Abstract
Surgical carotid endarterectomy (CEA) was long considered the standard approach for the treatment of atherosclerotic carotid artery disease. This was based on results of several randomized trials demonstrating its effectiveness over the best medical therapy. In the past two decades, patients identified high-risk for surgery were offered carotid artery stenting (CAS) as a less invasive option. Despite its initial limitations, CAS has evolved into an elaborate method currently considered to be equivalent and in selected patients even preferable to CEA. However, outcomes of both procedures are highly operator dependent and a simple stratifying method to prioritize CAS, CEA or medical therapy only has not yet been proposed. In addition, recently published randomized trials highlighted the importance of proper patient selection and rigorous training contributing to low absolute rates of (procedural) adverse events. This review discusses the history and evidence for carotid revascularization and briefly presents technical aspects and innovations in CAS.
Keywords: carotid stenting, carotid endarterectomy, emboli protection device, transcranial Doppler
Introduction
Stroke has become the second most common cause of death in industrialized nations and is a major cause of long-term disability worldwide [1]. Currently, it is estimated that stenosis of the internal carotid artery may be responsible for up to 15–20% of all strokes or transient ischaemic attacks [2]. In order to prevent stroke, carotid endarterectomy (CEA) has been used extensively as a primary option to eliminate both hemodynamic stenosis as well as the source of cerebral atheroemboli. Over time, carotid artery stenting (CAS) has emerged as an alternative and less invasive treatment. Importantly, it has been shown that the outcomes of both CAS and CEA are comparable after 30 days of follow-up [3, 4] and therefore the efficacy and safety of both procedures are particularly dependent on periprocedural results.
Rationale for carotid revascularization
In the early 1950s, DeBakey and Eastcott et al. pioneered the first carotid endarterectomies [5, 6]. Thereafter, CEA has evolved over the years and has become a standard for the treatment of atherosclerotic carotid artery disease.
Following wide acceptance in the late 1950s, the number of performed procedures steadily increased until the mid 1980s, when criticism over inappropriate indications and the unacceptable rate of periprocedural adverse events was reported, requiring better evidence to justify interventions [7].
North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST) were the first well-constructed, multicenter, randomized controlled trials which found a clear benefit of CEA compared to medical treatment in patients with high-degree symptomatic internal carotid stenosis [8, 9]. In summary, the benefit was unequivocal in patients with carotid stenosis of 70% or greater and was proportionate to severity of stenosis. In patients with 50–69% stenosis, the benefit was less, albeit still significant. No benefit from surgery was demonstrated in patients with less than 50% stenosis.
Unlike symptomatic patients, carotid revascularization is a matter of debate in patients with asymptomatic stenosis. Asymptomatic Carotid Atherosclerosis Study (ACAS) and Asymptomatic Carotid Surgery Trial (ACST) trials reported reduction in estimated 5-year risk of ipsilateral stroke or any perioperative stroke or death in patients with carotid stenosis of 60% or greater who underwent CEA [10, 11]. One should, however, keep in mind that these studies were performed at times when optimal medical treatment of vascular risk factors was not available. Currently, it is recommended that carotid revascularization in asymptomatic patients with > 70% stenosis should be guided by an assessment of individual risk factors and should include a thorough discussion of the risks and benefits of the procedure with an understanding of patient preferences [12].
Evidence for carotid artery stenting
Percutaneous transluminal balloon angioplasty for carotid artery stenosis was first reported by Mathias in 1981 [13]. Despite showing favourable results, simple balloon angioplasties were associated with a number of complications such as vessel wall recoil, angiographically evident intimal dissection, and plaque dislodgement with particulate embolization. Immediately after reporting promising results of stent-supported coronary angioplasties, stents were applied in carotid interventions and the first series of patients undergoing CAS were reported [14]. Since 1994, CAS has been investigated as an alternative treatment to CEA in multiple randomized studies.
