Abstract
Introduction
Systemic sclerosis (scleroderma, SSc) is a severe chronic connective tissue disease caused by immune system disorders and changes in the structure and functions of blood vessels, which consequently leads to enhanced tissue fibrosis. The aim of the study was to evaluate changes in the organ of vision in systemic sclerosis patients.
Material and methods
Overall the study involved 27 patients with systemic sclerosis. The control group comprised 27 age- and gender-matched healthy individuals. All the study subjects underwent complete ophthalmological examination that in systemic sclerosis patients additionally involved fluorescein angiography.
Results
Ophthalmological examination revealed higher incidence of the following abnormalities in the study group, compared to the control: symptoms of dry eye syndrome (19 eyes, p < 0.02), astigmatism(in 30 eyes, p < 0.01), posterior subcapsular cataract (10 eyes, p < 0.05), increased intraocular pressure (> 21 mm Hg were observed in 11 eyes, p < 0.002) and vascular abnormalities within fundus in fluorescein angiography (20 eyes).
Conclusions
In patients with systemic sclerosis numerous abnormalities within the vision of organ may be found. Regular ophthalmological examinations are essential among the mentioned group. The examination should be particularly focused on the presence of retinal vascular abnormalities.
Keywords: scleroderma, eye complications, retinal changes
Introduction
Systemic sclerosis (scleroderma, SSc) is a severe chronic connective tissue disease caused by immune system disorders and changed structure and functions of blood vessels [1].
There are two major forms of systemic sclerosis: a limited one (lSSc), which involves cutaneous manifestations that mainly affect the hands, arms and face, where skin hardening lesions do not exceed 1/3 of the forearm length; and diffuse systemic sclerosis (dSSc), which is rapidly progressing and affects a large area of the skin and one or more internal organs. Apart from these basic variants, some transitional forms and systemic sclerosis without skin lesions may be observed [2].
In both clinical variants of systemic sclerosis (lSSc and dSSc) lesions in internal organs give similar symptoms [3]. Approximately 90% of patients with lower oesophageal stenosis suffer from problems with swallowing and reflux. Pulmonary fibrosis that occurs in over 40% of patients results in the development of circulatory and respiratory failure, while bronchial lesions may promote development of cancer [4, 5]. Myocardial fibrosis may result in conduction and rhythm disorders. Pericardial fibrosis commonly leads to the deterioration of cardiac performance. Lesions involving renal vessels and parenchyma that occur in approx. 40% of patients result in malignant hypertension and/or renal cirrhosis, and may result in serious general complications and death [2, 3, 6].
There are few reports available concerning ophthalmological complications in the course of systemic sclerosis. The aim of our paper was to evaluate changes in the organ of vision in systemic sclerosis patients [7–12].
Material and methods
Overall the study involved 27 patients with systemic sclerosis (mean age: 54.5 ±11.1 years, Me = 52 years), including 17 patients with lSSc and 10 with dSSc. Disease duration was 3–22 years (mean 11.0 ±7.5 years, Me = 8 years). The limited systemic sclerosis patients (16 women and 1 man, aged 38–77 years) received vasodilating drugs (calcium channel blockers, or benzodiazepines or angiotensin receptor antagonists, including pentoxifylline sometimes) and vitamin E, and periodic procaine penicillin injections were used. The diffuse systemic sclerosis patients (8 women and 2 men, aged 35–70 years) used immunosuppressive therapy (low doses of corticosteroids – prednisone 0.5 mg/kg bw/day) alone or in combination with cytostatic drug (cyclophosphamide 1.5 mg/kg bw/day).
Considering the similar character of organ complications in particular forms of systemic sclerosis, the group of scleroderma patients was treated as a whole, without division into two major forms.
The control group comprised 27 age- and gender-matched healthy individuals (mean age: 55.7 ±9.7 years; Me = 55 years).
Ethical approval was obtained from the Ethics Committee of the Medical University of Lodz (number of the approval RNN/332/06/KB from 26.09.2006). All participants gave informed consent.
