.g. TNF-α, IL-1α/β), and upregulation of chemoattractants of T cells, B cells, neutrophils and macrophages. Interestingly, neutrophils from naïve animals are more closely related to splenic G-MDSC than to TANs. Unlike neutrophils from naïve mice, splenic G-MDSC are immune suppressive and express higher levels of M-CFSR, CD244, arginase, MPO and ROS, with a lower phagocytic activity [32]. A recent study has reported that tumor-promoting neutrophils are recruited to the tumors by tumor-derived oxysterols in a CXCR2-dependent manner, promoting tumor growth and neoangiogenesis [33]. Future studies will further unveil the exact origin of MDSC and its relation to other myeloid subsets in cancer.
MDSC mechanisms of suppression
Tumor-infiltrating immune cells with a suppressive phenotype includes regulatory T cells (Tregs), γδ T cells [34, 35], suppressive TAMs, and MDSC. The detailed mechanisms of MDSC suppression were recently described elsewhere [16, 36, 37]. Herein, we briefly highlight the main suppressive mechanisms employed by MDSC in cancer.
Although MDSC subsets share the ability to suppress T cell activation, their different mechanisms of function, recruitment and expansion in tumor-bearing animals are being constantly highlighted. In general, MDSC expansion is induced by tumor factors such as GM-CSF [38], stem-cell factor (SCF-1)[39], prostanglandin E2 (PGE2) [40], cyclooxigenase-2 (COX-2) [41], vascular endothelial growth factor (VEGF) [42], macrophage colony-stimulating factor (M-CSF) and IL-6 [43]. MDSC suppress T cell activity mainly by modulating L-arginine metabolism through the upregulation of arginase-1 (Arg1) and inducible nitric oxide synthase (iNOS) in M-MDSC, and Arg1 and reactive oxygen species (ROS) upregulation in G-MSDC [37]. Arg1 catalyzes the conversion of L-arginine to L-ornithine, while iNOS mediates NO production from L-arginine, ultimately leading to the production of urea [37]. L-arginine starvation induces the loss of the CD3ζ chain [44], and inhibition of T cell-cycle progression by restraining the upregulation of cyclin D3 and cyclin-dependent kinase 4 (cdk4) [45]. In addition, NO induces Fas-dependent apoptosis [46] and inhibits proteins downstream IL-2 receptor [47]. Moreover, reactions between NO and ROS, such as superoxide ions, produce peroxinitrite that impact CD8+ T cell activity by inducing the nitration of CD8+ T-cell receptor (TCR) restraining its recognition by the MHC peptide [36]. MDSC also deprive T cell from cystein, an essential amino acid required for T cell activation [48]. T cells do not produce cysteine and depends on APCs for cysteine supply after they uptake cystine. However, MDSC limit cystine pool available for APCs depriving T cells from cysteine [48].
M-MDSC and G-MDSC suppressive activities are thought to also diverge in the expression and phosphorylation of signal transducers and activators of transcription (STATs). G-MDSC suppressive functions are thought to be mainly mediated by STAT3 phosphorylation, while STAT1 seems to play a main role in M-MDSC suppressive biology [36]. Current studies show that STAT3 phosphorylation is a key event in regulating G-MDSC suppressive activity and inhibition of differentiation through various mechanisms. STAT3 phosphorylation enhances the production of ROS through the activation of calcium-binding proteins S100A9 and S100A8 [49] which are involved in the formation of NAPDH oxidase complex (Nox-2) [50]. In addition, upregulation of Nox-2 subunits, such as p47phox and gp91phox, are directly related to the enhanced production of ROS by G-MDSC augmenting its suppressive activity [36]. STAT3 not only promotes the suppressive activity and expansion of MDSC, but also induces tumorigenesis by mediating the production of myeloid-derived angiogenic factors such as VEGF [51]. STAT3 immunosuppressive functions of MDSC can also be mediated by Hsp72 from tumor-derived exosomes [52]. On the other hand, M-MDSC suppressive activities are mediated by factors that regulate Arg1 and iNOS production, such as STAT1. STAT1 activation is mediated by IFN-γ and IL-1β and is thought to play an important role in M-MDSC suppressive activity due to the fact that blocking IFN-γ or disrupting STAT1 partially reduced M-MDSC suppressive functions [14]. However, a recent study demonstrated that expression of IFN-γ and IL-4Rα is not required for T-cell suppression by MDSC, since MDSC from IFN-γ−/−, IFN-γR−/− and IL-4Rα−/− tumor-bearing mice suppress CD8+ and CD4+ T cells [53].
MDSC immunosuppression also extends to a vast network of immune cells including Tregs, macrophages, and NK cells. MDSC induce the recruitment and expansion of Tregs in tumor-bearing mice through the production of IL-10 and TGF-β [54], dependent on CD40-CD40L interaction [55]. IL-10 production by MDSC also decreases IL-12 production in macrophages enhancing Th2 responses in tumor-bearers [56]. However, a controversial report demonstrated that TGF-β mediated generation of induced Tregs (iTregs) (CD4+CD25+Foxp3+) and proliferation of natural Tregs (nTregs) is impaired by G-MDSC [57]. NK cell activity is also suppressed by MDSC. MDSC from the liver and spleen of tumor-bearing mice inhibit NK cell cytotoxicity, NKG2D expression and IFN-γ through membrane-bound TGF-β in tumor-bearers [58] or NKp30 receptor expression on NK cells in patients with hepatocellular carcinoma [59]. Interestingly, a recent report has demonstrated that NK cells can be converted to MDSC in the presence of GM-CSF [60]. However, this finding has to be further confirmed since NK cells share a common lymphoid progenitor with T and B cells but not myeloid cells [18].