The SAPPHIRE randomized trial specifically enrolled high-risk patients to compare CEA to CAS with an emboli protection device (EPD) [15]. The trial was stopped prematurely because of slow enrolment while many potential participants were excluded because they were considered to be at exceedingly high risk for complications if randomized to surgery. In patients with symptomatic stenosis, the occurrence of primary endpoint (composite of stroke, myocardial infarction (MI) or death) was similar (16.8% in CAS vs. 16.5% in CEA). In asymptomatic patients, fewer primary endpoints occurred after CAS (9.9% vs. 21.5%). The protocol required the collection of cardiac serum biomarker data for diagnosis of periprocedural MI, the majority of which were asymptomatic events.
Stent-protected Percutaneous Angioplasty of the Carotid vs. Endarterectomy (SPACE) was a non-inferiority trial in which 1200 symptomatic patients with > 50% stenosis were randomized to endarterectomy or stenting [3]. The primary endpoint (ipsilateral stroke or death within 30 days) occurred in 6.84% of stented patients and 6.34% of patients undergoing CEA.
In contrast to SAPPHIRE, the Endarterectomy Versus Angioplasty in Patients with Severe Symptomatic Carotid Stenosis (EVA-3S study) was terminated prematurely due to a significant difference in the 30-day complication rate favouring carotid surgery [4]. However, both SPACE and EVA-3S were criticised because of involving CAS operators with limited experience (e.g. in EVA-3S only 12 CAS procedures or 35 stenting procedures in other vessels were required).
The Stenting versus endarterectomy for treatment of carotid-artery stenosis (CREST) study published in 2010 [16] is considered to be the most informative CAS trial published to date, especially because only experienced and well-trained operators were involved. The primary endpoint was a composite of stroke, MI or death from any cause during the periprocedural period (30 days) or any ipsilateral stroke within 4 years after randomization. Overall 2502 symptomatic and asymptomatic patients were randomized and observed over a period of 2.5 years. There was no significant difference in the estimated 4-year rates of primary endpoint between the stenting and endarterectomy group (7.2% vs. 6.8%, respectively). Despite the similarity in primary outcome, there were differences in rates of the component periprocedural events. Stroke was more frequent with CAS (4.1% vs. 2.3%, p = 0.01), and MI was more likely after CEA (2.3% vs. 1.1%, p = 0.03). Although the absolute rates of either component events were low, it was suggested that the quality of life was impacted significantly by stroke but not by MI.
Taken together, the results of the so far published studies show a lower and in some cases similar complication rate for CEA compared with CAS. In high-volume centres with well-trained operators, it was shown that CAS can be equivalent in terms of benefit and complications [17]. However, despite the results of SAPPHIRE and CREST trials, the evidence suggesting an optimal treatment method in asymptomatic patients on optimal medical therapy is still limited. Currently, several randomized trials in standard surgical risk asymptomatic patients are in progress (ACT-1, ACST-2, SPACE-2). Hopefully, the results of these trials will soon shed some light into various opinions concerning this state of the field.
Patient selection
Carotid artery stenting represents one of the final frontiers for percutaneous endoluminal intervention. Apart from operator training, it is equally essential to carefully select patients in order to reach favourable outcomes [18].
Usually, CAS has been offered predominantly to patients considered high risk for open surgical procedure [19]. But, over time, a number of articles have dealt with stroke predictors in relation to anatomy, lesion characteristics and patient status, modifying the selection criteria for both CAS and CEA [20–23]. Tables I and II summarize high-risk patient and lesion/approach characteristics.
Table I.
Patient-related high-risk criteria
| CEA-related |
| History of open heart surgery |
| Need of open heart surgery within 30 days |
| History of myocardial infarction |
| Known multi-vessel coronary artery disease |
| Left ventricular dysfunction with ejection fraction < 40% |
| Severe bronchopulmonary disease |
| Severe renal disease |
| Contralateral laryngeal nerve palsy |
| CAS-related |
| Advanced age (> 70 years) |
| Prior stroke |
| Decreased cerebrovascular reserve |
| High risk of bleeding |
Table II.