All the study subjects underwent complete ophthalmological examination involving visual acuity assessment with Snellen charts, examination of anterior and posterior eye segments with the slit lamp, Schirmer I test, assessment of intraocular pressure with Goldmann applanation tonometer and ultrasound assessment of vitreous body (Humphrey Instruments A/B-Scan System, with 10 MHz head, B mode). In the event any change of optic disc appearance was found, additionally the vision field was examined (Humphrey analyser).
The eyelid skin fibrosis was estimated similar to the whole body skin fibrosis as the skin fold recognition difficulty, swelling of the eyelids and difficulty in eversion of the eyelids. The examination in all subjects (in study and control group) was performed by one physician.
The criteria for the diagnosis of keratoconjunctivitis sicca were based on the results of the anterior eye segment examination (bulbar and tarsal conjunctival and perilimbal area hyperaemia assessment) and corneal fluorescein staining ≥ 3 degree on CCLRU scale (Cornea and Contact Lens Research Unit, School of Optometry and Vision Science, The University of New South Wales, Sydney, Australia) as well as the test results of lissamine green conjunctival staining ≥ 4 on the Bijsterveld scale. In addition the results of Schirmer I test (≤ 5 mm) were taken into consideration.
Moreover, retinal blood circulation was investigated in all the patients with fluorescein angiography. The investigation involved the use of fundus camera TRC 50 EX (Topcon) following pupil dilatation with 1% tropicamide solution (eye drops) and administration of 5 ml of 10% fluorescein solution into the basilic vein. The investigation was recorded for approx. 10 min.
Statistical analysis
Statistical analysis of the study groups involved calculation of structure factors. The factors were not expressed as percentages, due to small groups, but as fractions. For quantitative variables arithmetic mean (), median (Me) and standard deviation (SD), as a measure of dispersion, were calculated. Minimum and maximum values also were provided.
In the analysis of vision defects, for the results of Schirmer test I, the χ2 test of independence was used, and in the case of a small size of the analysed group Fisher's exact test was employed. To compare the age of the subjects, duration of the disease, and intraocular pressure values the Mann-Whitney test was applied. The adopted significance level was p ≤ 0.05.
Results
No statistically significant differences in age or gender were found between the study groups (p > 0.05). Full visual acuity, corrected for distant vision (1.0), was found in 28 eyes of 21 patients (f = 0.7) with systemic sclerosis. Visual acuity in the range 0.9–0.5 was noted in 12 eyes (f = 0.22), and in the range < 0.5–0.1 in 4 eyes (f = 0.07). Analysis of visual acuity with optimal correction did not reveal statistically significant differences between the study and control group (p > 0.05).
The most common refractive error in the study group was compound myopic astigmatism – 16/54 eyes (f = 0.3), while myopia was the most common in the control group – 12/54 eyes (f = 0.22) (Table I).
Table I.
Incidence of refractive errors in all the eyes in systemic sclerosis group and control group
| Refractive error | Study group | Control group | Value of p | ||
|---|---|---|---|---|---|
| Number of eyes (n = 54) | Fraction f | Number of eyes (n = 54) | Fraction f | ||
| Myopia | 2 | 0.04 | 12 | 0.22 | < 0.01* |
| Hyperopia | 6 | 0.11 | 4 | 0.07 | > 0.05 |
| Astigmatism (total) | 30 | 0.55 | 17 | 0.31 | < 0.01* |
| Emmetropia | 16 | 0.3 | 21 | 0.4 | > 0.05 |
| Altogether | 54 | 1 | 54 | 1 | – |
Statistically significant
The most common complaints reported by SSc patients referred to eye fatigue in 24 patients (f = 0.89), dry conjunctiva in 23 (f = 0.85), burning eyes in 21 (f = 0.78), stinging sensation in 17 (f = 0.63) and lacrimation in 5 (f = 0.18).
Hardening and thickening of palpebral skin was noted in 25 (f = 0.93) SSc patients. Eyelid telangiectasias was noted in 19 (f = 0.7) patients, and chronic blepharitis in 16 (f = 0.6). Superficial conjunctival hyperaemia was noted in 20 (f = 0.74), and varicose dilatation of subconjunctival and episcleral blood vessels in 8 (f = 0.29) and 6 (f = 0.22) SSc patients, respectively. Conjunctival fibrosis was found in 7 SSc patients (f = 0.26).
