Abstract
Immunoglobulins and immune cells are critical components of colostral immunity; however, their transfer to and function in the neonate, especially maternal lymphocytes, is unclear. Cell-mediated and antibody-mediated immunity in sow blood and colostrum and piglet blood before (PS) and after (AS) suckling were assessed to investigate transfer and function of maternal immunity in the piglet. CD4, CD8, and γδ lymphocytes were found in sow blood and colostrum and piglet blood PS and AS; each had a unique T lymphocyte profile. Immunoglobulins were detected in sow blood, colostrum, and in piglet blood AS; the immunoglobulin profile of piglet serum AS mimicked that of sow serum. These results suggest selectivity in lymphocyte concentration into colostrum and subsequent lymphocyte transfer into the neonate, but that immunoglobulin transfer is unimpeded. Assessment of colostral natural killer activity and antigen-specific proliferation revealed that colostral cells are capable of influencing the innate and specific immune response of neonatal pigs.
Keywords: colostrum, T lymphocyte, passive transfer, maternally derived immunity, neonate, swine
1. Introduction
Maternal immunity is transferred to offspring in utero across the placenta or after birth via the ingestion of mammary secretions. Neonatal piglets first receive maternal immunity in the form of colostrum since the porcine placenta prohibits transfer of immunity in utero. Therefore, successful absorption of colostrum is essential for disease prevention and growth of healthy pigs. Immunomodulatory and antimicrobial factors including antibodies and a variety of cells are integral parts of colostrum (reviewed in Wagstrom et al., 2003; Salmon et al., 2009). Maternal immunoglobulins are concentrated from the blood into colostrum in the mammary gland via an Fc-receptor dependent mechanism (Hammer and Mossmann, 1978). Lymphocytes derived from the common mucosal immune system migrate to the mammary gland (Salmon, 1987) and can be found in colostrum (Harp and Moon, 1988).
Cell-mediated immunity (CMI) is a necessary component of disease control, but the cellular contribution to colostrum has traditionally been overlooked in favor of non-cellular factors such as immunoglobulins. Colostrum is rich in cells; there are more than 1×106 cells per ml, and it is estimated that piglets obtain 5.0×108–7.0×108 maternal cells daily (Evans et al., 1982; Magnussen, 1999). A relatively high percentage of these cells are lymphocytic (15–25%; Le Jan, 1994). Using technetium-labeled cells (Tuboly et al., 1988) or fluorescein-labeled cells (Williams, 1993), it was demonstrated that maternal colostral cells cross the neonatal intestinal epithelium and are found in the neonate’s circulation and immune tissues. However, heat-killed cells and cells from a source other than the piglet’s own mother do not transverse the intestinal barrier (Tuboly et al. 1988, Williams, 1993). In contrast to immune cells, colostral immunoglobulins cross the neonatal intestinal epithelium and enter the circulation independent of source, whether it be immunoglobulins from another dam or from another species (Klobasa et al. 1981); however the efficiency of cross species immunoglobulin transfer may be increased in the presence of porcine colostrum (Jensen et al., 2001; reviewed in Sanglid, 2003). The mechanism involved in selective lymphoid cell transfer remains to be clarified.
Colostral lymphocytes express activation markers (Park et al. 1992), suggesting that they are functional. The ability of human colostral cells to respond to bacterial and viral antigen stimulation (Parmely, 1976; Ogra and Ogra, 1978) and to produce cytokines has been observed in vitro (Kohl et al., 1982; Skansen-Saphir et al., 1993). However, studies on the ability of colostral cells to respond in the recipient have largely relied on mitogenic responses (Riedel-Caspari and Schmidt 1991; Williams 1993).
The present study was undertaken to assess CMI and antibody-mediated immunity (AMI) in sow blood, colostrum, and piglet blood to investigate differences in transfer of specific immune components and the role of those immune components, specifically colostral lymphocytes, in immune development of young pigs. The results show that colostral lymphocytes are selectively transferred into piglet blood and that these colostral lymphocytes are capable of influencing the innate and adaptive immune response of neonatal pigs. These results have important implications for sow herd management and piglet health.
2. Materials and Methods
2.1 Animals
Animals (English Belle; GAP Genetics, Winnipeg, MB Canada) were housed at a commercial farm and treated in accordance with the University’s Institutional Animal Care and Use Committee regulations. Sows were fed commercial corn-soybean based diets and water was provided ad libitum. Randomly chosen sows (12 of a group of 24) were vaccinated with a commercially available, federally approved Mycoplasma hyopneumoniae (M. hyopneumoniae; Respisure, Pfizer Animal Health, Kalamazoo, MI, USA) vaccine. Vaccination was performed at 5 and 3 weeks prior to farrowing according to the vaccine manufacturer’s instructions.
All farrowings were monitored and piglets were placed into plastic tubs immediately after birth to prohibit suckling. Piglets were returned to their dams immediately following blood collection. Cross-fostering was not practiced. Sows and their litters were kept in individual crates; farrowing rooms were maintained at 18°C. Heat lamps provided additional heat to newborn piglets for 72 h and subsequent extra heat was provided by heat-pads (Osborne, NE, USA).
2.2 Sample Collection
Blood was collected from sows prior to farrowing and from their piglets pre-suckling (PS) and 24 h after (AS) colostrum ingestion. Blood for the PS piglet sample was collected within 1 hour of birth. All blood samples were collected from the jugular vein into sterile EDTA Vacutainer tubes (Becton Dickinson, Rutherford, NJ, USA). Colostrum was collected manually into sterile 50 ml conical tubes from all functional teats for a total volume of 25–30 ml. Teats were scrubbed with alcohol and gloves were worn to minimize contamination. Colostrum was obtained within 1 h of farrowing.
2.3 Lymphocyte purification
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by density centrifugation using Lymphocyte Separation Media (Cellgro, Inc, VA, USA; Bautista et al., 1999). Colostral mononuclear cells (CMC) were purified as described (Le Jan, 1994) with modifications. Colostrum was diluted 1:3 in sterile PBS and centrifuged at 800 × g for 10 min. The cell pellet was retained and the supernatant was centrifuged again at the same specifications. The cell pellets were combined and washed 3× and resuspended in PBS then centrifuged on Lymphocyte Separation Media for 35 min at room temperature at 1000 × g. The lymphocyte130 enriched layer was retained and washed with PBS. Isolated PBMC and mononuclear colostral cells were resuspended in RPMI medium supplemented with 10% heat-inactivated, irradiated fetal calf serum, 2mM L-glutamine, 100 U penicillin G per ml, and 100 µg of streptomycin per ml. The serum portions of blood and whey portions of colostrum were retained and stored at −80C until IgG, IgA, and M. hyopneumoniae-specific antibody analyses were performed.
2.4 Lymphocyte phenotyping
Cells were enumerated using a hemocytometer and viability was assessed by trypan blue exclusion. Lymphocytes were phenotyped as described (Yang and Parkhouse, 1996) with modifications. Anti-pig CD8-PE, CD4-PE, and γδ-FITC antibodies (VMRD, Inc Pullman, WA, USA) were added at a concentration of 1 µg/106 target cells in 200 µl of PBS. Cells were allowed to incubate for 30 min in the dark at room temperature. Samples were washed and immediately analyzed via flow cytometry. A Facs Caliber flow cytometer was used and the FL-4 laser was calibrated using the manufacturer’s calibration beads (Becton Dickinson Immunocytometry System, San Jose, CA, USA). Non-stained cells were used to establish a base line for phenotyping. Event acquisition was set at 10,000 within the regions encompassing PE or FITC depending on subset being analyzed.