Lesion/approach-related high-risk criteria
| CEA-related |
| Significant contralateral carotid disease |
| Restenosis after carotid endarterectomy |
| Prior neck surgery or irradiation |
| High lesion behind mandible or low lesion that would require thoracic exposure |
| CAS-related |
| Aortic arch type II or III |
| Bovine aortic arch |
| Aortic arch calcification |
| Severe calcification or ulceration at the level of the lesion |
| Ostial lesions |
| Long lesions (> 15 mm) |
| Need for predilation |
Particularly, patients with extensive accumulation of scar tissue following previous neck surgery (including CEA) or irradiation present a surgical challenge and therefore are considered preferable candidates for CAS [24, 25]. It is also problematic to operate on patients with high carotid bifurcation (especially lesions located above the second cervical vertebra) or lesions below the clavicle [26, 27]. On the other hand, difficult arch anatomy and advanced atherosclerotic changes of the lesion or alongside the access route might excessively increase the risk of CAS [28, 29].
One must also be very reserved in indicating patients with multiple clinical high risk factors, which might influence the patient's long-term prognosis per se despite favourable procedural outcomes [18, 30]. In such case, the benefit of CAS over medical treatment must be critically evaluated, especially in asymptomatic patients.
Most importantly, for many years, advanced age was considered to be a high risk criterion indicating less invasive endovascular treatment. However, the SPACE and CREST trials proved this concept to be misleading and favoured patients older than 70–-75 years to be better treated with CEA [16, 31].
Technical aspects
The efficacy of CAS in preventing stroke depends on the ability of the operator to achieve complication-free results. Meticulous knowledge of the technique and its innovations as well as proper selection of instruments is therefore necessary.
Overall, CAS can be divided into 5 phases – wiring, EPD placement, predilation, stent deployment and postdilation. The procedure is usually performed through the femoral access with continuous monitoring of haemodynamics. Following diagnostic angiography and anatomical evaluation, selection of proper EPD must be done.
In early reports, predilation of the stenosis (usually with a 4-mm balloon) was vigorously recommended, suggesting less “scissoring” effect of the stent struts on plaque [32]. However, later observational studies proved direct (non-predilated) CAS to be safe [33, 34], and thereafter predilation has not been routinely performed in order to minimize endovascular manoeuvres and the risk of potential embolization.
Interestingly, there is ongoing debate over superiority of stent designs used. Balloon-expandable stents have been abandoned because of higher risk of deformation (external crushing) and covered stents showed a high risk of restenosis [35]. Therefore, only self-expanding stents are available for routine CAS. These can be further divided into open-cell design and closed-cell design stents, which differ in the size of free cell area between the stent struts (Figure 1). Whereas closed-cell design stents have a dense, metallic mesh and thus may provide more effective plaque coverage, open-cell design stents are more flexible and conformable due to a lower number of bridges between the different rings. Notably, Bosiers et al. found in their non-randomized study that patients (particularly symptomatic) who received closed-cell carotid stents exhibited lower risk for subacute neurologic events [36]. However, no randomized trial has yet supported this hypothesis, while Schillinger et al. [37] did not show superiority of either type of stent in a large scale study of 1684 patients treated in 10 European centres (51% closed-cell stent design).
Figure 1.
