Abstract
Objective:
The objective of this study was to identify the common etiological pathogens causing community acquired pneumonia (CAP) in our hospital and sensitivity patterns to the common antibiotics used.
Materials and Methods:
This study was undertaken in a 750 bedded multi-specialty referral hospital in Kerala catering to both urban and semi-urban populations. It is a prospective study of patients who attended the medical out-patient department and those admitted with a clinical diagnosis of CAP, during the year 2009. Data were collected based on detailed patient interview, clinical examination and laboratory investigations. The latter included sputum culture and sensitivity pattern. These were tabulated and percentage incidence of etiological pathogens calculated. The antimicrobial sensitivity pattern was also classified by percentage and expressed as bar diagram.
Results:
The study showed Streptococcus pneumoniae to be the most common etiological agent for CAP, in our hospital setting. The other organisms isolated in order of frequency were Klebsiella pneumoniae, Pseudomonas aeruginosa, Alpha hemolytic streptococci, Escherichia coli, Beta hemolytic streptococci and atypical coli. S. pneumoniae was most sensitive to linezolid, followed by amoxicillin-clavulanate (augmentin), cloxacillin and ceftriaxone. Overall, the common pathogens causing CAP showed highest sensitivity to amikacin, followed by ofloxacin, gentamycin, amoxicillin-clavulanate (augmentin), ceftriaxone and linezolid. The least sensitivity rates were shown to amoxicillin and cefoperazone.
Conclusion:
In a hospital setting, empirical management for cases of CAP is not advisable. The present study has shown S. pneumoniae as the most likely pathogen and either linezolid or amikacin as the most likely effective antimicrobial in cases of CAP, in our setting.
Keywords: Community acquired, microbial sensitivity, pathogens, pneumonia
Introduction
Community acquired pneumonia (CAP) is defined as an acute infection of the pulmonary parenchyma that is associated with at least some symptoms of acute infection, accompanied by the presence of an acute infiltrate on a chest radiograph or auscultatory findings consistent with pneumonia, in a patient not hospitalized or residing in a long-term care facility for 14 days before the onset of symptoms.[1] In the absence of a chest X-ray, the British Thoracic Society defines pneumonia as symptoms of an acute lower respiratory tract infection, including a cough and at least one other lower respiratory tract symptom, together with at least one systemic symptom and new focal signs on chest examination.[2]
The incidence of CAP is lowest in the age group of 18-24 years and highest in the under-5 and over-65 groups. The mortality rates are disproportionately high in the those aged more than 65 years.[3] The average mortality for hospitalized patients with CAP is 14%.[4]
Given the emergence of antibiotic resistance and the potential hazards of antibiotic treatment failures, a definitive microbiological diagnosis is desirable. The common etiological agents causing CAP include Streptococcus pneumoniae (20-60%), Hemophilus influenza (3-10%), Chlamydia pneumoniae (4-6%), Mycoplasma pneumoniae (1-6%), Legionella (2-8%), Staphylococcus aureus (3-5%), Gram-negative bacilli (3-5%), viruses (2-13%). In 40-60% cases, no cause is identified and in 2-5% cases, two or more pathogens are identified.[5]
The main objectives of investigating patients with a clinical diagnosis of pneumonia are to obtain radiological confirmation of the diagnosis, to exclude other conditions that may mimic pneumonia, to obtain a microbiological diagnosis, to assess the severity of pneumonia and to identify the development of complications.
Treatment is often empirical and based on the clinical and radiological diagnosis directed at the most common organisms. CAP remains an important public health problem. Hence, its antimicrobial treatment should be based on the distribution of etiological pathogens and their incidence in the community. Local and national resistance patterns and prior exposure should be taken into consideration. The decision to hospitalize is based on the prognostic criteria.
The present study is an attempt to identify and delineate the microbiological and antimicrobial sensitivity characteristics of CAP in a given community and hospital setting.
Materials and Methods
The study was conducted for dissertation purpose at Lourdes Hospital, Ernakulam which is a 750 bedded multispecialty referral hospital catering to both urban and semi-urban population.