2.5 Natural Killer assay
The natural killer (NK) assay was performed as described (Olin et al., 2005a). K562 cells were membrane stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; 10 µM concentration; Immunochemistry Technologies, LLC, Bloomington, MN, USA). CFSE-stained K562 cells were added to PBMC or CMC at a ratio of 1:50. Samples were incubated for 4 h at 37°C in 5% CO2. The live/dead cell stain 7-Aminoactinomycin D (7AAD: Immunochemistry Technologies, LLC, Bloomington, MN, USA) was added and the samples were placed on ice for 15 min and analyzed via flow cytometry. Non-stained effector (PBMC or CMC) and target cells (K562) were used to establish a baseline for NK activity. CFSE stained K562 cells in suspension with non-stained PBMC or CMC were used to verify the separation of effector and target cells. Water-lysed K562 cells stained with 7AAD were used to calibrate acquisition of live and dead K562 cells. Event acquisition was set for 10,000 events in a region encompassing the CFSE positive, and 7AAD positive and negative quadrants (upper and lower right quadrants only). NK activity was assessed by flow cytometry and analyzed by CellquestPro (Olin et al., 2005b).
2.6 Antigen specific blastogenesis
Mononuclear cells isolated from colostrum and blood were stained with CFSE as stated above and plated in duplicate in v-bottom 96-well-plates at a concentration of 5×105 cells per well. Cells were stimulated with 10 µg/ml purified M. hyopneumoniae antigen as described (Thacker et al., 2000); M. hyopneumoniae antigen was prepared as described (Bandrick et al., 2008). Nonstimulated cultures served as negative controls and concanavalin A (conA) stimulated cultures (5 µg/ml) served as positive controls. Experimental, negative, and positive controls were analyzed for each animal. M. hyopneumoniae-specific proliferation data are described as the percentage of mononuclear cells proliferating to M. hyopneumoniae antigen minus the percentage of cells spontaneously proliferating in the negative control (M. hyopneumoniae-stimulated proliferation - nonstimulated proliferation). Proliferation was assessed by flow cytometry and analyzed by CellQuestPro.
2.7 IgG and IgA quantitation
ELISAs were performed to quantitate the immunoglobulin concentrations in blood and colostrum. ELISA reagents and antibodies from Bethyl Laboratories (Montgomery, TX, USA) were used for IgA quantitation (Foss and Murtaugh 1999). ELISA reagents from Immunochemistry Technologies, LLC (Bloomington, MN, USA) and antibodies from Immunology Consultants Laboratory, Inc (Newberg, OR, USA) were used for IgG quantitation. Plates were coated with either anti-swine IgG (4 µg/ml) or anti-swine IgA (10 µg/ml) and incubated overnight at 4° C. Plates were washed 4× and blocked for 1 h. Diluted samples were added to the plates and incubated for 1 h before 4× washing and addition of the secondary antibody. The secondary antibody was incubated for 1 h in the dark, the plates were washed 4× and read on a plate reader at absorption 540 nm.
2.8 Antigen specific antibody detection
ELISAs were performed on colostral whey and serum collected from sows and from serum collected from piglets, both before and after colostrum ingestion. The HerdCheck ELISA (Idexx Laboratories, Westbrook, Maine, USA) was performed per manufacturer’s instructions (Erlandson et al., 2005). Positive and negative status was determined based on sample to positive (S:P) ratio: S:P = (sample OD - negative control OD)/(positive control OD - negative control OD). S:P ratios >0.4 were classified as positive; S:P ratios < or = 0.4 were classified as negative. All samples were run in duplicate and sample means were used to determine the sample S:P ratio.