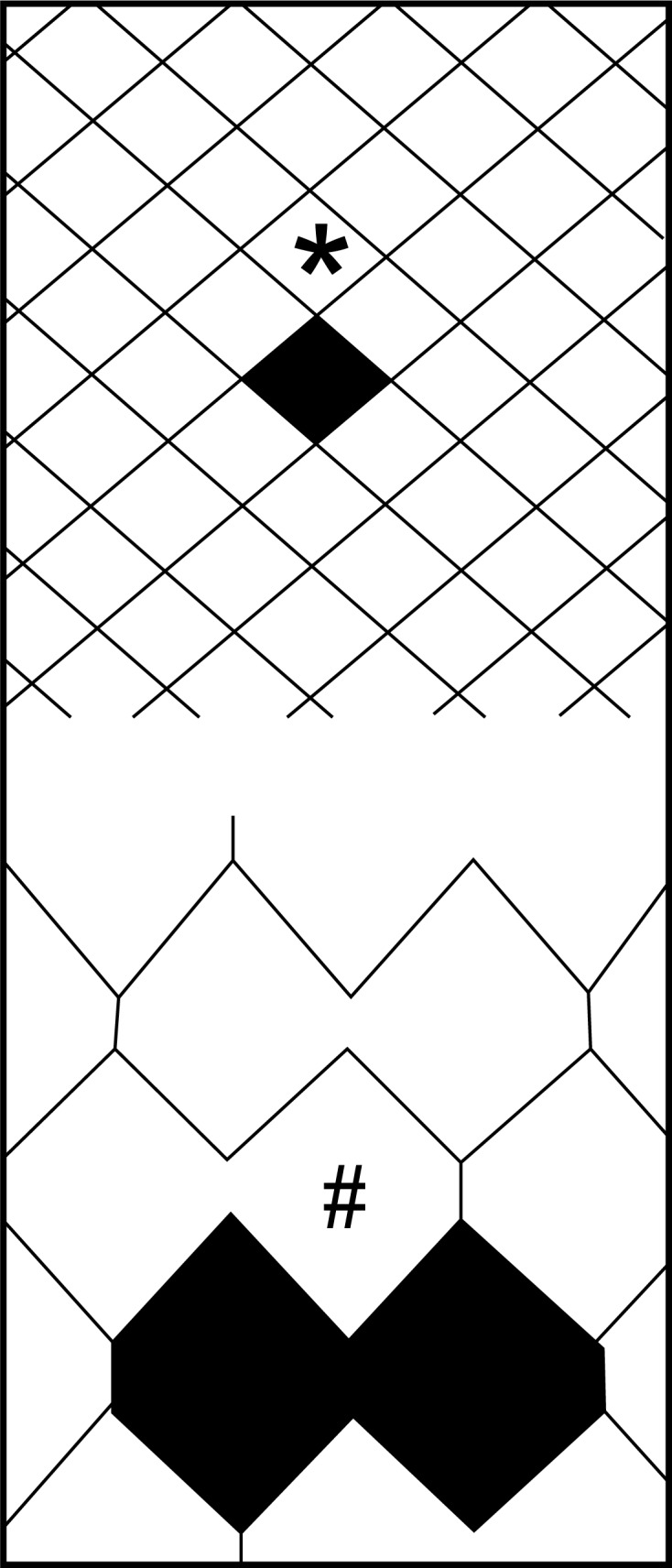
Schematic view of stent-cell designs: (*) – closed-cell design vs. (#) – open-cell design
In current praxis, the choice of the optimal carotid stent depends mainly on arterial anatomy and lesion morphology. When treating a tortuous anatomy, stents with a flexible open-cell configuration are preferred in order to prevent kinking of the artery either proximal or distal to the stent (Figure 2). Lesions with suspected high emboligenicity are best covered with closed-cell design stents.
Figure 2.
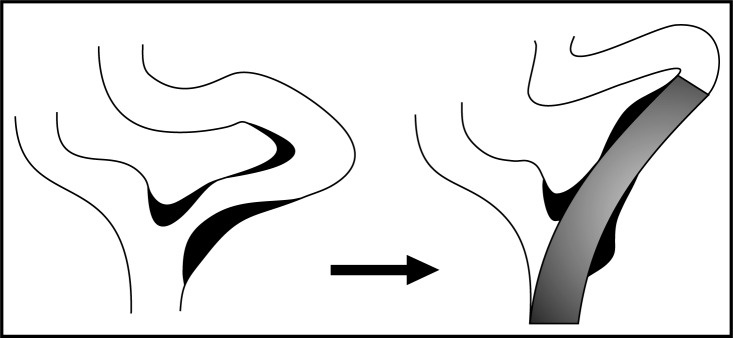
Schematic view of internal carotid artery kinking distal to the stent
In the majority of patients, stents need to be postdilated at the end of the procedure. Residual stenosis of less than 30% with restoration of normal blood flow denotes a technically successful procedure [38]. In rare exceptions, it was shown to be safe to omit postdilation when optimal expansion of the stenosis was reached with stent deployment only [39].
After the intervention, patients are transferred to a monitored unit and discharged the following day if no complications occur. Aspirin 100 mg daily is continued indefinitely and clopidogrel 75 mg daily is continued for 30 days with a loading dose administered within 24 h before the procedure. Ultrasound follow-up is scheduled for 30 days, 6 and 12 months, and then yearly to document continued patency of the stent.