It was a prospective study of all patients who attended the medical out-patient department and were diagnosed with CAP. Furthermore, those patients admitted with a clinical diagnosis of CAP were included. There was no control group involved in this study.
The duration of the study was from January 2009 to December 2009.
Data collection was by detailed history, clinical examination and sputum culture and sensitivity pattern. For the latter, the zone of inhibition was calculated by the diameter in mm. Data analysis was by representation as tabulation and bar diagrams and calculation, in percentages, of etiological pathogens and sensitivity patterns.
The exclusion criteria were patients below 16 years of age, immunocompromised patients, hospital acquired pneumonia (onset after 4 days of hospitalization), aspiration pneumonia, patients with cystic fibrosis or tuberculosis and pregnant women.
Statistical analysis was out of the scope of this particular study.
Results
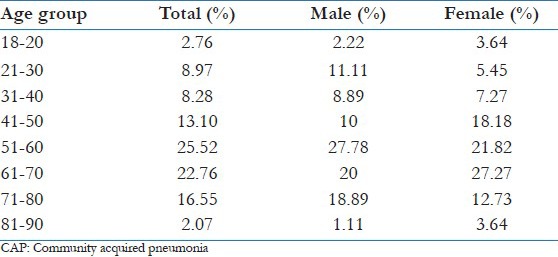
A total of 145 patients were included in the study. There were 90 males (62.07%) and 55 females (37.93%). Ages ranged from 18 to 90 years [Table 1]. The highest incidence was in the 51-60 years group.
Table 1.
Age and gender distribution of CAP, in the studied population
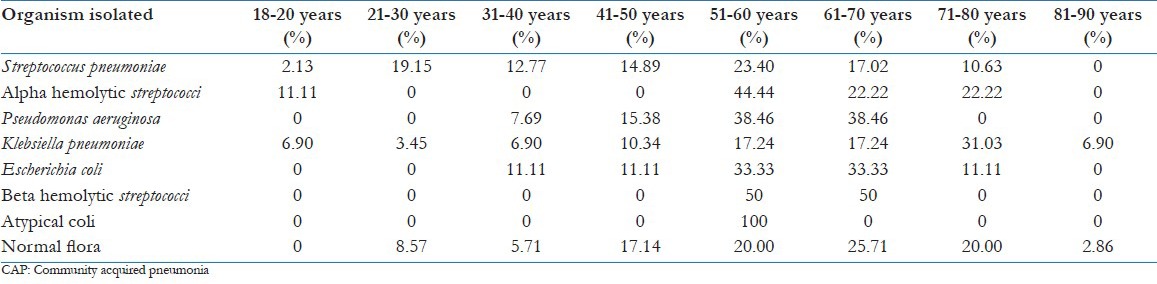
Seven pathogens were isolated by sputum culture [detailed in Table 2 and Figure 1]. The rate of incidence of the common pathogens based on the distribution in different age groups varied as shown in Table 3. No pathogen was identified in 35 patients (24.14%).
Table 2.
Incidence of etiological organisms of CAP, with gender distribution
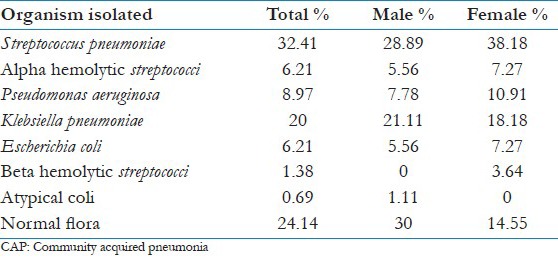
Figure 1.