Therapeutic Targeting of MDSC
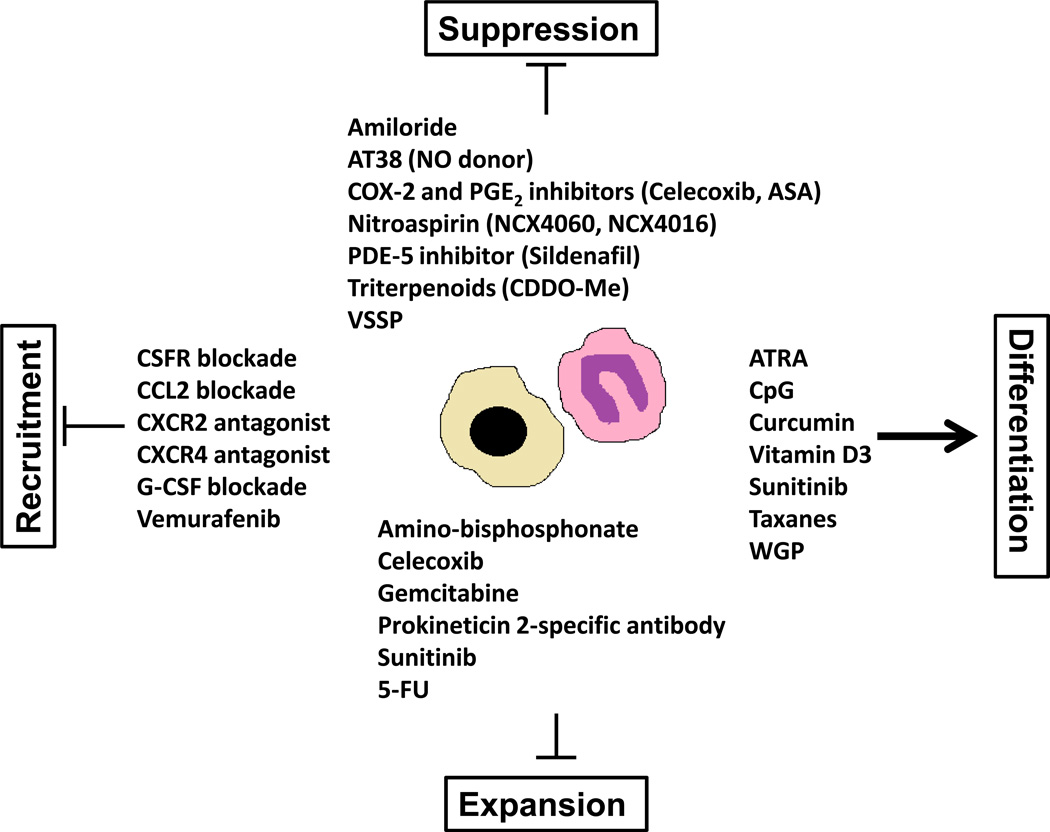
It is now being greatly highlighted that exploring immunosuppressive regulation by MDSC in the tumor microenvironment will bring a new paradigm in our understanding of cancer as well as for devising novel immunotherapeutic approaches. In recent years, many approaches have been developed with the goal of abolishing their suppressive activity in vivo as a therapeutic intervention in cancer. In the following, we discuss different therapeutic strategies applied in the modulation of MDSC in tumor-bearing mice and cancer patients including inhibition of MDSC suppressive function, expansion, recruitment, and induction of MDSC differentiation (Figure 1).
Figure 1.
The main therapeutic compounds targeting MDSC suppression, expansion, recruitment and differentiation in cancer.
I. Inhibition of MDSC suppressive function
1. Inhibitor of Reactive Nitrogen Species (RNS) (AT38)
Murine
RNS produced by MDSC and tumor cells such as peroxynitrite anion induces the nitration of the chemokine CCL2 preventing the migration of CTLs to the tumor core. Targeting of RNS with AT38 ([3-(aminocarbonyl) furoxan-4-yl] methyl salicylate) in mice bearing subcutaneous colon carcinoma expressing GM-CSF (C26GM), or thymoma expressing OVA (EG7-OVA), or spontaneous prostate cancer (TRAMP mice), downregulated Arg1, iNOS and peroxinitrite in MDSC, enhanced survival and improved the efficacy of adoptive transferred tumor-specific CTLs [61].
2. Nitroaspirin
Murine
NO-donating aspirin (NO aspirin) consists of an aspirin molecule covalently linked to a NO donor group. The effects of different NO-donating aspirins (NCX4060, NCX4016) on MDSC suppressive activity in cancer were studied by De Santo et al [62]. In BALB/c mice inoculated with a colon adenocarcinoma expressing GM-CSF (C26-GM), treatment with NCX4060 or NCX4016 restored T lymphocyte proliferation in MLR reactions or T cells induced with anti-CD3 and anti-CD28 in the presence of MDSC, increased CTL activity and reduced MDSC Arg1 and NOS activity, in vitro and in vivo. Despite these effects on MDSC suppressive function, treatment of tumor-bearing mice with NCX4016 orally did not significantly decrease tumor burden or prolonged survival except only when coupled to a recombinant DNA vaccine in two vaccination models:
Vaccination of BALB/c mice with a plasmid encoding the full-length env gene (pcDNA3-env), challenged with colon carcinoma CT26 (gp70 positive) subcutaneously, and orally treated with NXC4016.
Immunization of BALB/c mice with a plasmid DNA encoding p186 (extracellular and transmembrane portion of HER-2/neu) prior to challenging with a mammary carcinoma cell line N2C.
3. Phosphodiesterase-5 Inhibitors (Sildenafil)
Murine
Inhibition of cGMP phosphodiesterase gene family 5 (PDE-5) induces apoptosis of colon cancer cells through the induction of cGMP protein kinase (PKG) in colon tumor cells [63] or apoptosis of B-cell chronic lymphocytic leukemia (B-CLL) cells in a caspase- dependent manner [64]. Treatment of mice with Sildenafil, a PDE-5 inhibitor, delayed the progression of tumors in mice inoculated with different tumor cell lines including colon carcinoma (CT26WT), colon carcinoma expressing GM-CSF (C26GM), mammary adenocarcinoma (TS/A), MCA203 fibrosarcoma [65], and a mouse transgenic melanoma model [66]. Interestingly, delayed tumor progression was associated with the modulation of T cell immune suppression by MDSC towards a less suppressive phenotype. Treatment of tumor-bearing mice with Sildenafil given in the drinking water, decreased the expression of IL-4Rα, Arg1, NOS2 in tumor infiltrating CD11b+ cells, increased the efficacy of adoptive T cell transfer therapy (ACT), induced the infiltration of tumor infiltrating lymphocytes (TILs) including CD8+ and CD4+ T lymphocytes [65, 66], partially restored the expression of CD3ζ chain in CD8+ and CD4+ T lymphocytes infiltrating skin melanomas and metastatic lymph nodes, increased IL-2 levels and decreased the levels of IL-1β, VEGF, GM-CSF, IL-6, S100A9, and the chemokines CCL2 (MCP-1) and CCL3 (MIP-1α) in metastatic lymph nodes in melanoma-bearing mice [66]. The exact mechanism of linking the accumulation of cGMP in MDSC with L-arginine metabolism is not fully elucidated. Although Sildenafil delayed tumor growth in different mice models, tumor eradication was not achieved suggesting the importance of the combined targeting of a network of immune cells with a suppressive phenotype in the tumor microenvironment.