Diameter and mobility of pupils, as well as eyeball mobility, were determined in all the study subjects. In one female SSc patient (in 1 eye) significant scleral thinning was found involving the perilimbal area inferiorly and superiorly.
Mean IOP values in the study group were 18.58 ±3.7 mm Hg (Me = 19 mm Hg) and were significantly higher than in the control group (mean 15.89 ±2.2 mm Hg, Me = 17 mm Hg, p < 0.001). Intraocular pressure valve exceeding 21 mm Hg was observed in 8 patients (in 11 eyes), exclusively in the systemic sclerosis group (p < 0.002).
In 19 eyes of SSc patients Schirmer I test results were low – below 10 mm, and in 5 eyes below 5 mm. In the control group all the results of the test were normal, and differences between groups were statistically significant (p < 0.02) (Table II).
Table II.
Results of Schirmer I test in study and control groups
| Schirmer I test Right and left eye [mm] | Study group | Control group | Together | Value of p | |||
|---|---|---|---|---|---|---|---|
| Number of eyes n | Fraction f | Number of eyes n | Fraction f | Number of eyes n | Fraction f | ||
| < 5 | 5 | 0.093 | 0 | 0.000 | 5 | 0.046 | > 0.05 |
| 5–10 | 14 | 0.259 | 0 | 0.000 | 14 | 0.130 | < 0.001* |
| > 10-15 | 19 | 0.352 | 3 | 0.056 | 22 | 0.204 | < 0.001* |
| > 15 | 16 | 0.296 | 51 | 0.944 | 67 | 0.620 | < 0.001* |
| Together | 54 | 1.000 | 54 | 1.000 | 108 | 1.000 | – |
Statistically significant
Keratoconjunctivitis sicca was found in 6 (f = 0.22) SSc patients. In one female SSc patient fatty deposits on corneal endothelium were observed. History confirmed recurrent uveitis in the course of systemic sclerosis.
Lens opacity was found in 14 (f = 0.52) patients (21 eyes), mostly in the form of posterior subcapsular cataract (in 10 eyes) (f = 0.18), nuclear cataract (in 8 eyes) (f = 0.15) and cortical cataract appearing as focal cystic opacities (in 3 eyes) (f = 0.05). Posterior subcapsular cataract was significantly more common in the study group as compared to the control group (p < 0.05).
Clinical assessment of SSc patients revealed degenerative lesions in the vitreous body structure in 39 eyes (f = 0.73) (22 patients, f = 0.81). In 15 eyes (f = 0.18) the lesions appeared as veil-like condensations, while in 16 eyes (f = 0.29) they appeared as single opacities at the base of the vitreous body. Complete posterior vitreous detachment was found in 8 eyes (f = 0.15).
Retinal lesions were found in 15 (f = 0.55) systemic sclerosis patients. In 10 patients (f = 0.37) changes in optic disc appearance were found in the form of dilatation of the optic cup above 0.6 (13 eyes, f = 0.24), optic disc drusen (2 eyes, f = 0.04) and temporal pallor of the optic disc (1 eye, f = 0.02). Visual field test in these patients revealed arcuate scotoma, increase of blind spot and paracentral scotoma, respectively.
In 14 eyes (f = 0.26) the dry form of age-related macular degeneration (AMD) was found. Fluorescein angiography performed in these patients (8 patients, f = 0.28) revealed foci of hyperfluorescence, slightly increasing with time, without leakage effect, that were consistent with soft drusen and window defects of retinal pigment epithelium. In 1 eye (f = 0.02) epiretinal membrane was found. Hard exudates in the posterior pole were found in 8 eyes (f = 0.15).
In 8 eyes in the patients of the study group (f = 0.15) foci of pigment epithelium were found peripherally on the optic disc. In 2 eyes (f = 0.04) peripheral foci of choroidal dystrophy were found, and in 6 eyes (f = 0.1) paving stone degeneration areas. “Salt and pepper” degenerative lesions were found in 4 eyes (f = 0.7).