2.9 Data Analysis
Flow cytometry data was analyzed using a dot plot separated into four quadrants. Percentages of cells killed by cytotoxic activity were calculated by dividing the upper right quadrant by the sum of the upper and lower right quadrants and multiplying by 100, minus the percentage of spontaneous dead cells obtained from target cell control tube. Lymphocyte subsets, NK activity, and immunoglobulin concentrations were analyzed by ANOVA with multiple comparisons. Significant interactions (P<0.05) were analyzed by Tukey’s HSD method and to correct for multiple comparisons. Significant pairwise interactions (within multiple comparisons or familywise testing) are described by the multiplicity adjusted P value (the smallest familywise significance level at which a pairwise comparison is significantly different) and were determined by HSD. When means are stated in the text, variation is described as SEM. Statistical analysis was performed using GraphPad Prism 5 (Graph Pad Software, Inc, CA, USA).
3 Results
3.1 CMI Components
3.1.1 T lymphocytes in Colostrum and Sow Blood
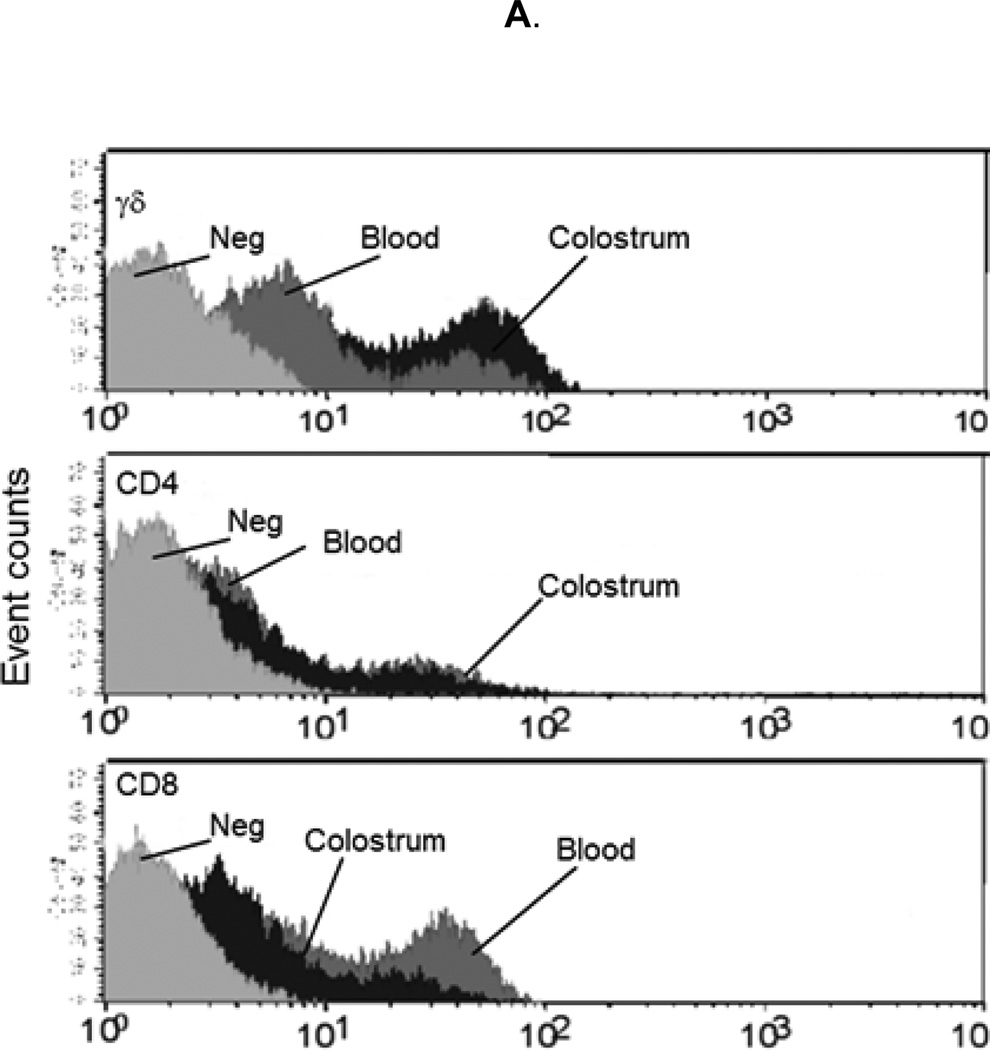
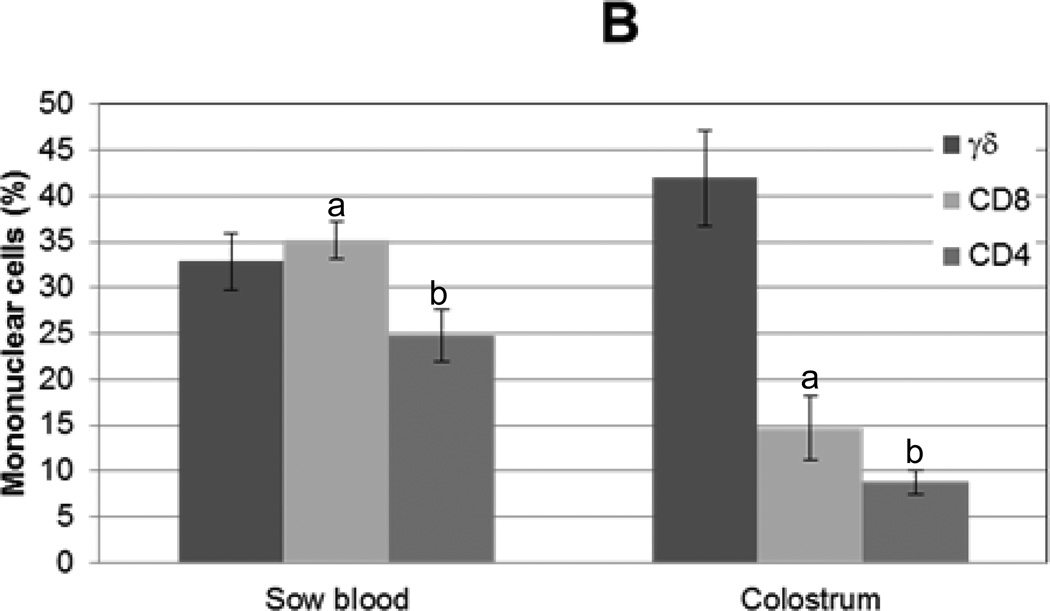
To assess the lymphocyte contribution to porcine colostrum and compare this to sow blood, isolated mononuclear cells were enumerated and viability was assessed. Viability was determined to be greater than 97% cells/ml for blood and 90% cells/ml for colostrum. Significantly more mononuclear cells were isolated from peripheral blood than from colostrum, 1.69×107 (+/−2.3×106) cells/ml and 4.07×106 (+/−1.29×106) cells/ml, respectively. CD4, CD8, and γδ T lymphocyte subpopulations were enumerated by flow cytometry (Fig 1A). All three T lymphocyte subsets were observed in sow peripheral blood and colostrum (Fig 1B). CD8 (35% +/− 2%) and γδ (33% +/− 4%) lymphocytes predominated in sow peripheral blood. In colostrum γδ lymphocytes (42% +/− 8%) alone predominated (Fig 1B). CD4 and CD8 lymphocytes constituted significantly lower proportions of cells in colostrum compared to sow blood (P=0.0127 and P=0.003, respectively). While the ratio of CD4 to CD8 lymphocytes in blood (0.7) and colostrum (0.6) were similar, CD4 and CD8 lymphocytes combined constituted approximately 60% of PBMC but only 25% of CMC.
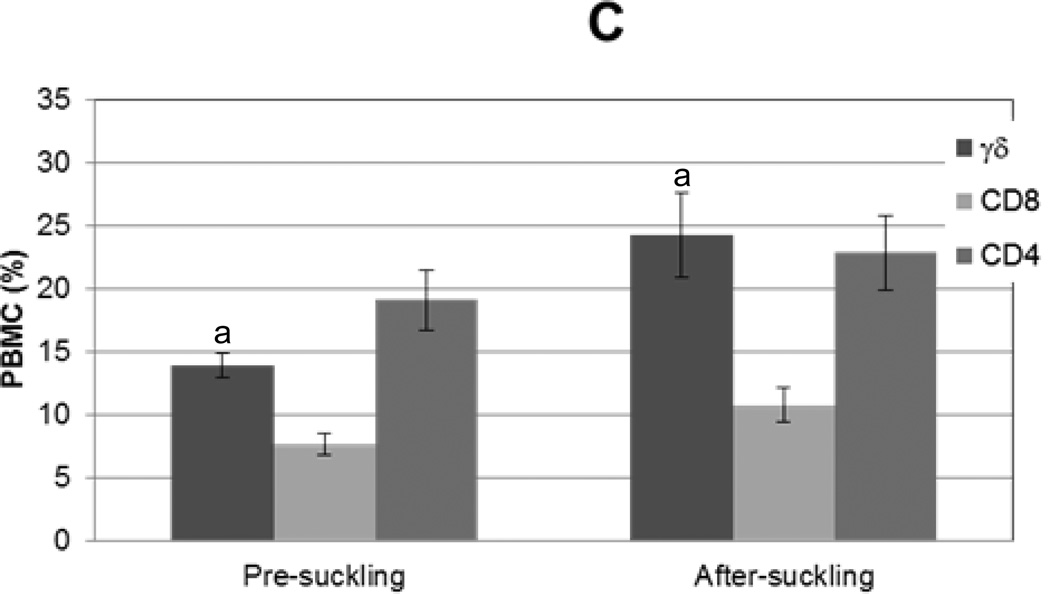
Figure 1. T lymphocyte distribution in sow blood and colostrum and in piglet blood before and after colostrum ingestion.
Mononuclear cells were isolated from sow blood or colostrum, phenotyped with swine specific γδ, CD8, or CD4 monoclonal antibodies (n=16), and analyzed via flow cytometry. (A) Representative flow cytometric analysis of T lymphocyte subpopulations in sow blood (dark gray) and colostrum (black); light gray represents unstained control. (B) Percentages of T lymphocyte subsets in sow blood and colostrum, n=16. (C) Percentages of T lymphocyte subsets in blood from piglets prior to colostrum ingestion (pre-suckling) and again at 24 h (after-suckling), n=34.Variance is given by standard error. Samples with same letters are significantly different; for samples marked “a,” p=0.001, for samples marked “b” p=0.0001.