Protection devices
Despite the improvement of CAS delivery systems, stents and the technique itself, it is not possible to completely eliminate the risk of distal embolization of the atherosclerotic fragments released during endovascular manipulation. Inevitably, this must have led to the development of EPDs.
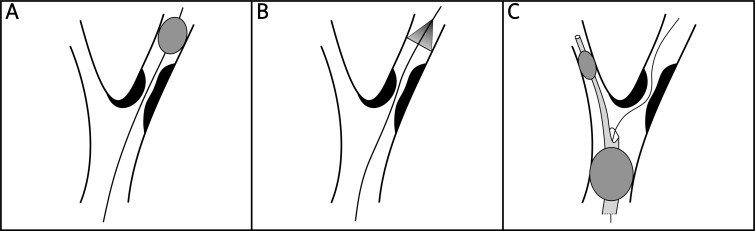
In 1990, Theron et al. made the first attempt to eliminate the embolic burden on cerebral circulation by occluding the distal carotid artery with a balloon (Figure 3 – panel A) [40]. This balloon contained embolic material, released during the intervention, within the proximal blood column. This was subsequently aspirated (or flushed into the external carotid artery) before re-establishing the flow.
Figure 3.
Schematic review of emboli protection devices. Guidewire threaded through stenosis of internal carotid artery in all panels. Panel A – distal balloon occlusion device. Panel B – distal filter protection device. Panel C – proximal protection device with distal occlusion balloon (smaller) inflated in external carotid artery and proximal occlusion balloon (larger) inflated in common carotid artery
By far the most widely used EPDs are based on distal filter placement (Figure 3 – panel B). The inherited advantage of the filter systems compared to the occlusion systems is the possibility to preserve blood flow and directly visualize the lesion during treatment. Although the use of a filter makes intuitive sense, the evidence that it actually reduces the incidence of stroke during CAS was repeatedly questioned. Interestingly, despite not being powered for such analysis, the ACCULINK for Revascularization of Carotids in High-Risk Patients (ARCHeR trial) failed to show a difference in 30-day stroke rates between the first phase (in which no EPDs were used) and the rest of the study [41]. However, several observational studies reported improved outcomes during protected CAS [42–44] and current guidelines suggest that EPD deployment during CAS can be beneficial to reduce the risk of stroke [45].
Promising means of protection against stroke are proximal protection systems (Figure 3 – panel C), which avoid the need for lesion crossing. Inflating a proximal balloon in the common carotid artery and distal balloon in the external carotid artery allows for flow reversal or flow arrest within the internal carotid artery with the possibility to withdraw debris during intervention. These systems are rapidly gaining popularity for several studies have reported on very low rates of complications [46–48]. It is believed that the results of CAS could even be improved with broad acceptance of proximal protection systems.
Imaging techniques (transcranial Doppler and magnetic resonance imaging)
Because adverse events of carotid revascularization are relatively sparse, clinicians have been seeking other means of monitoring the embolic load on the cerebral circulation. Such surrogates are transcranial Doppler (TCD) and magnetic resonance imaging (MRI).
Transcranial Doppler monitoring uses a dedicated Doppler probe, which is fixed to the head by a frame. This allows for continuous online monitoring of the middle cerebral artery flow disturbances as well as detection of microembolic signals (MES). These high intensity transient signals (HITS) are observed during both CAS and CEA and represent plaque debris/thrombus fragments dislodged during intervention or air emboli (typically observed during plain angiography).
Magnetic resonance imaging sensitively detects ischaemic areas resulting from occlusion of distal intracranial arteries. Enhancement techniques such as diffusion-weighted MRI (DWI) can detect diffusion abnormalities present in brain areas with acute ischaemia, thus making MRI the most sensitive technique for detection of ischaemic areas currently available.
Although multiple MES are detected during CAS by TCD and occasionally paralleled by new ischaemic brain lesions on DWI, the majority of patients remain clinically silent [49]. Therefore, the concept arises that the brain has sufficient resistance to clear the majority of these emboli. Ackerstaff et al. reported that this resistance can be broken by high embolic load within a short time (e.g. successive showers of emboli during postdilation) or by a macroembolus (embolic signal immediately followed by marked attenuation of MCA flow) [50].