In 15 patients (f = 0.55) abnormalities of retinal vessels concerning their calibre and shape, in the form of stenoses and increased tortuosity, were found.
Fluorescein angiography revealed delayed filling of choroidal lobules in 20 eyes of SSc patients (f = 0.37). Among other retinal vascular abnormalities, occlusion of the peripheral arterial vessel was found in 1 eye (f = 0.02), and presence of a sheathed venous vessel due to underlying inflammation also in 1 eye (f = 0.02); the latter finding was confirmed by fluorescein angiography. In 6 eyes (f = 0.1) thinning of choroidal capillaries and retinal pigment epithelium was noted.
In the study group, in 4 eyes (f = 0.07) ischemic areas surrounded by microaneurysms and intraretinal extravasation, dilatation of the vessel-free fovea region and diffuse macular oedema were observed. The lesions required laser photocoagulation. Peripherally on the fundus, areas lacking capillary perfusion and an enhanced capillary network were found (Figure 1–4).
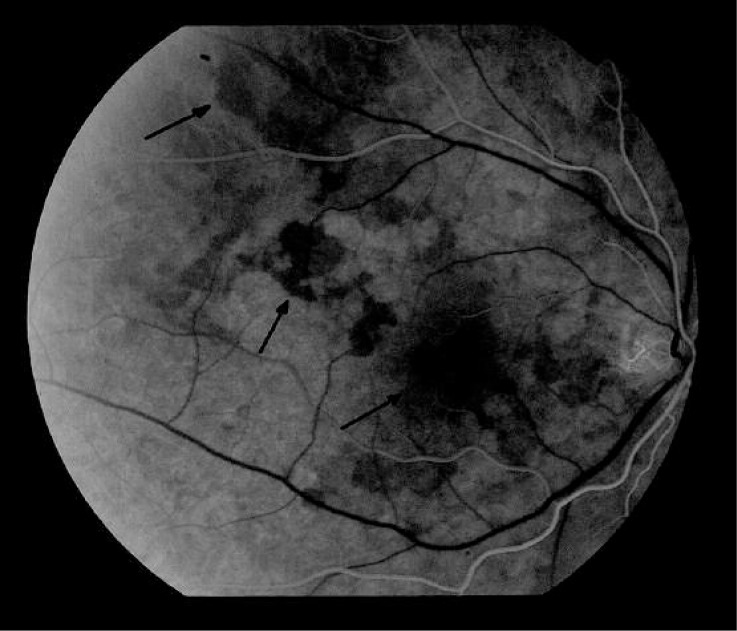
Figure 1.
Results of fluorescein angiography of the right eye of a patient, aged 64, diagnosed with systemic sclerosis 8 years ago. In the arterial phase delayed filling of choroidal lobules with fluorescein in the central macular and along the upper temporal arcade may be noted, that persists up to the venous phase
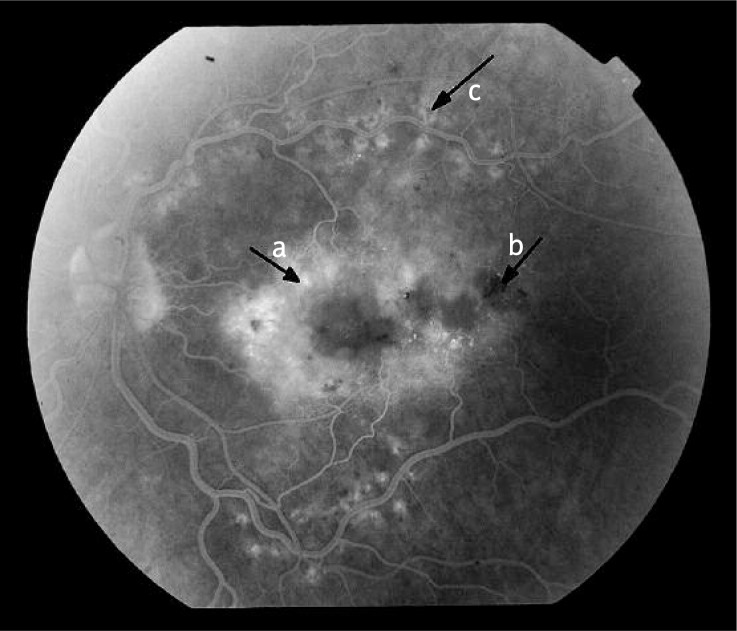
Figure 2.