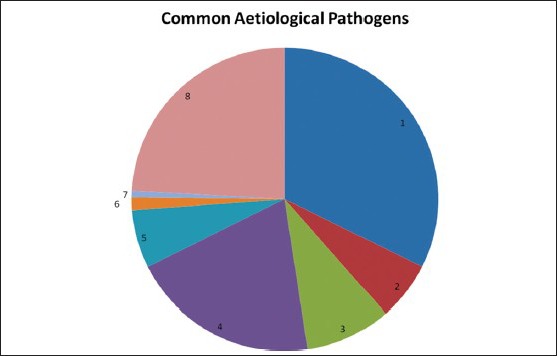
Pie Diagram showing the aetiological pathogens isolated from the sputum of CAP patients in this study
Table 3.
Incidence of etiological organisms of CAP, with age distribution
The different antimicrobial agents included in this study are mentioned in Annexure 1.
Annexure 1.
The observed antimicrobial sensitivity patterns of individual pathogens are shown in Table 4.
Table 4.
Observed incidence and antimicrobial sensitivity patterns of individual pathogens in the studied population
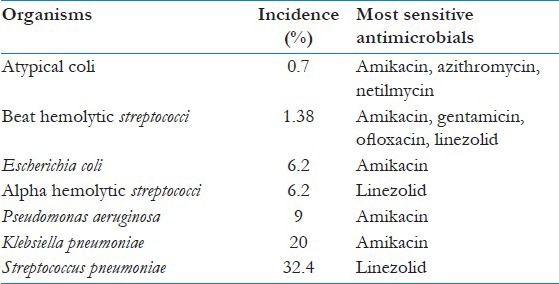
Figure 2 depcits the the antimicrobial sensitivity of the most common pathogen in this study (Streptococcus pneuominae).
Figure 2.
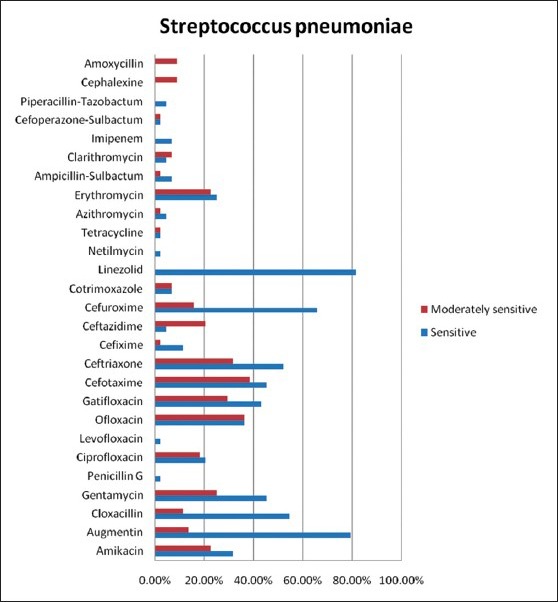
Bar diagram showing the antimicrobial sensitivity of the most common pathogen in this study (Streptococcus pneumoniae)
The rates of sensitivity to these antimicrobial agents shown by all the pathogens taken together are represented in terms of percentage [Figure 3]. Here, moderate sensitivity is not taken into consideration.
Figure 3.
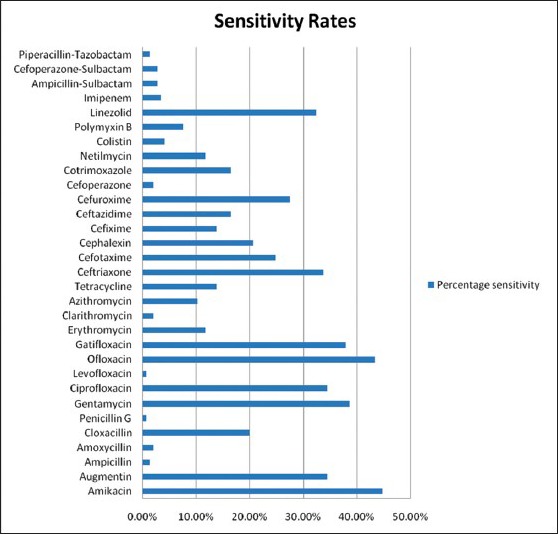
Bar diagram showing the overall sensitivity to the tested antimicrobial agents
Overall, the common pathogens causing CAP showed highest sensitivity to amikacin (44.84%), followed by ofloxacin (43.45%), gentamicin (38.62%), gatifloxacin (37.93%), augmentin and ciprofloxacin (34.48%), ceftriaxone (33.79%) and linezolid (32.41%). The least sensitivity rates are shown to levofloxacin and penicillin G (0.69%), ampicillin and piperacillin-tazobactam (1.38%) and clarithromycin, amoxicillin and cefoperazone (2.07%).