3.1.2 Neonatal peripheral T lymphocytes
Piglet PBMC were enumerated and CD4, CD8, and γδ subpopulations were assessed to compare the distribution of circulating T lymphocytes in pre- and post-colostral piglets. The number of PBMC isolated per ml of blood increased 63% from pre-colostrum (4.4 ×106 +/− 0.73×106 cells/ml) to after colostrum ingestion (7.0×106 +/− 0.71×106 cells/ml; p=0.018). The distribution of CD4, CD8, and γδ lymphocytes in piglet blood varied depending on colostrum ingestion status (Fig 1C). In the pre-colostral pig, CD4 (19% +/−2%) lymphocytes represented the largest percentage of lymphoid cell, while, in the post-colostral pig, γδ (24% +/−3%) and CD4 (23% +/− 3%) lymphocytes predominated. The percentage of γδ cells observed in PBMC of post-colostral piglets was greater than pre-colostral piglets (P=0.009), yet the percentage of CD4 and CD8 lymphocytes remained constant. The distribution of γδ and CD8 T lymphocytes found in piglet blood AS was different from what was found in sow blood (P=0.05 and P=0.001, respectively). The distribution of γδ and CD4 T lymphocytes found in piglet blood AS was different from what was found in colostrum (P=0.02 and P=0.004, respectively).
3.2 AMI Components
3.2.1 immunoglobulins in Colostrum and Sow Blood
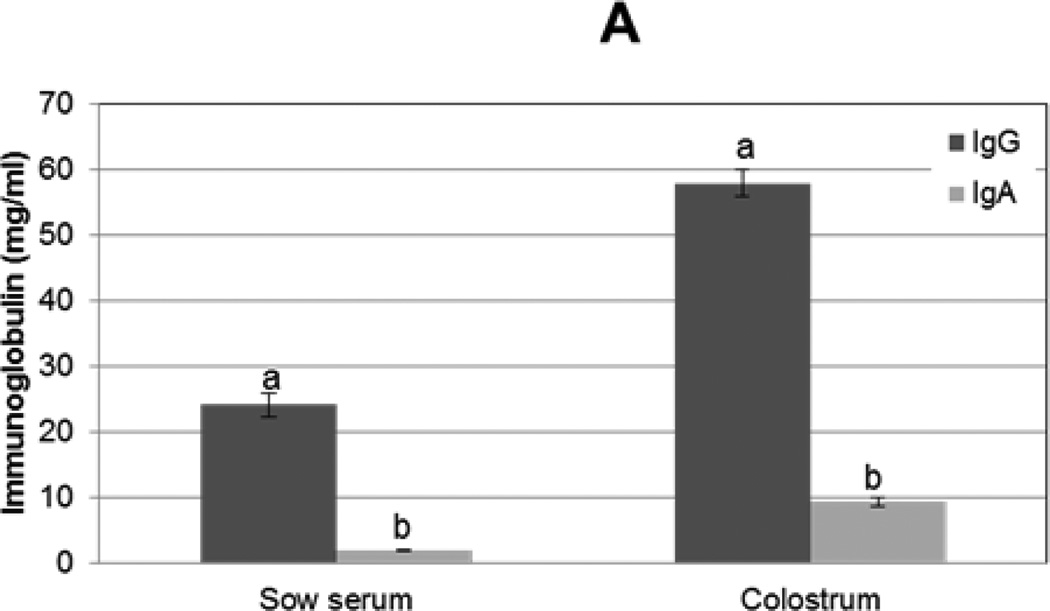
To compare the immunoglobulin concentrations in colostrum to serum, IgG and IgA sandwich ELISAs were performed on colostral whey and sow serum. Both IgG and IgA were found in greater concentrations in colostrum compared to serum (P<0.0001 and P<0.0001, respectively); approximately 2.5× more IgG and 5× more IgA was found in colostrum compared to serum (Fig 2A). Additionally, IgG predominated over IgA in colostrum (12 IgG: 1 IgA) and in serum (8 IgG: 1 IgA); however, the difference in ratio of IgG:IgA in colostrum versus serum was not significantly different.
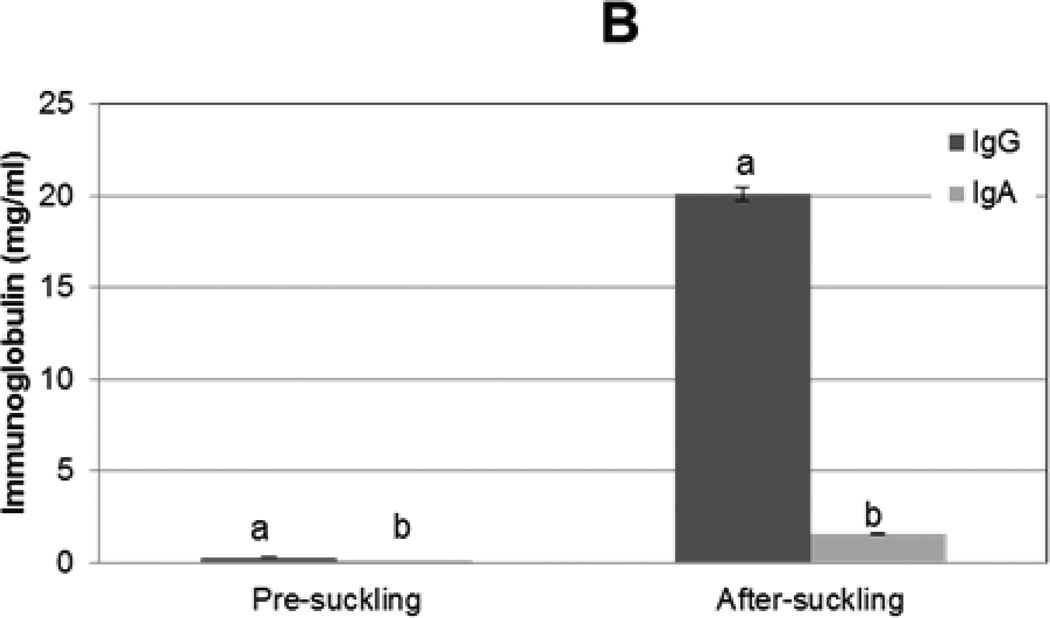
Figure 2. Immunoglobulin G and A concentrations in sow serum and colostrum and in piglet serum before and after colostrum ingestion.
IgG and IgA concentrations from (A) sow serum and colostral whey (n=16) and (B) in piglet serum before colostrum ingestion (pre-suckling) and 24 h later (after-suckling) (n=34) were determined by ELISA. Variance is given by standard error. Samples with same letters are significantly different, p<0.001
3.2.2 Neonatal serum immunoglobulins
To compare the concentrations of IgG and IgA in piglet serum before and after colostrum ingestion, blood samples were collected from pigs immediately following birth and 24 h later. IgG and IgA concentrations were determined by ELISA. Neither IgG nor IgA were detected in pre-colostral piglet serum samples. Approximately 20 (+/− 0.4) mg/ml IgG and 1.6 (+/− 0.04) mg/ml IgA were found in piglet serum post-suckling (Fig 2B). The immunoglobulin pattern detected in the post-colostral piglet mimics that found in sow serum.