Results of fluorescein angiography of the left eye of a patient, aged 61, diagnosed with systemic sclerosis 7 years ago. Areas lacking capillary perfusion (a) and enhanced capillary network (b) in the arterial-venous phase were found in the superior temporal quadrant
Figure 3.
Results of fluorescein angiography of the left eye of a patient, aged 61, diagnosed with systemic sclerosis 7 years ago. In the recirculation phase a diffuse, poorly circumscribed and intensive hyperfluorescence of the whole posterior pole was ob - served, that was consistent with diffuse macular oedema (a). In the projection of extravasations hypofluorescence foci (b), and within temporal arcades post-laser therapy foci (c) may be noted
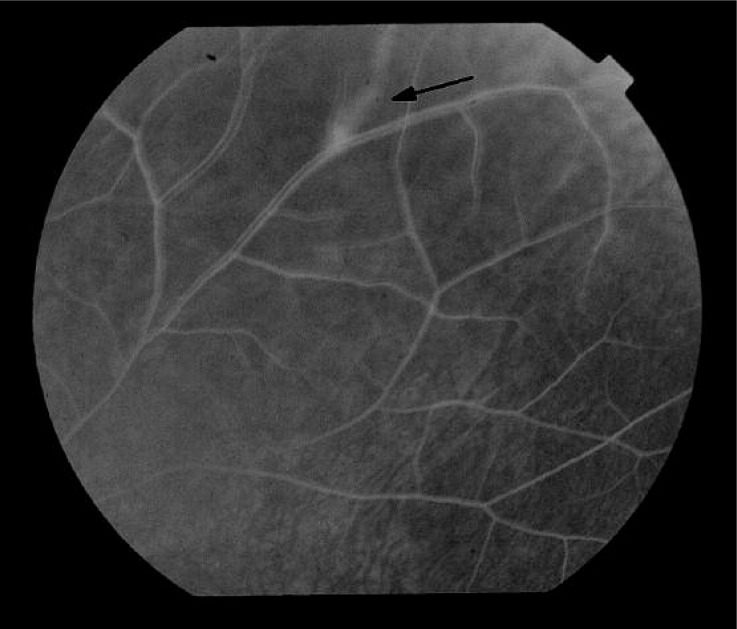
Figure 4.
Results of fluorescein angiography of the left eye of a patient, aged 60, diagnosed with systemic sclerosis 10 years ago. In the late venous phase inflammatory reaction within the bifurcation of the peripheral venous vessel with increasing dye leakage may be noted. Sheathed vessel with closed perfusion above the bifurcation (arrow)
Discussion
Systemic sclerosis is a severe chronic connective tissue disease, which results in involvement of numerous internal organs. Changes in the organ of vision are consequences of systemic complications of scleroderma or adverse effects of immunosuppressive treatment applied. Ocular symptoms may occur at any stage of the disease and may involve numerous ocular structures. Their course may be clinically latent or very intensive.
The most common clinical manifestations of soft tissue fibrosis and inflammation in the patients examined included increased tonus and telangiectasias of eyelid skin noted in the majority of the patients, while lesions involving conjunctival fibrosis were much less common. Results of this study are consistent with reports of other authors. The most commonly reported lesions include periorbital oedema, ectropion and ciliary madarosis [7].
With swollen and hardened eyelids, microorganism colonisation and eyelid margin inflammation are more common. In more advanced cases even restriction of eyelid mobility may occur. In systemic sclerosis patients, occlusion of tear ducts and reduced tear production and outflow may occur due to enhanced fibrosis, resulting in the lack of removal of pathogens from the conjunctival sac. There are cases of marginal ulcer [7, 8] as well as endophthalmitis caused by Propionibacterium acnes following cataract phacoemulsification in SSc patients reported in the literature [7]. Also eyeball retraction was observed in a SSc patient with concomitant Parry-Romberg syndrome that involves hemifacial atrophy due to an inflammatory process of muscles and soft tissues [9].