Most importantly, TCD and MRI have proved that EPDs can effectively counteract MES and DWI lesions [48, 51, 52]. Interestingly, while proximal protection devices significantly reduce the MES count (especially during flow arrest or the flow reversal period) [53], filter-type protection devices result in an increased number of TCD-detected emboli. A theoretical explanation is that a macroembolus is propelled into the filter and subsequently disintegrated into smaller particles which can pass through the micropores, thereby leading to an increase in the number of microemboli. The filters typically retain fragments larger than the pore size of around 100 µm but allow passage of smaller particles. This accounts for the lower number of macroemboli found compared to unprotected CAS [54].
In summary, despite failure to show a clear-cut correlation between embolism and neurological sequelae, these imaging techniques provide clinicians with important feedback and are essential for research purposes.
Conclusions
Carotid artery disease is an important contributor to stroke which carries high risk of morbidity and mortality. It has been repeatedly shown that patients with significant carotid artery disease profit from interventional therapy, especially if symptomatic. Carotid endarterectomy has long been considered to be the standard for treatment, while patients unsuitable or unfit for CEA were offered CAS as an alternative method. Despite slow acceptance, CAS has evolved into an elaborate technique considered to be equivalent to CEA in experienced hands. In addition, it is believed that the results of CAS could even improve with the broad acceptance of proximal protection devices. Therefore, the option of CAS should always come to the mind of all physicians involved in care of patients suffering from stroke or having significant carotid artery disease.
Acknowledgments
The preparation of this article was supported by grants from the Ministry of Health of the Czech Republic: 00064203 and NT13319.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. [Google Scholar]
- 4.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 5.DeBakey ME. Carotid endarterectomy revisited. J Endovasc Surg. 1996;3:4. doi: 10.1583/1074-6218(1996)003<0004:CER>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplagia. Lancet. 1954;267:994–6. doi: 10.1016/s0140-6736(54)90544-9. [DOI] [PubMed] [Google Scholar]
- 7.Warlow CP. Carotid endarterectomy: does it work? Stroke. 1984;15:1068–76. doi: 10.1161/01.str.15.6.1068. [DOI] [PubMed] [Google Scholar]
- 8.North American Symptomatic Carotid Artery Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 9.European Carotid Surgery Trialists′ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or mild (0-29%) carotid stenosis. Lancet. 1991;337:1235–43. [PubMed] [Google Scholar]
- 10.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 11.Halliday AW, Thomas D, Mansfield A. The Asymptomatic Carotid Surgery Trial (ACST). Rationale and design. Steering Committee. Eur J Vasc Surg. 1994;8:703–10. doi: 10.1016/s0950-821x(05)80650-4. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004;35:2425–7. doi: 10.1161/01.STR.0000141706.50170.a7. [DOI] [PubMed] [Google Scholar]
- 13.Mathias K. Perkutane transluminale Katheterbehandlung supraaortaler Arterienobstruktionen. Angiology. 1981;3:47–50. [Google Scholar]
- 14.Roubin GS, Yadav JS, Iyer SS, Vitek JJ. Carotid stent supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol. 1996;78:8–12. doi: 10.1016/s0002-9149(96)00487-0. [DOI] [PubMed] [Google Scholar]
- 15.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 16.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier B, Frank B, Wahl A, Diener HC. Secondary stroke prevention: patent foramen ovale, aortic plaque, and carotid stenosis. Eur Heart J. 2012;33:705–13. doi: 10.1093/eurheartj/ehr443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spacek M, Martinkovicova L, Zimolova P, Veselka J. Mid-term outcomes of carotid artery stenting in patients with angiographic string sign. Catheter Cardiovasc Interv. 2011;79:174–9. doi: 10.1002/ccd.23144. [DOI] [PubMed] [Google Scholar]