Human
PBMCs from patients with multiple myeloma (MM) or head and neck squamous cell carcinoma (NSCLC) stimulated in vitro with plate-coated anti-CD3/anti-CD28 Abs in the presence or absence of Sildenafil or Arg1 inhibitor (NorNOHA) or NOS2 inhibitor (L-NMMA) restored the proliferation of CD3 cells. Interestingly, Sildenafil only restored the proliferation of CD8+ T cells in PBMCs from NSCLC cancer patients. CD4+ T cell proliferation in PBMCs from MM patients was still lower than PBMCs from healthy donors [65]. More studies need to be performed to demonstrate the significance of these findings in humans, its toxicity, and its efficacy in the clearance of primary or metastatic tumors.
4. Triterpenoids (CDDO-Me)
Murine
Treatment of mice-bearing colon carcinoma (MC38), Lewis lung carcinoma (LLC) or EL-4 thymoma with a synthetic triterpenoid, CDDO-Me, abrogated MDSC suppressive function through the downregulation of ROS and inhibition of STAT3, and inhibited tumor growth. In addition, CDDO-Me enhanced the efficacy of survivin vaccine in vivo. However, CDDO-Me treatment did not affect Arg1 and NO production or the frequency of MDSC in the spleens of tumor-bearing mice [67].
Human
Treatment of patients with locally advanced (stage II-III) or metastatic (stage IV) pancreatic cancer with CDDO-Me and gemcitabine in a Phase I clinical trial had no effects in the frequencies of MDSC in the peripheral blood with an increased response of T cells from treated patients to tetanus toxoid and phytohemagglutinin. However, the effect of CDDO-Me treatment was not assessed in the absence of gemcitabine [67].
5. Very small size proteoliposomes (VSSP)
Murine
VSSP is a nanoparticulated adjuvant that promotes DC maturation and enhances CD8+ T cell effector function. Treatment of tumor-bearing mice with VSSP increases the accumulation of MDSC in the spleen. However, MDSC from the spleens of VSSP-treated mice are significantly less suppressive than non-treated mice, correlating with enhanced CTL activity in mice treated with VSSP. In addition, VSSP treatment induces the differentiation of MDSC into mature antigen-presenting cells (APC) [68]. More studies need to be performed to evaluate VSSP efficacy in the clinic.
6. Inhibition of exosome formation (Amiloride)
Murine
Amiloride inhibits exosome formation. It has been reported that membrane associated Hsp72 from tumor-derived exosomes (TDEs) induce MDSC suppressive activity by inducing the STAT3 phosphorylation in a TLR2/MyD88-dependent manner through autocrine IL-6. Treatment of mice-bearing CT26, TS/A, or EL4 tumors with exosome inhibitors such as amiloride, enhanced anti-tumor immune responses when combined with cyclophosphamide[52]. However, treatment of mice with exosome inhibitors alone did not reduce tumor growth.
Human
Treatment of patients with colorectal metastatic carcinoma with amiloride for 3 weeks abrogated the suppressive activity of MDSC from peripheral blood ex vivo, and decreased the ability of autologous serum to induce STAT3 phosphorylation in MDSC [52].
II. Inhibition of MDSC expansion
1. Gemcitabine and 5-Fluorouracil
Murine
Pyrimidine analogues, such as Gemcitabine (Gem) and 5-Fluorouracil (5-FU), have been currently used in the clinic to induce tumor cell death and hamper tumor growth. Their cytotoxic effects on MDSC in tumor-bearers have currently been described. Administration of Gem and/or 5-FU selectively induced apoptosis of Gr1+CD11b+ MDSC in the spleens and tumors of tumor-bearing mice, with no significant decrease in the levels of CD4+, CD8+, B cells, NK cells or macrophages and enhanced IFN-γ production by tumor-specific CD8+ T cells and NK cells [69–72]. The anti-tumor effects of Gem were enhanced when combined with other therapeutic protocols including chemotherapeutic drugs, such as cyclophosphamide [73] and rozigliazone, [74] that target Tregs and PPARγ respectively, or in combination with adenoviral based immunotherapy [75] or IFN-β treatment [69]. Despite promising effects in anti-tumor immunity, the ambivalent effects of chemotherapeutic drugs, including Gem and 5-FU, is yet to be further explored. Gem and 5-FU triggered the production of IL-1β in MDSC following the activation of the inflammasome (NOD-like receptor family, pyrin domain containing-3 protein (Nlrp3)-dependent caspase-1 activation complex) in a cathepsin-B dependent manner. Secreted IL-1β then stimulated the production of IL-17 by CD4+ T cells inducing the expression of angiogenic factors such as Eng and Pecam1 counteracting anti-tumor immunity [76].
2. Cyclooxygenase (COX-2) and Prostaglandin E2 (PGE2) inhibitors
Murine
COX-2 and PGE2 are produced by different human and murine cancer cells including LLC, renal carcinoma, colon carcinoma MCA-38 and head and neck tumors [41]. Targeting COX-2 and PGE2 with inhibitors, such as indomethacin [77], celecoxib [78] , meloxicam [79] and acetylsalicylic acid (ASA) [80], augmented tumor growth in different cancer models. COX-2 inhibitors reduced systemic levels of PGE2, modulating MDSC suppressive function, recruitment and induction. COX-2 inhibitors inhibited Arg1 expression in MDSC induced by tumor released factors such as COX-2 and PGE2, reduced ROS and NO levels, enhanced T cells anti-tumor responses and improved the efficacy of DC-based immunotherapy in tumor-bearing mice [41, 81].
ASA also regulated the recruitment of MDSC to the tumors by decreasing the levels of MDSC-attracting chemokine CCL2 and increasing the expression of CXCL10 and CTL infiltration in glioma [80]. COX-2 is a PGE2 –forming enzyme that can also be activated through PGE2 receptor EP2 in MDSC by PGE2 [40, 82]. Induction of EP2 in MDSC by PGE2 hampered their differentiation to mature APCs from the bone marrow [82], and induced the generation of MDSC from monocytes in vitro by blocking monocyte differentiation to mature CD1a+ DC and enhancing the expression of indoleamine 2,3-dioxygenase (IDO), IL-4Rα, NOS2 and IL-10 [83].