Discussion
A wide array of organisms can cause acute pneumonia and published reports vary in the organisms isolated due to differences in patient groups, presence of epidemic organisms and diligence of the investigation. If sputum is available and the patient has not had prior antibiotic treatment, then a gram stain is sufficient to identify the causative organism. Overnight culture will provide confirmation and the chance to perform susceptibility studies, allowing modification of empirical therapy. Culture is also helpful in establishing the pathogenicity of any isolates.[6,7] The role of sputum as a tool in the diagnostic work-up of patients with CAP remains controversial.
Key findings
S. pneumoniae was the most common etiological pathogen isolated in the present study. On the other hand, no pathogen was identified in almost 25% cases. Both these findings are in agreement with Indian and Western reports in the literature.[5,8] Almost as high as 50% normal flora has been reported in CAP. One study from Sher-i-Kashmir Institute of Medical Sciences, Srinagar has emphasized the need for further studies including the serological tests for Legionella, mycoplasma and viruses to identify the microbial etiology of CAP.[9]
For low-risk patients who may be safely treated in an ambulatory setting, the Infectious Diseases Society of America (IDSA)-updated guidelines recommend doxycycline, a macrolide or an antipneumococcal fluoroquinolone as preferred agents because these agents have activity against the most likely pathogens in this setting (S. pneumoniae, M. pneumoniae and Chlamydophila pneumoniae).[9] The IDSA has adopted the new antipneumococcal fluoroquinolones, namely levofloxacin, sparfloxacin, moxifloxacin and gatifloxacin, as preferred agents for the treatment of both ambulatory and hospitalized patients with CAP and for penicillin-resistant pneumococcal pneumonia.[10] For empiric treatment of the moderately ill-hospitalized patient, the IDSA recommends an extended-spectrum cephalosporin (cefotaxime, ceftriaxone) plus a macrolide or monotherapy with a fluoroquinolone.[11] For hospitalized patients admitted to the intensive care unit, the IDSA prefers agents that include combination therapy with an extended-spectrum cephalosporin plus a macrolide or a fluoroquinolone. Monotherapy with a fluoroquinolone is not recommended because data with seriously ill CAP patients are limited.
The Centers for Disease Control and Prevention (CDC) working group suggests that penicillin-resistant S. pneumoniae isolates are uncommon and activity against such organisms is unnecessary for empiric treatment.[12] Their recommendations for first-line therapy of CAP include a macrolide, doxycycline or an oral beta-lactam such as cefuroxime, amoxicillin or amoxicillin/clavulanate. The CDC recommends reserving the use of fluoroquinolones for treatment of Gram-negative pathogens, patients with beta-lactam allergy or treatment of penicillin-resistant pneumococcal pneumonia.[13,14] Differences exist among groups about the role of the fluoroquinolones. The major concern is the potential that extensive use may result in increased resistance. Although this argument may be raised regarding any antibiotic, the concern with the fluoroquinolones is that resistance to one agent affects all agents to some degree. Currently, pneumococcal resistance to the fluoroquinolones is low, but a report from Canada has demonstrated an association between increased fluoroquinolone use and resistance in S. pneumoniae.[15] In addition, the potential for development of resistance in other organisms (especially Pseudomonas aeruginosa) as a result of the indiscriminate use of fluoroquinolones poses a more ominous threat to this drug class.[16] Here, the common pathogens show an overall sensitivity of 43.45% to ofloxacin, 37.93% to gatifloxacin and 34.48% to ciprofloxacin.