- 19.Roubin GS, Iyer SR, Halkin A, et al. Realizing the potential of carotid artery stenting. Proposed paradigms for patient selection and procedural technique. Circulation. 2006;113:2021–30. doi: 10.1161/CIRCULATIONAHA.105.595512. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann R, Niessner A, Kypta A, et al. Risk score for peri-interventional complications of carotid artery stenting. Stroke. 2006;37:2557–61. doi: 10.1161/01.STR.0000240688.81918.32. [DOI] [PubMed] [Google Scholar]
- 21.Veselka J, Zimolova P, Martinkovicova L, et al. Comparison of mid-term outcomes of carotid artery stenting for moderate versus critical stenosis. Arch Med Sci. 2012;8:75–80. doi: 10.5114/aoms.2012.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadek M, Hynecek RL, Sambol EB, Ur-Rehman H, Kent KC, Faries PL. Carotid angioplasty and stenting, success relies on appropriate patient selection. J Vasc Surg. 2008;47:946–51. doi: 10.1016/j.jvs.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Estruch-Perez MJ, Plaza-Martinez A, Hernandez-Cadiz MJ, Soliveres-Ripoll J, Solaz-Roldan C, Morales-Suarez-Varela MM. Interaction of cerebrovascular disease and contralateral carotid occlusion in prediction of shunt insertion during carotid endarterectomy. Arch Med Sci. 2012;8:236–43. doi: 10.5114/aoms.2012.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrod-Kim P, Kadkhodayan Y, Derdeyn CP, Cross DT, 3rd, Moran CJ. Outcomes of carotid angioplasty and stenting for radiation-associated stenosis. AJNR Am J Neuroradiol. 2005;26:1781–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Favre JP, Nourissat A, Duprey A, Nourissat G, Albertini JN, Becquemin JP, Association Universitaire de Recherche en Chirurgie Vasculaire Endovascular treatment for carotid artery stenosis after neck irradiation. J Vasc Surg. 2008;48:852–8. doi: 10.1016/j.jvs.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Bryant MF. Anatomic considerations in carotid endarterectomy. Surg Clin North Am. 1974;54:1291–6. doi: 10.1016/s0039-6109(16)40484-6. [DOI] [PubMed] [Google Scholar]
- 27.Hans SS, Shah S, Hans B. Carotid endarterectomy for high plaques. Am J Surg. 1989;157:431–4. doi: 10.1016/0002-9610(89)90593-x. [DOI] [PubMed] [Google Scholar]
- 28.Setacci C, Chisci E, Setacci F, Iacoponi F, de Donato G, Rossi A. Siena carotid artery stenting score: a risk modelling study for individual patients. Stroke. 2010;41:1259–65. doi: 10.1161/STROKEAHA.110.578583. [DOI] [PubMed] [Google Scholar]
- 29.Spacek M, Veselka J. Bovine arch. Arch Med Sci. 2012;8:166–7. doi: 10.5114/aoms.2012.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69–77. doi: 10.1159/000086509. [DOI] [PubMed] [Google Scholar]
- 31.Stingele R, Berger J, Alfke K, et al. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 2008;7:216–22. doi: 10.1016/S1474-4422(08)70024-3. [DOI] [PubMed] [Google Scholar]
- 32.Vitek JJ, Roubin GS, Al-Mubarek N, New G, Iyer SS. Carotid artery stenting: technical considerations. AJNR Am J Neuroradiol. 2000;21:1736–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Veselka J, Cerna D, Zimolova P, et al. Thirty-day outcomes of direct carotid artery stenting with cerebral protection in high-risk patients. Circ J. 2007;71:1468–72. doi: 10.1253/circj.71.1468. [DOI] [PubMed] [Google Scholar]
- 34.Mathur A, Dorros G, Iyer SS, Vitek JJ, Yadav SS, Roubin GS. Palmaz stent compression in patients following carotid artery stenting. Cathet Cardiovasc Diagn. 1997;41:137–40. doi: 10.1002/(sici)1097-0304(199706)41:2<137::aid-ccd7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Schillinger M, Dick P, Wiest G, et al. Covered versus bare self-expanding stents for endovascular treatment of carotid artery stenosis: a stopped randomized trial. J Endovasc Ther. 2006;13:312–9. doi: 10.1583/06-1819.1. [DOI] [PubMed] [Google Scholar]