Human
CD14+ monocytes acquire MDSC phenotype through coculture with human melanoma cells through a COX-2-dependent mechanism [84]. In patients with advanced melanoma, inhibition of PGE2, COX-2, STAT3 and superoxide in MDSC restored T-cell proliferation in culture.
3. Sunitinib
Murine
Sunitinib, a tyrosine kinase inhibitor, targets a wide range of kinases including, platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptors (VEGFR1-VEGFR3), SCF, m-CSF and FMS-like tyrosine kinase 3 (FLT3) [85–87]. Sunitib is currently approved by the Food and Drug Administration (FDA) for the treatment of patients with gastrointestinal stromal tumors (GIST) that failed to respond to Imatinib [85, 88] and as a first-line treatment of patients with metastatic RCC [89, 90]. In addition to its angiogenic effects and targeting of tumor cells, sunitinib immune modulation of suppressor cells, such as MDSC and Tregs, has been recently described [91–93]. Sunitib treatment decreased the percentages of MDSC and Tregs in the spleen of tumor-bearers, reduced the expression of pro-tumorigenic factors in tumor infiltrating lymphocytes (TILs) such as IL-10, TGF-β, Foxp3, CTLA-4 and PDL-1, skewing suppressed T lymphocytes towards an anti-tumor Th1 phenotype, represented by the increased levels of IFN-γ and CTL responses [91]. Sunitib combined with IL-12 and 4-1BB activation prolonged survival of mice bearing MCA26 colon tumor, and increased the anti-tumor efficacy of OVA-loaded DC vaccines in mice bearing subcutaneous B16 melanoma expressing OVA [91, 94]. However, sunitinib modulation of tumor MDSC seems to be compromised in tumors expressing high levels of GM-CSF, such as 4T1 or human RCC cells [95, 96]. GM-CSF in the tumor microenvironment reprograms MDSC to act independently on STAT3 through the induction of STAT5 phosphorylation, bypassing the inhibition of STAT3 phosphorylation induced by sunitinib [92, 95, 96]. Another mechanism of tumor evasion from sunitinib treatment is the increased production of the chemokine stromal cell-derived factor-1 (SDF-1; CXCL12) in the tumor microenvironment following p53 activation induced by sunitinib, leading to increased levels of MDSC in RCC xenografts [97].
Human
The clinical outcome of sunitinib treatment in patients with metastatic RCC is limited to its ability to modulate immune suppression in the tumor rather than in the periphery [95, 96]. Despite the encouraging findings that sunitinib treatment decreases the levels of MDSC, Tregs and IFN-γ producing T lymphocytes in PBMCs of RCC patients [98, 99], its effects on intratumoral MDSC and proangiogenic factors such as MMP-9, MMP-8 and IL-8 in tumor explants were minimal [95, 96].
4. Blockade of stem cell factor (SCF; ckit ligand)
Murine
SCF is expressed by tumor cells, with a higher expression in large tumors. SCF production in the tumor microenvironment enhances the accumulation of MDSC in the tumor. SCF siRNA knockdown in tumor cells, and blocking SCFR-SCF binding with anti-ckit reduces MSDC expansion and infiltration in the BM and tumor, reduced angiogenesis, enhanced T cell proliferative responses and decreased Treg development in mice bearing MCA26 colon carcinoma [39].
5. Amino-biphosphonate (Zoledronate, Pamidronate)
Murine
Amino-bisphosphonates is a MMP-9 (metalloproteinase-9) inhibitor. The production of MMP-9 by tumor and stromal cells is directly correlated with VEGF production in the tumor microenvironment. Targeting MMP-9 by amino-biphosphate in mice with spontaneous mammary carcinoma reduced the levels of pro-MMP-9 and VEGF in the serum, tumor-enhanced BM hematopoiesis and improved anti-tumor responses induced by DNA vaccination [100].
6. Doxorubicin-cyclophosphamide chemotherapy
Human
MDSC (Lin−/low CD33+CD11b+ HLA-DR−) percentages and absolute numbers in the peripheral blood of breast cancer patients directly correlate with the stage of the disease; patients in stage IV with extensive metastatic tumor having the highest numbers of circulating MDSC and lowest T cell responses. Doxorubicin and cyclophosphamide are common chemotherapeutic drugs included for the treatment of breast cancer. Interestingly, patients treated with doxorubicin-cyclophosphamide displayed increased percentages of MDSC in the peripheral blood but not when followed with paclitaxel treatment, suggesting that treatment with doxorubicin-cyclophosphamide alone may not be favorable in the development of anti-tumor immune responses in patients with breast cancer [101].
III - Inhibition of MDSC recruitment
1. Vemurafenib
Human
Vemurafenib is a specific inhibitor of BRAFV600E [102], a mutation that causes constitutive activation of the MAP Kinase pathway, a common mutation in melanoma patients [103]. Treatment of advanced melanoma patients with Vemurafenib decreased the frequencies of M-MDSC (CD14+ HLA-DR−/low) and G-MDSC (CD66b+Arginase1+CD16−/low) in PBMCs [102]. The clinical course melanoma was correlated with an increase in the percentage or M-MDSC and G-MDSC, with patients responding more to treatment having lower percentages of M-MDSC, while G-MDSC percentages varied among patients. Vemurafenib treatment is thought to modulate the tumor microenvironment impacting MDSC induction since culturing PBMCs from healthy donors in a conditioned media from a primary melanoma cell line in the presence of Vemurafenib failed to induce CD14+HLA-DR−/low M-MDSC compared to cultures with no Vemurafenib [102]. However, it was not shown in this study whether Vemurafenib had direct effects on MDSC suppressive functions.
2. Anti-G-CSF (granulocyte- colony stimulating factor) and anti-Bv8 antibodies
Murine
Bv8 (prokineticin-2) is upregulated in MDSC upon G-CSF receptor activation by G-CSF [104]. In tumor-bearing mice Bv8 induced angiogenesis, MDSC mobilization from the BM to the tumor or distant sites establishing a pro-metastatic niche for the colonization of metastatic tumor cells [104, 105]. Overall, targeting the G-CSF-Bv8 axis in tumor-bearing animals with specific antibodies decreased MDSC infiltration in the tumor, G-MDSC recruitment to the lungs of tumor-bearing mice, and decreased angiogenesis, tumor growth and metastasis.
3. Anti-CSF-1 receptor (CSF1R) (GW2580)
Murine
GW2580 is a kinase inhibitor of CSF1R [106]. Targeting CSF1R with GW2580 in LLC-bearing mice decreased M-MDSC infiltration in the tumor with no effect on G-MDSC recruitment, decreased the expression of Arg1 and MMP-9 in the tumors, and angiogenesis. Interestingly, treatment of CSF-1 only impaired tumor growth when combined with VEGFR-2 (vascular endothelial growth factor-2 receptor) [107].