3.3 NK activity of CMC and PBMC
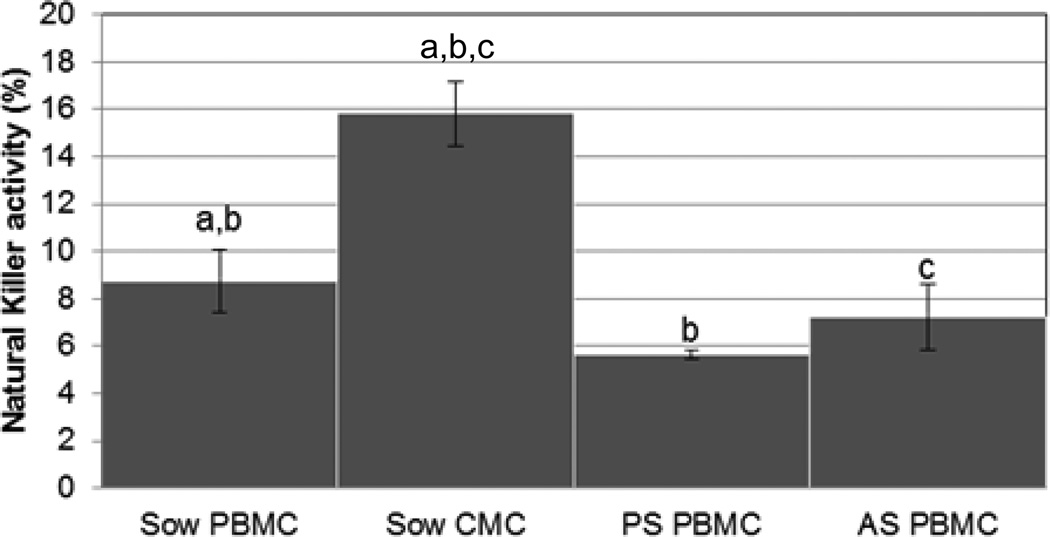
NK activity was used to assess the innate functional ability of CMC and PBMC. NK activity is a critical defense mechanism for killing intracellular pathogens in an antigen-nonspecific manner. NK activity is accomplished by activated NK cells, γδ T lymphocytes, and select CD8 lymphocytes (Charerntantanakul and Roth, 2007). CMC demonstrated the greatest amount of NK activity (16% +/− 1%) and this was significantly more NK activity than was observed for sow PBMC (9% +/− 1%; P=0.003) or any piglet sample (Fig 3). Sow PMBC demonstrated more NK activity than PBMC isolated from piglets PS (P=0.03) and similar activity to PBMC isolated from piglets AS (P=0.4).
Figure 3. Natural killer activity of colostral and peripheral mononuclear cells.
Innate natural killer activity was used to assess the functional ability of mononuclear cells from sow colostrum (CMC) and blood (PBMC) of sows and piglets, before (PS) and after (AS) colostrum ingestion. Variance is given by standard error. Samples with same letters are significantly different, p<0.05.
3.4 Antigen specific AMI and CMI in colostrum and blood
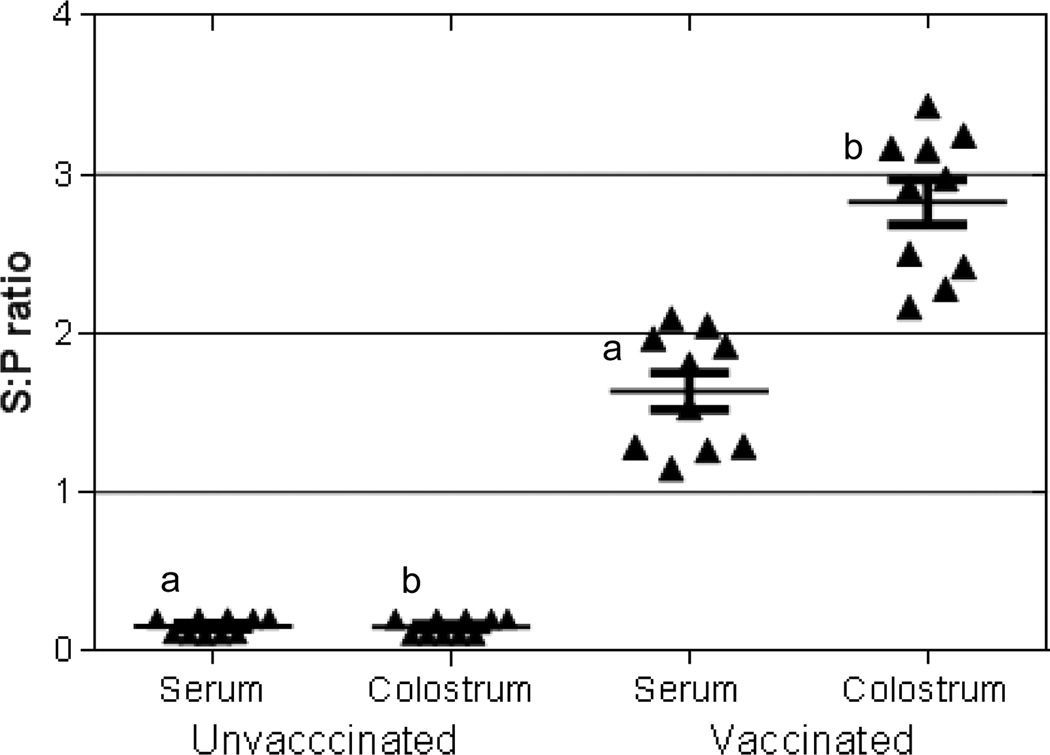
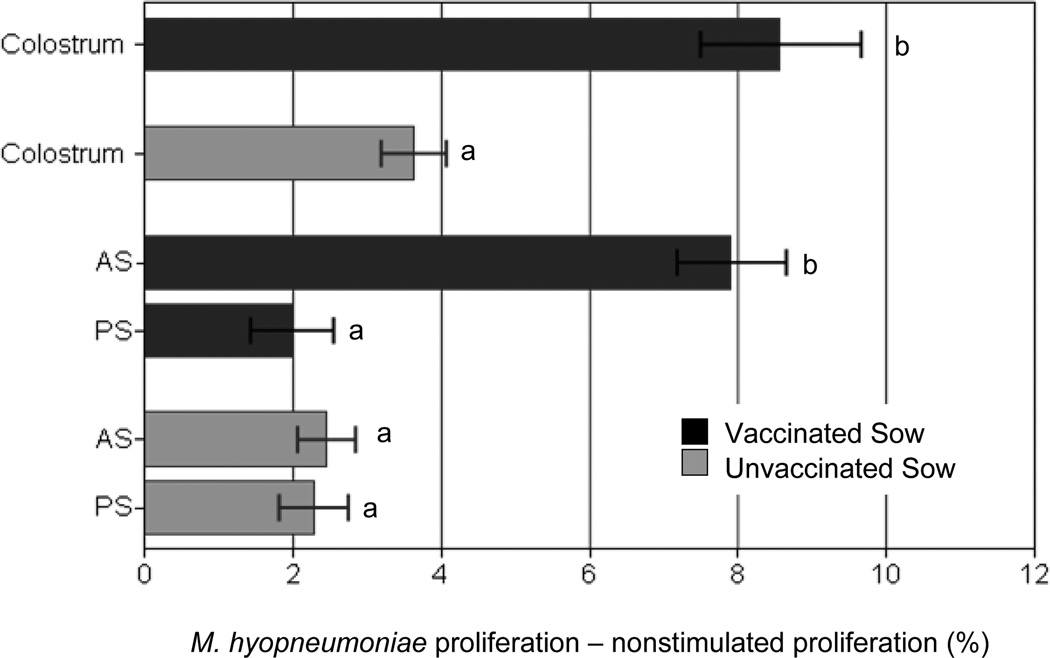
M. hyopneumoniae-specific antibodies and M. hyopneumoniae-specific lymphocyte proliferation were measured to assess and compare antigen-specific AMI and CMI in colostrum. Antibodies specific to M. hyopneumoniae were found in serum and colostrum of vaccinated sows (Fig 4). In addition, M. hyopneumoniae-specific antibodies were detected in the serum of piglets from vaccinated dams after colostrum ingestion but not from piglets of unvaccinated dams (data not shown). Pre-colostral piglets and unvaccinated animals had M. hyopneumoniae-antibody S:P values <0.4 and were classified as negative. Colostral lymphocytes from vaccinated sows proliferated in response to M. hyopneumoniae stimulation and this response was mimicked in offspring of vaccinated sows after suckling only (Fig. 5). Antigen-specific proliferation was not detected from PBMC of pre-colostral piglets, from CMC of non-vaccinated sows, or from PBMC of their offspring (before and after colostrum ingestion).