In the patients studied, symptoms and signs of dry eye syndrome, that appeared as keratoconjunctivitis sicca in as many as 22% of them, were observed significantly more commonly than in the control group. Dry eye syndrome is believed to be caused by fibrosis-related impairment of lacrimal gland secretion, namely water portion of the tear film or lipid layer disorder due to chronic blepharitis and Meibomian gland dysfunction, as well as increased evaporation of tears from the ocular surface in the case of restricted eyelid mobility and thus reduced blinking.
In our own studies involving SSc patients, astigmatism, which occurred in over half of the eyes and sometimes was determined as 5 dioptres, was observed significantly more commonly than in the control group. Also in the analysis of vision defects performed in other studies a high incidence of astigmatism was stressed [10, 11], which may be explained by corneal thickening. Significant increase of corneal thickness occurs in early stages of SSc, the most prominent changes being observed within the first 8 years of disease duration [12, 13]. Literature data indicate an association between systemic sclerosis, and keratoconus and pellucid marginal degeneration [10]. No case of keratoconus was observed in our patients.
In approx. 16–35% of scleroderma patients central and peripheral nervous system disorders occur [7]. Damage to the nervous system may involve cranial nerves, mostly in the form of trigeminal neuropathy. The median nerve is the most commonly affected peripheral nerve in SSc patients. Changes in the nervous system secondary to systemic sclerosis may result in weakness of the extraocular muscles, in particular the superior rectus one [7, 11, 14]. In the literature cases of bilateral retrobulbar neuritis [15], optic disc oedema [9, 10, 16] and optic nerve atrophy with concomitant hemiparesis [17] in SSc patient are described. Apparent temporal pallor of the optic disc observed in a SSc patient in our own studies led us to perform neurological examination of the patient. Head magnetic resonance imaging (MRI) revealed disseminated demyelinisation foci consistent with multiple sclerosis. Some papers indicate an association between the disease and systemic sclerosis [18].
There are reports on autonomic system disorders in SSc patients. Superiority of sympathetic system is one of the causes of Raynaud’ sign, paroxysmal vasoconstriction, mostly within hands, as a reaction to stress and low temperature. According to literature data, in SSc patients frequently dilated pupils at rest were observed, and in challenge tests, following stimulation with substance P, more prominent narrowing than in healthy individuals was observed, irrespective of baseline pupil diameter [17] that indicates autonomic system disorders. No pupil-related changes or eyeball mobility changes were observed in the SSc patient group.
In our own studies, thinning of a significant area of the sclera was found in a systemic sclerosis patient. Such a symptom was also noted by other authors [19]. Recurrent nodular episcleritis is highlighted among complications in SSc patients [19, 20].
Incidentally uveitis is noted in the course of various autoimmune diseases [9, 20, 21]. In our study, 1 patient had a history of past recurrent uveitis.
In our study cataracts at various stages were observed in over 50% of the patients, posterior subcapsular form being the most common. Higher incidence of this cataract form in SSc patients is believed to result from lens nutrition disorders secondary to narrowing of ciliary body vessels and/or complications of general steroid treatment applied in such cases [22, 23].
Significantly higher IOP in SSc patients as compared to the control group may result from fibrosis-restricted outflow of aqueous humour and/or chronic general corticosteroid use. According to other authors, normal tension glaucoma occurs more frequently in SSc patients than in the general population, promoted by vascular changes within the optic nerve [9, 11, 24].
In our group of SSc patients atrophic lesions of retinal pigment epithelium within the posterior pole occurred more frequently. Other authors’ studies show that foci of pigment epithelium degeneration occur in 25–50% of SSc patients, while in other autoimmune diseases, such as systemic lupus erythematosus, Sjögren's syndrome or mixed conjunctive tissue disease, their incidence is 4.5%. Focal atrophy of retinal pigment epithelium is most likely caused by choroidal circulation disorders [25, 26]. Also serous detachment of retinal pigment epithelium may be noted in the course of systemic sclerosis [27].