4. Anti-CCL2 antibody
Murine
Inflammatory monocytes expressing Gr-1 and CCR2 (CCL2 chemokine receptor), in response to CCL2 produced by tumor cells and stroma are preferentially recruited to pulmonary metastases but not to primary tumor. Ablation of CCL2-CCR2 signaling with anti-CCL2 antibody promoted tumor survival and decreased tumor metastasis by blocking the recruitment of inflammatory monocytes to the lungs. Induction of tumor metastasis in the lungs by inflammatory monocytes was dependent on the expression of VEGFA, since inhibition of VEGFA expression in inflammatory monocytes abrogated the index of metastases in the lungs [108].
5. CXCR2 and CXCR4 antagonists
Murine
Tumors with Tgfbr2 deletion induced the recruitment of MDSC through the production of CXCL5 and SDF-1, that chemoattracted MDSC expressing CXCR2 and CXCR4. In mice inoculated with 4T1 mammary carcinoma, blockade of CXCR2 and CXCR4 significantly decreased lung metastasis with no substantial difference in the growth of the primary tumors [109].
IV - Induction of MDSC differentiation
1. Vitamin D3
Human
Differentiation of suppressive CD34+ myeloid progenitors to DC by the differentiation-inducing hormone 1α, 25-hydroxyvitamin D3, has been reported in mice bearing Lewis Lung Carcinoma (LLC) and patients with NSCLC [110, 111]. In a study conducted by Kulbersh et al [111], 17 NSCLC patients were either treated (11 patients) or untreated (6 patients) with 1α, 25-hydroxyvitamin D3 for 3 weeks prior to surgery. Analysis of CD34+ and dendritic cells by immunohistochemistry in the NSCLC tissues revealed reduced infiltration of intratumoral CD34+ cells and immature DC-SIGN+ dendritic cells and increased numbers of intratumoral DC-LAMP+ mature DC. To assess the clinical significance of such findings, a clinical trial was conducted in 32 newly diagnosed patients with NSCLC, with 16 patients left untreated or and the other 16 treated with 1α, 25-hydroxyvitamin D3 for 3 weeks prior to surgical removal of the tumor [112]. Immunohistochemical analysis of the tumor revealed a significant increase in the levels of intratumoral CD4+, CD8+ T lymphocytes and cells expressing the activation markers CD69 in patients treated with 1α, 25-hydroxyvitamin D3. To assess the significance of these findings NSCLC patients were monitored for tumor recurrence after surgery, with median recurrence being 181 days in the untreated group compared to 620 days in the treated group, emphasizing the importance of combining immune therapy by targeting suppressive cells to surgical or chemotherapy procedures in the treatment of cancer. In another study to determine the effects of 1α, 25-hydroxyvitamin D3 in the cytokine profiles in the plasma and tumor tissue of NSCLC patients, it was reported that 1α, 25-hydroxyvitamin D3 differently modulates the cytokine milieu in the plasma compared to tumor tissue, increasing the levels of IL-6, IL-10, IL-2, IFN-γ, TNF-α in the tumor tissue, and increased levels of IL-8, VEGF, IL-1α and IL-1β in the plasma but not tumors of treated patients compared to the levels in untreated patients [113]. Although some studies have assessed the effects of 1α, 25-hydroxyvitamin D3 on the cytokine profile of human monocytes and macrophages in vitro [114], more studies need to be performed to link the effects of different cytokine milieu in the tumor and plasma of cancer patients in differentiation of immunosuppressive cells to mature DC.
In a recent report, fibrocytes expressing CD45+CD34+HLA-DR+ with a suppressive phenotype were found to expand in patients with metastatic pediatric sarcomas, demonstrating both features of neutrophilic and monocytic cells, suppressed anti-CD3 induced T cell proliferation through IDO, and its expansion correlated with an increased Th2 phenotype in patients [115]. Since 1α, 25-hydroxyvitamin D3 is believed to skew CD34+ differentiation to mature DC, their effect in other types of cancers on suppressive CD34+ cells is still to be elucidated.
2. Taxanes (Docetaxel and Paclitaxel)
Docetaxel and Paclitaxel are semi-synthetic taxanes with anti-tumor properties. These drugs target tubulin in rapidly dividing cells, stabilizing microtubules during cell division leading to cell arrest and cell death. It has been reported in several studies that these taxanes modulate immune responses in cancer patients and tumor-bearing mice [116, 117]. Herein, we summarize the main effects of Docetaxel and Paclitaxel on MDSC in murine tumor models and cancer patients.
Murine
In a murine squamous cell carcinoma model, SCC VII/SF, docetaxel in conjunction with vitamin D3 increased the numbers of intratumoral active T cells compared to vitamin D3 alone. However, docetaxel had no effect on the levels of CD34+ cells in the spleens and lymph nodes, and no difference in tumor weight was found between untreated mice or mice treated with vitamin D3 and/or docetaxel. It is important to note that the efficacy of vitamin D3 treatment in clinical trials on the recurrence of NSCLC in patients was assessed pos surgery [113], suggesting that additional procedures are required along with immune therapy in the treatment of cancer.
In a mammary carcinoma model, 4T1-Neu, intraperitoneal treatment of tumor-bearing mice significantly decreased tumor growth through the modulation of MDSC [118]. Docetaxel treatment decreased the percentage of splenic MDSC, decreased its suppressive activity, increased T cells CTL activity, upregulated the expression of CCR7 (M1 marker), MHC II, CD11c, CD86 in MDSC, and preferentially induced cell death of Mannose Receptor (MR+; M2 marker) MDSC. Incubation of MDSC for 6 hours decreased STAT3 phosphorylation, suggesting that docetaxel directly modulates MDSC signaling. Consistent with these findings, treatment of B16-melanoma-bearing mice with docetaxel following total body irradiation (TBI), improved T cell transfer and dendritic cell therapy, improving CTL function in vaccinated mice, by targeting highly suppressive MDSC and blocking their rapid reconstitution following TBI [119]. However, the mechanisms of docetaxel effects on MDSC differentiation and the presence of possible receptors have not been identified.