The CDC working group recommends amoxicillin-clavulanate (augmentin) as one of the first-line antimicrobials. In our study, S. pneumoniae, which is the commonest etiological agent, showed nearly, 80% sensitivity to amoxicillin-clavulanate. Overall, the common pathogens showed 34.48% sensitivity to this drug. Pneumococcal susceptibility has changed significantly over the past decade. Despite four decades of using penicillin, only modest rates of reduced susceptibility to penicillin were reported in the 1980s. Strains with minimum inhibitory concentrations (MICs) >0.1 mg/mL accounted for 3.8% of isolates in the 1980's; by 1994-1995, the rate was 24% and by 1997 it was 43.8%. Penicillin resistance is also associated with resistance to other antimicrobial classes, including cephalosporins, macrolides, tetracyclines and trimethoprim/sulfamethoxazole. Antibiotics less affected by this broad-spectrum resistance include vancomycin, the fluoroquinolones, clindamycin, chloramphenicol and rifampin.[17,18,19] Penicillin susceptibility should be tested in all significant pneumococcal isolates. Strains are considered as sensitive if the MIC is <0.1 mg/mL.
It is observed from various studies that most penicillin resistance is “relative resistance” and is readily treatable with penicillin and/or beta-lactams. Most of the highly penicillin-resistant S. pneumoniae infections may also be treated with beta-lactams. Alternately, doxycycline or respiratory quinolones may be used. Vancomycin is rarely, if ever, needed. Very highly penicillin-resistant S. pneumoniae (MIC 6 μg/mL) strains are a rare cause of CAP, but remain susceptible to ceftriaxone. Studies suggest that empiric macrolide monotherapy should be avoided because approximately 25% of S. pneumoniae strains are naturally resistant to all macrolides.[20] In our study, the common pathogens showed low overall sensitivity rates to macrolides. The rates of sensitivity were 11.72% to erythromycin, 10.34% to azithromycin and 2.07% to clarithromycin.
Preferred monotherapy for CAP includes doxycycline or a respiratory quinolone. This is the most cost-effective way to optimally treat CAP. No increased resistance is noted with extensive use. It is well-tolerated in both oral and intravenous (IV) forms. It is ideal for IV-to-oral switch monotherapy in terms of patient compliance, safety and cost.[21,22]
The timing of initial antimicrobial administration may be an important predictor of outcome. Mortality gradually increased with progressive delays between the time a patient presented and the time the initial dose of an antibiotic was administered. This difference reached statistical significance when the delay exceeded 8 h. Many studies conclude that antibiotics should be initiated within 4 h after patient presentation.[23]
Interpretation and implications in the context of the totality of evidence
A final conclusion about the superiority of one antibacterial regimen over another in hospitalized patients with CAP cannot be drawn on the basis of the limited data available. Monotherapy coverage of both typical and atypical pathogens in CAP is preferred over double-drug therapy. It is less expensive and as effective as double-drug regimens.
Strengths
Comprehensive inclusion of the entire possible patient group, in the given community setting.
Limitations
Serological tests to identify some rare organisms were not carried out in this study. Some of these may have been reported as normal flora.
Antimicrobials tested for sensitivity in this study have not included the newer, costlier drugs.
Conclusions
CAP remains an important public health problem. Hence, its appropriate antimicrobial treatment is a relevant issue. Ideally, this has to be culture and sensitivity based.
We have attempted to identify these in our community and study the pathogens and their sensitivity. The present study has shown S. pneumoniae as the most likely pathogen and either linezolid or amikacin as the most likely effective antimicrobial in cases of CAP, in our setting. Keeping the IDSA and CDC recommendations in mind, we suggest that the inferences from our study should be considered when initiating antimicrobial therapy in our local setting.