Figure 4. Mycoplasma hyopneumoniae specific antibodies in serum and colostrum of unvaccinated and vaccinated sows.
Mycoplasma hyopneumoniae specific antibodies were measured in serum and colostrum of unvaccinated (n=10) and vaccinated (n=10) sows using the Idexx HerdCheck ELISA. Positive and negative antibody status was based on sample to positive (S:P) ratio. Samples with the same letters are significantly different, p<0.05
Figure 5. Antigen specific proliferation by mononuclear cells isolated from piglets before and after colostrum ingestion and from sow colostrum.
Mononucelar cells from sow colostrum (CMC; n=10) and from piglet blood (PBMC; n=20) before (PS) and after (AS) colostrum ingestion were stimulated with Mycoplasma hyopneumoniae antigen. Proliferation was assessed by flow cytometry. Antigen specific proliferation was compared between colostrum of vaccinated and unvaccinated sows and piglets receiving colostrum from vaccinated and unvaccinated sows. Variation is expressed as standard error; samples with different letters indicate significance at p<0.05.
4. Discussion
The epitheliochorial nature of the porcine placenta prohibits transfer of maternal immune cells and immunoglobulins to the fetus. Therefore, piglets are born agammaglobulinemic and must rely on the successful absorption of colostral components to acquire maternal immunity. Maternal immunity serves to protect young pigs while their own immune systems develop. The amount of maternal immunity in the post-suckling pig depends on the concentration of immune and other bioreactive products in colostrum, the amount of colostrum ingested, and timing of gut closure (Rooke and Bland, 2002). It is important to define the events of passive immunity in the neonate to be able to best manage and improve the health of young animals.
T lymphocyte subpopulations in colostrum did not mimic what was seen in sow blood, indicating that the mononuclear cell portion of colostrum may be selectively concentrated. While the relative ratio of CD8:CD4 cells remained similar across blood and colostrum, in accordance with Magnussen (1999) and Le Jan (1994), there were a significantly greater percentages of CD8 and CD4 lymphocytes in blood compared to colostrum (P=0.001 and P=0.001, respectively). The differences in lymphocyte subpopulations from blood to colostrum are highlighted by a significant increase in the percentage of γδ T lymphocytes, which constitute almost half of the mononuclear cells found in colostrum. The disparity in the T lymphocyte contribution to colostrum and blood indicate that there is selectivity in the lymphocyte contribution to colostrum. While other reports have suggested that γδ T lymphocytes contribute to porcine colostrum (Wagstrom et al. 2000), this is the first report to show γδ T lymphocytes are indeed in porcine colostrum. Interestingly, γδ T lymphocytes were the only lymphocytes analyzed to increase significantly after suckling in the piglet.
Pigs have a significant population of circulating γδ T lymphocytes. The presence of these lymphocytes in colostrum is anticipated and of interest because of their unique immunologic activities. γδ T lymphocytes respond to antigens differently than αβ T cells. γδ T lymphocytes are MHC unrestricted and have the ability to recognize unprocessed non-protein antigens (Tanaka et al., 1994). In addition, γδ T lymphocytes in murine and bovine species possess pattern recognition receptors (PRR; Mokuno et al., 2000; Hedges, 2003). Whether porcine γδ T lymphocytes possess PRR or toll-like receptors is unknown (Takamatsu, 2006); however scavenger receptors on porcine γδ T lymphocytes have been demonstrated (Carr et al., 1994). Moreover, γδ T lymphocytes are thought to have NK activity and antigen presentation abilities (Bandes et al., 2005; Sinkora et al., 2005). Having mature antigen presenting cells (APC) would be advantageous to the neonate since neonatal APC are immature and require greater stimulation to function at the level of a mature animal (Adkins, 2004). γδ T lymphocytes’ ability to “bridge” the innate and adaptive immune responses would be an asset for the maturing immune system of the piglet.
The increase in γδ T lymphocytes in piglets PS to AS may be due to the high percentage of colostral γδ T lymphocytes, and the static CD4 and CD8 percentages in the piglet PS to AS may be because these colostral cells were not present in sufficient quantities to affect a change in the piglet. However, the percentage of CD4 and CD8 T lymphocytes present in post-colostral piglets is not easily explained by numbers alone since one CD4 and CD8 T lymphocyte percentages would be expected to increase, corresponding to their levels in colostrum as seemed to occur for γδ T lymphocytes. This finding may indicate a release from stores or production of γδ T lymphocytes birth, or that there may be selectivity in colostral cell uptake by the piglet. While the mechanism of maternal lymphocyte transport across the neonatal intestinal epithelium is unknown, selectivity in lymphocyte transfer across the neonatal intestinal epithelium has been suggested by other studies (Tuboly et al., 1988; Williams, 1993). Transfer may be a receptor-mediated event and may be related to cellular phenotypic changes incurred while in the colostral environment (Reber et al., 2006).
IgG and IgA were more concentrated in colostrum than sow serum, and the distribution pattern of IgG to IgA across colostrum and sow serum were similar. These findings are further evidence that colostral immunoglobulins are nonselectively concentrated from sow serum in the mammary gland (Porter, 1969; Clarke and Hardy, 1971; Curtis and Bourne, 1971). Levels of IgG and IgA in the post-colostral piglet suggest that absorption of colostral immunoglobulins was nonselective since the serum IgG and IgA levels of post-colostral piglets mimicked the distribution of IgG and IgA in sow colostrum. These findings are in agreement with Burton and Smith (1977). As expected, IgG and IgA were not detected in the serum of blood from piglets prior to colostrum ingestion. Since fetuses have the ability to produce IgG while gestating (Tlaskalova-Hogenova et al., 1994), the lack of IgG in pre-colostral piglet serum indicates that the piglets were not facing antigen challenge in utero.
To assess the ability of colostral cells to participate in an innate immune response, NK activity was measured. Colostral lymphocytes demonstrated the greatest amount of NK activity across all samples. While no clear difference was observed in NK activity between PBMC isolated from pigs that had ingested colostrum compared to those that did not, this does not necessarily indicate non-function of colostral cells in the recipient. Neonates have higher numbers of circulating natural killer cells than adults (Yabuhara et al., 1990), yet reports have indicated that NK activity in neonates is lower than in adults (Baley and Schacter, 1985; Lin et al., 2004). Consequently, the contribution by colostrum may not be significant enough to enhance measurable NK activity in the post-colostral piglet. Additionally, the presence of an inhibitory factor in colostrum could explain the lack of difference. Corticosteroids found in colostrum have been shown to be immunosuppressive and influence immune activity in the neonate (Borghetti et al., 2006). The potential Th2 bias of newborns and increased apoptosis of Th1 cells may also contribute to the lack in difference of NK activity (Adkins et al., 1996).