Combination of ultra low-doses of Paclitaxel with a peptide vaccine derived from the melanoma antigen Tyrosine related protein 2 (TRP2) enhanced vaccine efficiency in healthy mice. The vaccine efficiency was associated with an increase in the levels of TRP-2 specific T cells in the spleen, and correlated with decreased levels of Tregs and immature myeloid cells, increased levels of effector CD8+ and CD4+ T lymphocytes in the bone marrow and spleen, IFNγ-producing NK cells in the bone marrow and NKT cells in the lymph nodes [120]. Stimulation of MDSC in vitro with Paclitaxel in ultra-low doses induced the expression of CD11c, CD86 and CD40 in a TLR4-independent manner [121], suggesting that the decrease in the levels of MDSC in Paclitaxel-treated mice was due to its differentiation to DC, however the levels of DC in tumor-bearing mice treated with Paclitaxel was not speculated.
Human
The levels of circulating MDSC was assessed in a clinical trial involving forty-one women diagnosed with HER-2 neu negative breast cancer in stages II-IIIa and received three chemotherapeutic drugs: doxorubicin-cyclophosphamide followed by docetaxel every 3 weeks followed by NOV-002, a disodium glutathione disulfide [122]. It was found that patients who achieved pathologic complete response (pCR) (defined as no metastatic tumor in the axillary lymph nodes, no invasive tumor in the breast or and invasive tumor ≤ 10mm in dimension) had lower levels of circulating MDSC (Lin−HLA-DR−CD11b+CD33+) in the blood compared to patients who did not achieve pCR. In this study 15 out of 39 patients achieved a pCR. Suggesting that MDSC targeting may increase the efficacy of chemotherapy regimens currently used in the clinic.
3. All trans-Retinoic s (ATRA)
Murine
Retinoic acid was previously shown to induce granulocytic differentiation of cells from patients with acute promyelocytic leukemia in vitro [123]. In mice bearing subcutaneous C3 fibrosarcoma or DA3-HA mammary adenocarcinoma, treatment with subcutaneously implanted ATRA pellets induced the differentiation of adoptively transferred MDSC to DC (CD11c+ IAb+), macrophages (F4/80+) and granulocytes (Gr1+CD11b−), and improved CD4+ T cell responses [124]. Although treatment of tumor-bearing mice with ATRA decreased the percentages of MDSC (Gr1+CD11b+) in the spleens and bone marrow of tumor-bearing mice, it did not decrease tumor growth except only when combined with a DC cell vaccine transduced with Ad-p53 [124]. ATRA-induced differentiation of MDSC from tumor-bearing mice and patients with renal carcinoma was shown to be through the activation of glutathione synthase (GSS) and accumulation of glutathione (GSH) leading to a neutralization of reactive oxygen species (ROS) in an ERK1/2-dependent mechanism [125]. In addition, ATRA enhanced the immunogenicity, and differentiation of MDSC loaded with NKT cell ligand α-GalCer in a mechanism dependent on GSH activation in MDSC and IFN-γ production by NKT cells [126].
Human
ATRA induction of MDSC differentiation in murine models was extended to clinical trials in patients with metastatic renal cell carcinoma (RCC) and small cell lung cancer (SCLC) [127, 128]. In RCC patients treated with ATRA prior to IL-2 treatment had reduced numbers of MDSC, a higher myeloid/lymphoid dendritic cell ratio, and decreased the suppressive activity of mononuclear cells [127]. Interestingly, IL-2 treatment abrogated ATRA effect. In vitro culture of MDSC from patients with metastatic RCC with ATRA induced its differentiation to functional APCs, and abrogated its immune suppression [129]. Similarly, in patients with SCLC, ATRA treatment increased DC vaccine efficacy decreasing MDSC levels in the peripheral blood and improving antigen-specific CD8+ T cell responses [128].
4. TLR9 activation by CpG
Murine
Activation of TLR9 receptor in MDSC by CpG induced MDSC maturation and differentiation, and abrogated MDSC suppressive function, especially G-MDSC, in mice-bearing subcutaneous C26 tumors and in CEA424-Tag mice autochthonous gastric tumors. MDSC differentiation and inhibition of suppressive function were promoted by IFN-α produced by plasmacytoid DC (pDC) in vitro. However, the effect of IFN-α on MDSC differentiation and function needs to be further elucidated [130].
5. Curcumin
Murine
In a human gastric cancer xenograft model and a mouse colon cancer allograft model, treatment with curcumin in the diet or intraperitoneally decreased the percentage of MDSC in the tumor, spleen and blood. G-MDSC percentage was decreased upon curcumin treatment, while M-MDSC differentiated to an M1-like phenotype with an increased expression in CXCR7. Curcumin treatment of MDSC cocultured with cancer cells or myofibroblast-conditioned medium in the presence of IL-1β inhibited p-Stat3 and IL-6 production by MDSC. However, it was not determined in this study whether curcumin impacted MDSC suppressive function [131].
6. Whole-glucan particles (WGP)
Murine
Treatment of M-MDSC from LLC-bearing mice in vitro with WGP induced a population of cells expressing CD11c+ F4/80+ Ly6Clow. M-MDSC suppressive function was also decreased upon incubation with WGP. Oral treatment of LLC-bearing mice with WGP significantly reduced the percentage of Gr-1+ CD11b+ cells and Tregs, and increased the percentage of macrophages, DC and effector CD8+ T cells in vivo, with a significant decrease in tumor burden [132]. The effects of WGP on M-MDSC were dependent on Syk and NF-κB p65 signaling.
The main therapeutic approaches applied up to date in the clinic to target MDSC in cancer patients are summarized in Table 1.
Table 1.