Acknowledgments
The authors would like to thank our Microbiologist, Mr. Toby Antony, Medical Supdt., Lourdes Hospital, Dr. Paul Puthuran, and Biostatistician, Ms. Yamuna S.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37:1405–33. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macfarlane J British thoracic society standards of care committee. BTS guidelines for the management of community acquired pneumonia in adults. Thorax. 2001;56(Suppl 4):IV1–64. doi: 10.1136/thorax.56.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett JG, Breiman RF, Mandell LA, File TM., Jr Community-acquired pneumonia in adults: Guidelines for management. The infectious diseases society of America. Clin Infect Dis. 1998;26:811–38. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 4.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–41. [PubMed] [Google Scholar]
- 5.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;333:1618–24. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MJ, Martin DE. Quantitative sputum culture as a means of excluding false positive reports in the routine microbiology laboratory. J Clin Pathol. 1972;25:697–700. doi: 10.1136/jcp.25.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guckian JC, Christensen WD. Quantitative culture and gram stain of sputum in pneumonia. Am Rev Respir Dis. 1978;118:997–1005. doi: 10.1164/arrd.1978.118.6.997. [DOI] [PubMed] [Google Scholar]
- 8.Bansal S, Kashyap S, Pal LS, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 9.Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Lung India. 2010;27:54–7. doi: 10.4103/0970-2113.63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious diseases society of America. Clin Infect Dis. 2000;31:347–82. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR, Musher DM, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: A report from the drug-resistant Streptococcus pneumoniae Therapeutic working group. Arch Intern Med. 2000;160:1399–408. doi: 10.1001/archinte.160.10.1399. [DOI] [PubMed] [Google Scholar]
- 12.Stahl JE, Barza M, DesJardin J, Martin R, Eckman MH. Effect of macrolides as part of initial empiric therapy on length of stay in patients hospitalized with community-acquired pneumonia. Arch Intern Med. 1999;159:2576–80. doi: 10.1001/archinte.159.21.2576. [DOI] [PubMed] [Google Scholar]
- 13.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–44. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 14.Westley BP, Chan PA. Questions remain regarding mandatory use of macrolides in community-acquired pneumonia. Intensive Care Med. 2010;36:1787. doi: 10.1007/s00134-010-1950-1. [DOI] [PubMed] [Google Scholar]
- 15.Restrepo MI, Frei CR. Health economics of use fluoroquinolones to treat patients with community-acquired pneumonia. Am J Med. 2010;123:S39–46. doi: 10.1016/j.amjmed.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Ailani RK, Agastya G, Ailani RK, Mukunda BN, Shekar R. Doxycycline is a cost-effective therapy for hospitalized patients with community-acquired pneumonia. Arch Intern Med. 1999;159:266–70. doi: 10.1001/archinte.159.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Hooper DC. Expanding uses of fluoroquinolones: Opportunities and challenges. Ann Intern Med. 1998;129:908–10. doi: 10.7326/0003-4819-129-11_part_1-199812010-00015. [DOI] [PubMed] [Google Scholar]
- 18.Appelbaum PC, Klepser ME. Role of the newer fluoroquinolones against penicillin-resistant Streptococcus pneumoniae. Infect Dis Clin Pract. 1999;8:374–82. [Google Scholar]
- 19.Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian bacterial surveillance network. N Engl J Med. 1999;341:233–9. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 20.Low DE, Scheld WM. Strategies for stemming the tide of antimicrobial resistance. JAMA. 1998;279:394–5. doi: 10.1001/jama.279.5.394. [DOI] [PubMed] [Google Scholar]
- 21.Thornsberry C, Ogilvie PT, Holley HP, Sahm DF. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: A prospective US study. Antimicrobial agents and chemotherapy. 1999;43:2612–23. doi: 10.1128/aac.43.11.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doern GV, Pfaller MA, Kugler K, Freeman J, Jones RN. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program. Clin Infect Dis. 1998;27:764–70. doi: 10.1086/514953. [DOI] [PubMed] [Google Scholar]
- 23.Mufson MA. Penicillin-resistant Streptococcus pneumoniae increasingly threatens the patient and challenges the physician. Clin Infect Dis. 1998;27:771–3. doi: 10.1086/514954. [DOI] [PubMed] [Google Scholar]