Antigen-specific proliferation was measured to assess the ability of primed colostral cells that have crossed into the neonate’s circulation to respond in an antigen-specific manner. Findings shown here indicate that antigen-specific cells transfer from colostrum into the neonate’s circulation. Additionally, these antigen-specific cells are functional, as evidenced by M. hyopneumoniae-specific proliferation by PBMC isolated from one-day-old colostrum-fed piglets. In pigs αβ T lymphocytes co-expressing CD4 and CD8 (reviewed in Charerntantanakul and Roth, 2007) are effector memory cells and stimulation with their specific antigen induces these double positive (DP) lymphocytes to proliferate (Saalmuller et al., 1987; Dillender and Lunney, 1993; Zuckermann and Husmann, 1996). Most γδ T lymphocytes do not express CD4 (Sinkora et al., 2005) or CD8 (Yang and Parkhouse, 1996). It is likely that many of the colostral lymphocytes responding in the piglet are effector memory DP cells.
Antigen-specific proliferation by PBMC from offspring of vaccinated dams and not from unvaccinated dams indicates that transfer of primed maternal cells may influence the neonate’s response to environmental antigens (Donovan et al. 2007, Bandrick et al. 2008). Reports in humans and rodents suggest that maternally derived T lymphocytes do not interfere with active immune development in the newborn, as is the case for maternal immunoglobulin, and in fact may be useful in overcoming the suppressive effects of passive immunoglobulin (Cherry et al., 1973; Gans et al., 1999; Siegrist 2001). Future studies should emphasize the mechanism of maternal cell transfer, the role of transferred cells in neonatal immune development and passive interference, and the ability of transferred cells to protect young pigs from specific pathogens.
Highlights.
T lymphocytes from colostrum may transfer into the piglet circulation.
Colostral immunoglobulin transfer into piglet blood unimpeded.
γδ lymphocytes are found in colostrum.
Offspring of vaccinated dams have antigen-specific lymphoproliferation 1d of age.
Maternal cells may influence the neonate’s immune response.
Acknowledgements
The authors would like to thank Dr. Mike Olin for assistance with flow cytometry, Dr. Alejandrina Da Silva for the mycoplasma antibody quantitation, and Dr. Brian Lee and Immunochemistry Technologies, LLC for assistance with ELISA setup and generous donation of ELISA reagents. The authors gratefully acknowledge the staff of the University of Minnesota, Southern Research and Outreach Center, for their help during sampling sessions. MB was in part supported from NIH T32 DA07097. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Abbreviations
- PS
Pre-suckling
- AS
After-suckling
- CMI
Cell-mediated immunity
- AMI
Antibody-mediated immunity
- M. hyopneumoniae
Mycoplasma hyopneumoniae
- NK
Natural killer
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- 7AAD
7-Aminoactinomycin D
- ELISA
Enzyme linked immunosorbant assay
- S:P ratio
Sample to positive ratio
- PBMC
Peripheral blood mononuclear cell
- CMC
Colostral mononuclear cells
- APC
Antigen presenting cell
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- conA
concanavalin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meggan Bandrick, Email: bandrick@umn.edu.
Claudia Ariza-Nieto, Email: cariza@corpoica.org.co.
Samuel K. Baidoo, Email: skbaidoo@umn.edu.
Thomas W. Molitor, Email: molit001@umn.edu.
References
- 1.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Reviews Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B, Chun K, Hamilton K, Nassiri M. Naive murine neonatal T cells undergo apoptosis in response to primary stimulation. J Immunol. 1996;157:1343–1349. [PubMed] [Google Scholar]
- 3.Bandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 4.Bandrick M, Pieters M, Pijoan C, Molitor TW. Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglets. Clin. Vaccine Immunol. 2008;15:540–543. doi: 10.1128/CVI.00466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. [PubMed] [Google Scholar]
- 6.Bautista AE, Suarez MP, Molitor TW. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Arch Virol. 1999;144:117–134. doi: 10.1007/s007050050489. [DOI] [PubMed] [Google Scholar]
- 7.Bertotto A, Castellucci G, Fabietti G, Scalise F, Vaccaro R. Lymphocytes bearing the T cell receptor gamma delta in human breast milk. Arch Disease in Childhood. 1990;65:1274–1275. doi: 10.1136/adc.65.11.1274-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghetti P, De Angelis E, Saleri R, Cavalli V, Cacchioli A, Corradi A, Mocchegiani E, Martelli P. Peripheral T lymphocyte changes in neonatal piglets: Relationship with growth hormone (GH), prolactin (PRL) and cortisol changes. Vet Immunol & Immunolpath. 2006;110:17–25. doi: 10.1016/j.vetimm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Burton KA, Smith MW. Endocytosis and immunoglobulin transport across the small intestine of the new-born pig. J. Physiol. 1977;270:473–488. doi: 10.1113/jphysiol.1977.sp011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr MM, Howard CJ, Sopp P, Manser JM, Parsons KR. Expression on porcine gamma delta lymphocytes of a phylogenetically conserved surface antigen previously restricted in expression to ruminant gamma delta T lymphocytes. Immunol. 1994;81:36–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Charerntantanakul W, Roth JA. Biology of porcine T lymphocytes. An Hlth Res Rev. 2007;7:81–96. doi: 10.1017/S1466252307001235. [DOI] [PubMed] [Google Scholar]
- 12.Cherry JD, Feigin RD, Shackelford PG, Hinthorn DR, Schmidt RR. A clinical and serological study of 103 children with measles vaccine failure. J Pediatr. 1973;82:802–808. doi: 10.1016/s0022-3476(73)80070-8. [DOI] [PubMed] [Google Scholar]
- 13.Clarke RM, Hardy RN. Histological changes in the small intestine of the young pig and their relationship to macromolecular uptake. J. Anat. 1971;108:63–77. [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis J, Bourne FJ. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochim Biophys Acta. 1971;236:319–332. doi: 10.1016/0005-2795(71)90181-4. [DOI] [PubMed] [Google Scholar]
- 15.Dillender MJ, Lunney JK. Characteristics of T lymphocyte cell lines established from NIH minipigs challenge inoculated with Trichinella spiralis. Vet Immunol Immunopathol. 1993;35:301–319. doi: 10.1016/0165-2427(93)90041-2. [DOI] [PubMed] [Google Scholar]
- 16.Donovan DC, Reber AJ, Gabbard JD, Aceves-Avila M, Galland KL, Holbert KA, Ely LO, Hurley DJ. Effects of maternal cells transferred with colostrum on cellular responses to pathogen antigens in neonatal calves. Am J Vet Res. 2007;68:778–782. doi: 10.2460/ajvr.68.7.778. [DOI] [PubMed] [Google Scholar]
- 17.Erlandson KR, Evans RB, Thacker BJ, Wegner MW, Thacker EL. Evaluation of three serum antibody enzyme-linked immunosorbent assays for Mycoplasma hyopneumoniae. J Swine Health and Production. 2005;13:198–203. [Google Scholar]
- 18.Evans PA, Newby TJ, Stokes CR, Bourne FJ. A Study of cells in the mammary secretions of sows. Vet. Immunol. Immunopathol. 1982;3:515–527. doi: 10.1016/0165-2427(82)90017-4. [DOI] [PubMed] [Google Scholar]
- 19.Foss DL, Murtaugh MP. Mucosal immunogenicity and adjuvanticity of cholera toxin in swine. Vaccine. 1999;17:788–801. doi: 10.1016/s0264-410x(98)00263-1. [DOI] [PubMed] [Google Scholar]