Therapeutic targeting of MDSC in human cancer
| Therapeutic drugs | Effect on MDSC | Overall Results | Tissue/type of cancer | Method | Ref. |
|---|---|---|---|---|---|
|
Phosphodiesterase-5 inhibitor (Sildenafil) |
Inhibit MDSC suppression |
Restore T cell proliferation | PBMCs; MM and NSCLC |
Sildenafil added in vitro to PBMC cultures |
[65] |
|
Triterpenoid (CDDO- Me) + Gemcitabine |
Inhibit MDSC suppression |
|
PBMCs; Stage II-IV pancreatic cancer |
Phase I trial T cell activation assessed ex vivo |
[67] |
|
Inhibitor of exosome formation (Amiloride) |
Inhibit MDSC suppression |
Decrease pStat3 in MDSC | PBMCs; colorectal metastatic carcinoma |
Phase I trial
|
[52] |
|
COX-2 and PGE-2 inhibitors (Celecoxib) |
Inhibit MDSC induction, expansion and suppression |
Restore CD3 proliferation | PBMCs; melanoma | COX-2 and PGE-2 inhibitors added in vitro to CD14+ and T cell cocultures |
[84] |
|
Dexorubicin- cyclophosphamide |
Killing tumor cells and Treg modulation |
|
PBMCs; StageI/II, stage III and stage IV breast cancer |
Percentages of MDSC and suppressive assays assessed ex vivo after each cycle of chemotherapy |
[101] |
|
Vemurafenib (BRAF V600E inhibitor) |
Inhibit MAP kinase pathway in tumor cells |
|
PBMCs; Advanced melanoma |
Percentages of MDSC and suppressive assays assessed ex vivo in patients treated with Vemurafenib |
[102] |
|
Vitamin D3 (1α,25- hydroxyvitamin D3) |
Differentiation of suppressive CD34+ myeloid progenitors to DC |
|
Tumor tissue; NSCLC |
Immunohistochemical analysis of the tumor of patients treated 3 weeks prior surgery |
[110–115] |
|
doxorubicin- cyclophosphamide + docetaxel every 3 weeks followed by NOV-002, a disodium glutathione disulfide |
Targeting of tumor cells and immune cells (T cells, MDSC) |
|
PBMCs; HER-2 neu negative breast cancer in stages II- IIIa |
Percentages of MDSC in PBMCs assessed after reatment |
[116,117] |
| ATRA (Vesanoid)+IL2 | Prior to IL-2 treatment ATRA Induces MDSC differentiation to functional APC and decreases MDSC suppressive activity |
|
PBMCs; metastatic renal cell carcinoma, SCLC |
Clinical trial Among 18 patients, there were 1 complete response, no partial responses, 11 stable diseases and 3 progression. 3 patients had IL-2 treatment discontinued. |
[127,128] |
|
Sunitinib (tyrosine kinase inhibitor) |
Inhibition of MDSC expansion |
|
PBMCs; RCC |
|
[95,96,98,99] |
Conclusion Remarks and Future Perspectives
Chemotherapeutic protocols already established in the clinic mainly include drugs that target tumor cell proliferation and induce tumor cell death. The success of current chemotherapeutic protocols in the clinic has been limited due to the ability of cancer cells to develop resistance and evade immunesurvaillance. MDSC have been widely described in the literature and it is currently accepted that targeting MDSC in the clinic is vital for the development of effective anti-tumor immunity.
One of the main challenges in MDSC research is its heterogeneity and the absence of a universal marker for immunosuppression. The availability of a wide spectrum of markers to describe different MDSC subsets in mice and the absence of a Gr-1 homologue in humans have limited the success of MDSC targeting in the clinic. The absence of unique markers has led to the lack of consensus in the literature, especially in human subject studies. In addition, due to limitations in the acquisition of tumor tissue from cancer patients, most studies describing MDSC phenotype, suppressive activity and response to therapeutic treatment in human cancer patients are restricted to the peripheral blood. Current data from tumor-bearing mice and clinical trials have revealed MDSC plasticity, with discripancies in the responses to therapy between tumor-infiltrating MDSC and peripheral MDSC (e.g. spleens of tumor-bearing mice, peripheral blood of cancer patients). The presence of tumor-infiltrating MDSC in the tumor microenvironment has rendered it more resistant to therapy in the clinic.
Thus, clinical trials designed to study the effect of therapeutic drugs on MDSC function and phenotype should consider analyzing the peripheral blood and tumor tissue. In addition, follow up of patients involved in clinical trials should be clearly reported in studies targeting MDSC to augment the significance of MDSC targeting in cancer and determine whether modulation of MDSC provides the overall survival benefit for cancer patients.
Acknowledgments
Source of Funding: This work was supported by NIH R01CA150947, R01CA086412, P01CA163223 and Kentucky Lung Cancer Research Program.
Footnotes
Conflict of interest: The authors declare no financial conflict of interest with this work.
References
- 1.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18(4):175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 3.Maier T, Holda JH, Claman HN. Murine natural suppressor cells in the newborn, in bone marrow, and after cyclophosphamide. Genetic variations and dependence on IFN-gamma. J Immunol. 1989;143(2):491–498. [PubMed] [Google Scholar]
- 4.Schmidt-Wolf IG, et al. T-cell subsets and suppressor cells in human bone marrow. Blood. 1992;80(12):3242–3250. [PubMed] [Google Scholar]
- 5.Angulo I, et al. Involvement of nitric oxide in bone marrow-derived natural suppressor activity. Its dependence on IFN-gamma. J Immunol. 1995;155(1):15–26. [PubMed] [Google Scholar]
- 6.Angulo I, et al. Early myeloid cells are high producers of nitric oxide upon CD40 plus IFN-gamma stimulation through a mechanism dependent on endogenous TNF-alpha and IL-1alpha. Eur J Immunol. 2000;30(5):1263–1271. doi: 10.1002/(SICI)1521-4141(200005)30:5<1263::AID-IMMU1263>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Wright MA, et al. Stimulation of immune suppressive CD34+ cells from normal bone marrow by Lewis lung carcinoma tumors. Cancer Immunol Immunother. 1998;46(5):253–260. doi: 10.1007/s002620050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffe ML, Arai H, Nabel GJ. Mechanisms of tumor-induced immunosuppression: evidence for contact-dependent T cell suppression by monocytes. Mol Med. 1996;2(6):692–701. [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161(10):5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162(10):5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, et al. Identification of a CD11b(+)/Gr−1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96(12):3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 12.Kusmartsev SA, Li Y, Chen SH. Gr−1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165(2):779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 15.Youn JI, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gros A, et al. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18(19):5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akashi K, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 19.Hettinger J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14(8):821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 20.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marigo I, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Ma G, et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34(3):385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varol C, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204(1):171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 25.Ancuta P, et al. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna RN, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12(8):778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugel S, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2(3):628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Youn JI, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14(3):211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridlender ZG, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7(2):e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youn JI, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raccosta L, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013 doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J, et al. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190(5):2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng G, et al. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serafini P, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 39.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha P, et al. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez PC, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–4166. [PubMed] [Google Scholar]
- 43.Bunt SK, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez PC, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannick JB, et al. Fas-induced caspase denitrosylation. Science. 1999;284(5414):651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 47.Bingisser RM, et al. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160(12):5729–5734. [PubMed] [Google Scholar]
- 48.Srivastava MK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foell D, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 50.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kujawski M, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118(10):3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha P, et al. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-gamma and IL-4Ralpha. Eur J Immunol. 2012;42(8):2052–2059. doi: 10.1002/eji.201142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B, et al. Gr−1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 55.Serafini P, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha P, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 57.Centuori SM, et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol. 2012;92(5):987–997. doi: 10.1189/jlb.0911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 59.Hoechst B, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang CY, et al. Tumor microenvironmental conversion of natural killer cells into myeloid-derived suppressor cells. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0545. [DOI] [PubMed] [Google Scholar]