- 36.Bosiers M, de Donato G, Deloose K, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg. 2007;33:135–41. doi: 10.1016/j.ejvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Schillinger M, Gschwendtner M, Reimers B, et al. Does carotid stent cell design matter? Stroke. 2008;39:905–9. doi: 10.1161/STROKEAHA.107.499145. [DOI] [PubMed] [Google Scholar]
- 38.Piamsomboon C, Roubin GS, Liu MW, et al. Relationship between oversizing of self-expanding stents and late loss index in carotid stenting. Cathet Cardiovasc Diagn. 1998;45:139–43. doi: 10.1002/(sici)1097-0304(199810)45:2<139::aid-ccd7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 39.Spacek M, Zimolova P, Veselka J. Carotid artery stenting without post-dilation. J Interv Cardiol. 2012;25:190–6. doi: 10.1111/j.1540-8183.2011.00694.x. [DOI] [PubMed] [Google Scholar]
- 40.Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology. 1996;201:627–37. doi: 10.1148/radiology.201.3.8939208. [DOI] [PubMed] [Google Scholar]
- 41.Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–64. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 42.Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60:259–66. doi: 10.1002/ccd.10645. [DOI] [PubMed] [Google Scholar]
- 43.Theiss W, Hermanek P, Mathias K, et al. Pro-CAS: a prospective registry of carotid angioplasty and stenting. Stroke. 2004;35:2134–9. doi: 10.1161/01.STR.0000135763.62131.6a. [DOI] [PubMed] [Google Scholar]
- 44.Kastrup A, Gröschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34:813–9. doi: 10.1161/01.STR.0000058160.53040.5F. [DOI] [PubMed] [Google Scholar]
- 45.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2011;57:e16–94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Clair DG, Hopkins NL, Mehta M, et al. EMPiRE Clinical Study Investigators. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPIRE Clinical Study. Catheter Cardiovasc Interv. 2011;77:420–9. doi: 10.1002/ccd.22789. [DOI] [PubMed] [Google Scholar]
- 47.Parodi JC, Schönholz C, Parodi FE, Sicard G, Ferreira LM. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. J Cardiovasc Surg (Torino) 2007;48:117–24. [PubMed] [Google Scholar]
- 48.Bijuklic K, Wandler A, Hazizi F, Schofer J. The PROFI Study (Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting): a prospective randomized trial. J Am Coll Cardiol. 2012;59:1383–9. doi: 10.1016/j.jacc.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 49.Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion-weighted MR imaging in carotid angioplasty and stenting with protection by the reversed carotid arterial flow. AJNR Am J Neuroradiol. 2006;27:753–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Ackerstaff RG, Suttorp MJ, Van Den Berg JC, et al. Antonius Carotid Endarterectomy, Angioplasty, and Stenting Study Group. Prediction of early cerebral outcome by transcranial Doppler monitoring in carotid bifurcation angioplasty and stenting. J Vasc Surg. 2005;41:618–24. doi: 10.1016/j.jvs.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 51.Cosottini M, Michelassi MC, Puglioli M, et al. Silent cerebral ischemia detected with diffusion-weighted imaging in patients treated with protected and unprotected carotid artery stenting. Stroke. 2005;36:2389–93. doi: 10.1161/01.STR.0000185676.05358.f2. [DOI] [PubMed] [Google Scholar]
- 52.Rubartelli P, Brusa G, Arrigo A, et al. Transcranial Doppler monitoring during stenting of the carotid bifurcation: evaluation of two different distal protection devices in preventing embolization. J Endovasc Ther. 2006;13:436–42. doi: 10.1583/05-1804MR.1. [DOI] [PubMed] [Google Scholar]
- 53.Montorsi P, Caputi L, Galli S, et al. Microembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protection. J Am Coll Cardiol. 2011;58:1656–63. doi: 10.1016/j.jacc.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Vos JA, Van Den Berg JC, Ernst SM, et al. Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology. 2005;234:493–9. doi: 10.1148/radiol.2342040119. [DOI] [PubMed] [Google Scholar]