- 20.Gans HA, Maldonado Y, Yasukawa LL, Beeler J, Audet S, Rinki MM, DeHovitz R, Arvin AM. IL-12, IFN-γ, and T cell proliferation to measles in immunized infants. J Immunol. 1999;162:5569–5575. [PubMed] [Google Scholar]
- 21.Hammer DK, Mossmann H. The importance of membrane receptors in the transfer of immunoglobulins from plasma to the colostrum. Ann. Recherches Vet. 1978;9:229–234. [PubMed] [Google Scholar]
- 22.Harp JA, Moon HW. Lymphocyte localization in lymph nodes of swine: changes induced by lactation. Vet. Immunol. Immunopathol. 1988;18:219–227. doi: 10.1016/0165-2427(88)90066-9. [DOI] [PubMed] [Google Scholar]
- 23.Hedges JF, Graff JC, Jutila MA. Transcriptional profiling of γδ T cells. J. Immunol. 2003;171:4959–4964. doi: 10.4049/jimmunol.171.10.4959. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AR, Elnif J, Burrin DG, Sangild PT. Development of intestinal immunoglobulin absorption and enzyme activities in neonatal pigs is diet dependent. J Nutrition. 2001;131:3259–3265. doi: 10.1093/jn/131.12.3259. [DOI] [PubMed] [Google Scholar]
- 25.Klobasa F, Werhahn E, Butler JE. Regulations of humoral immunity in the piglet by immunoglobins of maternal origin. Res. Vet. Sci. 1981;31:195–206. [PubMed] [Google Scholar]
- 26.Kohl S, Pickering LK, Loo LS. Virus-induced colostral cell cytokine stimulation of human leukocyte natural killer cytotoxicity. Infect Immun. 1982;36:691–695. doi: 10.1128/iai.36.2.691-695.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SJ, Cheng PJ, Huang YJ, Ming-Ling Kuo ML. Evaluation of cytotoxic function and apoptosis in interleukin (IL)-12/IL-15-treated umbilical cord or adult peripheral blood natural killer cells by a propidium-iodide based flow cytometry. Ped Allergy & Immunol. 2004;15:79–85. doi: 10.1046/j.0905-6157.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Jan C. A study by flow cytometry of lymphocytes in sow colostrum. Res Vet Sci. 1994;57:300–304. doi: 10.1016/0034-5288(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 29.Magnussen U. Longitudinal study of lymphocyte subsets and major histocomatibility complex class II expressing cells in mammary glands of sows. Am J Vet Res. 1999;60:546–548. [PubMed] [Google Scholar]
- 30.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Wasizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of toll-like receptor 2 on γδ T cells bearing invariant Vγ6/Vδ1 induced by Escherichia coli infection in mice. J. Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 31.Olin MR, Hwa Choi K, Lee J, Molitor TW. Gamma delta T-lymphocyte cytotoxic activity against Mycobacterium bovis analyzed by flow cytometry. J Immunol Methods. 2005a;297:1–11. doi: 10.1016/j.jim.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Olin MR, Batista L, Xiao Z, Dee SA, Murtaugh MP, Pijoan CC, Molitor TW. Gamma delta lymphocyte response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 2005b;18:490–949. doi: 10.1089/vim.2005.18.490. [DOI] [PubMed] [Google Scholar]
- 33.Ogra SS, Ogra PL. Immunologic aspects of human colostrum and milk. II. Characteristics of lymphocyte reactivity and distribution of E-rosette forming cells at different times after the onset of lactation. J Pediatr. 1978;92:550–555. doi: 10.1016/s0022-3476(78)80286-8. [DOI] [PubMed] [Google Scholar]
- 34.Parmely MJ, Beer AE, Billingham RE. In vitro studies on the T-lymphocyte population of human milk. J Exp Med. 1976;144:358–370. doi: 10.1084/jem.144.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park YH, Fox LK, Hamilton MJ, Daws WC. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992;75:998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4. [DOI] [PubMed] [Google Scholar]
- 36.Porter P. Transfer of immunoglobulins IgG, IgA and IgM to lacteal secretions in the parturient sow and their absorption by the neonatal piglet. Biochimica et biophysica acta. 1969;181:381–392. doi: 10.1016/0005-2795(69)90271-2. [DOI] [PubMed] [Google Scholar]
- 37.Riedel-Caspari G, Schmidt FW. The influence of colostral leukocytes on the immune system of the neonatal calf. I. Effects on lymphocyte responses. Dtsch tierarzt Wschr. 1991;98:102–107. [PubMed] [Google Scholar]
- 38.Reber AJ, Lockwood A, Hippen AR, Hurley DJ. Colostrum induced phenotypic and trafficking changes in maternal mononuclear cells in a peripheral blood leukocyte model for study of leukocyte transfer to the neonatal calf. Vet Immunol Immunopath. 2006;109:139–150. doi: 10.1016/j.vetimm.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Rooke JA, Bland IM. The acquisition of passive immunity in the new-born piglet. Livestock Prod Sci. 2002;78:13–23. [Google Scholar]
- 40.Saalmuller A, Reddehase MJ, Buhring HJ, Jonjic S, Koszinowski UH. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987;17:1297–1301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 41.Salmon H. The intestinal and mammary immune system in pigs. Vet Immunol Immunopath. 1987;17:367–388. doi: 10.1016/0165-2427(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 42.Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol. 2009;33:384–393. doi: 10.1016/j.dci.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Sanglid PT. Uptake of colostral immunoglobulins by the compromised newborn farm animal. Acta Veterinaria Scandinavica. Supplementum. 2003;98:105–122. doi: 10.1186/1751-0147-44-s1-s105. [DOI] [PubMed] [Google Scholar]
- 44.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 45.Šinkora M, Šinkorová J, Holtmeier W. Development of γδ thymocyte subsets during prenatal and postnatal ontogeny. Immunol. 2005;115:544–555. doi: 10.1111/j.1365-2567.2005.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skansen-Saphir U, Lindfors A, Andersson U. Cytokine production in mononuclear cells of human milk studied at the single-cell level. Pediatric Research. 1993;34:213–216. doi: 10.1203/00006450-199308000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV. Porcine γδ T cells: Possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol and Immunopathol. 2006;112:49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am J Vet Res. 2000;61:1384–1389. doi: 10.2460/ajvr.2000.61.1384. [DOI] [PubMed] [Google Scholar]
- 50.Tlaskalova-Hogenova H, Mandel L, Trebichavsky I, Kovaru F, Barot R, Sterzl J. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol. 1994;43:135–142. doi: 10.1016/0165-2427(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 51.Tuboly S, Bernath S, Glavits R, Medveczky I. Intestinal absorption of colostral lymphoid cells in newborn pigs. Vet Immunol Immunopath. 1988;20:75–85. doi: 10.1016/0165-2427(88)90027-x. [DOI] [PubMed] [Google Scholar]
- 52.Wagstrom EA, Yoon KJ, Zimmerman JJ. Immune component in porcine mammary secretions. Viral Immunol. 2000;13:383–397. doi: 10.1089/08828240050144699. [DOI] [PubMed] [Google Scholar]
- 53.Williams PP. Immunomodulating effects of intestinal maternal colostral leukocytes by neonatal pigs. Can J Vet Res. 1993;57:1–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Yabuhara A, Kawai H, Komiyama A. Development of natural killer cytotoxicity during childhood: marked increases in number of natural killer cells with adequate cytotoxic abilities during infancy to early childhood. Pediatr Res. 1990;28:316–322. doi: 10.1203/00006450-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Yang H, Parkhouse RME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunol. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunol. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]