- 61.Molon B, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Santo C, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102(11):4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, et al. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299(2):583–592. [PubMed] [Google Scholar]
- 64.Sarfati M, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101(1):265–269. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 65.Serafini P, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer C, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108(41):17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagaraj S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez A, et al. Inhibition of tumor-induced myeloid-derived suppressor cell function by a nanoparticulated adjuvant. J Immunol. 2011;186(1):264–274. doi: 10.4049/jimmunol.1001465. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki E, et al. Gemcitabine selectively eliminates splenic Gr−1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 70.Vincent J, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 71.Mundy-Bosse BL, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71(15):5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le HK, et al. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Tongu M, et al. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2013;62(2):383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bunt SK, et al. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62(2):225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fridlender ZG, et al. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18(11):1947–1959. doi: 10.1038/mt.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruchard M, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 77.Levin G, et al. Indomethacin inhibits the accumulation of tumor cells in mouse lungs and subsequent growth of lung metastases. Chemotherapy. 2000;46(6):429–437. doi: 10.1159/000007323. [DOI] [PubMed] [Google Scholar]
- 78.Leahy KM, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62(3):625–631. [PubMed] [Google Scholar]
- 79.Tsubouchi Y, et al. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000;20(5a):2867–2872. [PubMed] [Google Scholar]
- 80.Fujita M, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veltman JD, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eruslanov E, et al. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. J Leukoc Biol. 2010;88(5):839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obermajer N, Kalinski P. Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplant Res. 2012;1(1):15. doi: 10.1186/2047-1440-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Y, et al. Melanoma-Educated CD14+ Cells Acquire a Myeloid-Derived Suppressor Cell Phenotype through COX-2-Dependent Mechanisms. Cancer Res. 2013;73(13):3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 85.George S. Sunitinib, a multitargeted tyrosine kinase inhibitor, in the management of gastrointestinal stromal tumor. Curr Oncol Rep. 2007;9(4):323–327. doi: 10.1007/s11912-007-0040-1. [DOI] [PubMed] [Google Scholar]
- 86.Illmer T, Ehninger G. FLT3 kinase inhibitors in the management of acute myeloid leukemia. Clin Lymphoma Myeloma. 2007;8(Suppl 1):S24–S34. doi: 10.3816/clm.2007.s.030. [DOI] [PubMed] [Google Scholar]
- 87.Roskoski R., Jr Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356(2):323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 88.Rajendra R, Pollack SM, Jones RL. Management of gastrointestinal stromal tumors. Future Oncol. 2013;9(2):193–206. doi: 10.2217/fon.12.178. [DOI] [PubMed] [Google Scholar]
- 89.Vazquez S, et al. Sunitinib: the first to arrive at first-line metastatic renal cell carcinoma. Adv Ther. 2012;29(3):202–217. doi: 10.1007/s12325-011-0099-9. [DOI] [PubMed] [Google Scholar]
- 90.Molina AM, et al. Long-Term Response to Sunitinib Therapy for Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer. 2013 doi: 10.1016/j.clgc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozao-Choy J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xin H, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69(6):2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abe F, et al. Therapeutic activity of sunitinib for Her2/neu induced mammary cancer in FVB mice. Int Immunopharmacol. 2010;10(1):140–145. doi: 10.1016/j.intimp.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 94.Bose A, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2011;129(9):2158–2170. doi: 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finke J, et al. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panka DJ, et al. Effects of HDM2 antagonism on sunitinib resistance, p53 activation, SDF-1 induction, and tumor infiltration by CD11b+/Gr−1+ myeloid derived suppressor cells. Mol Cancer. 2013;12:17. doi: 10.1186/1476-4598-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 99.van Cruijsen H, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14(18):5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 100.Melani C, et al. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67(23):11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schilling B, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133(7):1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 103.Curtin JA, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 104.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450(7171):825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 106.Conway JG, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102(44):16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Priceman SJ, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115(7):1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr−1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young MR, et al. Skewed differentiation of bone marrow CD34+ cells of tumor bearers from dendritic toward monocytic cells, and the redirection of differentiation toward dendritic cells by 1alpha,25-dihydroxyvitamin D3. Int J Immunopharmacol. 1999;21(10):675–688. doi: 10.1016/s0192-0561(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 111.Kulbersh JS, et al. 1alpha,25-Dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory CD34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. 2009;140(2):235–240. doi: 10.1016/j.otohns.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walsh JE, et al. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71(7):659–665. doi: 10.1016/j.humimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walker DD, et al. Immunological modulation by 1alpha,25-dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine. 2012;58(3):448–454. doi: 10.1016/j.cyto.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Rosa M, et al. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280(1):36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Zhang H, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013 doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsavaris N, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87(1):21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Young MR, Lathers DM. Combination docetaxel plus vitamin D(3) as an immune therapy in animals bearing squamous cell carcinomas. Otolaryngol Head Neck Surg. 2005;133(4):611–618. doi: 10.1016/j.otohns.2005.05.658. [DOI] [PubMed] [Google Scholar]
- 118.Kodumudi KN, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kodumudi KN, et al. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189(11):5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sevko A, et al. Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J Immunotoxicol. 2012;9(3):275–281. doi: 10.3109/1547691X.2012.655343. [DOI] [PubMed] [Google Scholar]
- 121.Michels T, et al. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol. 2012;9(3):292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montero AJ, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132(1):215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Breitman TR, Collins SJ, Keene BR. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981;57(6):1000–1004. [PubMed] [Google Scholar]
- 124.Kusmartsev S, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63(15):4441–4449. [PubMed] [Google Scholar]
- 125.Nefedova Y, et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67(22):11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 126.Lee JM, et al. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer. 2012;131(3):741–751. doi: 10.1002/ijc.26411. [DOI] [PubMed] [Google Scholar]
- 127.Mirza N, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iclozan C, et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62(5):909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kusmartsev S, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 130.Zoglmeier C, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17(7):1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 131.Tu SP, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012;5(2):205–215. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian J, et al. beta-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol. 2013;43(5):1220–1230. doi: 10.1002/eji.201242841. [DOI] [PubMed] [Google Scholar]