Fluorescein angiography commonly revealed retinal circulation changes in the form of delayed choroidal filling in the posterior pole. Literature data indicate that choroidal circulation disorders occur in over 33% of SSc patients [28, 29].
In our studies, as well as in those of other authors, dilatation of venous vessels, intraretinal extravasation, hard exudates [10, 29] and macular oedema were found in the eye fundus of SSc patients. Carbohydrate metabolism imbalance was ruled out in these patients.
It should be noted that in the course of systemic sclerosis numerous complications within internal organs occur, including myocardial fibrosis, pulmonary fibrosis and secondary polycythaemia, which may promote development of thrombi in retinal veins [10, 16, 30]. On the other hand, fibrosis of blood vessel may result in the occlusion of retinal arterial vessels [31]. Only one case of retinal venous occlusion and sheathed vessel was observed among our SSc patients.
Interestingly, even with significantly advanced ischaemic retinopathy secondary to scleroderma, no case of retinal neovascularisation was found. It may result from angiogenesis impairment that is typical in the course of scleroderma. Moreover, a difference between skin and retinal vascular lesions in stressed. In in vitro experiments, apoptosis of endothelial cells of skin and cartilage vessels was found in histological specimens taken from combs of chickens with genetically induced systemic sclerosis, while no such lesions were found in retinal microcirculation [32]. According to the authors, the fact confirms the different course of immune reaction in the retinal capillary bed. To date, only two cases of proliferative retinopathy in SSc patients with concomitant autoimmune diseases, including dermatomyositis and polymyositis, have been described in the literature [33].
Ocular symptoms are relatively common complications of systemic sclerosis, and may result in serious, irreversible changes in the organ of vision. When comparing our results of ophthalmological examination of SSc patients with other authors’ results, a decreasing number of ocular SSc complications may be noted relative to data from the 1970s and 1980s. This is related to significant progress in the diagnostics and treatment of systemic sclerosis. Currently reports on vision loss due to systemic sclerosis come mostly from poorly developed countries.
Considering the wide spectrum of ocular symptoms of systemic sclerosis and the possibility of complications of immunosuppressive treatment applied, the role of regular ophthalmological check-up should be stressed; the examination should be particularly focused on the presence of retinal vascular abnormalities [7–10].
Acknowledgments
Study conducted with own research of the Medical Univesity of Lodz, grant no. 502-15-636.
References
- 1.Dziankowska-Bartkowiak B, Gerlicz-Kowalczuk Z, Waszczykowska E. Angiogenin and SDF-1alpha serum concentration in patients with systemic sclerosis in relation to clinical status. Arch Med Sci. 2011;1:92–6. doi: 10.5114/aoms.2011.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadashkevich O, Davis P, Fritzler MJ. A proposal of criteria for the classification of systemic sclerosis. Med Sci Monit. 2004;10:615–21. [PubMed] [Google Scholar]
- 3.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma Lung Study Group. Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66:1641–7. doi: 10.1136/ard.2007.069518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plastiras SC, Karadimitrakis SP, Kampolis C, Moutsopoulos HM, Tzelepis GE. Determinants of pulmonary arterial hypertension in scleroderma. Semin Arthritis Rheum. 2007;36:393–6. doi: 10.1016/j.semarthrit.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Chmiel K, Krela-Kaźmierczak I, Łykowska-Szuber L, et al. Association between dermatological diseases and pathological changes in the gastrointestinal tract. Postep Derm Alergol. 2011;28:506–13. [Google Scholar]
- 6.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis,1972 -2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert DM, Jakobiec FA. Vol. 5. Philadelphia: W.B.Saunders Company; 2000. Principles and practice of ophthalmology; pp. 4589–95. [Google Scholar]
- 8.Horie K, Nishi M, Sawa M, Mochizuki M. A case of peripheral corneal ulcer accompanied by progressive systemic sclerosis. Nippon Ganka Gakkai Zasshi. 1992;96:922–9. [PubMed] [Google Scholar]
- 9.Zulian F, Vallongo C, Woo P, et al. Juvenile Scleroderma Working Group of the Pediatric Rheumatology European Society (PRES). Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873–81. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 10.Sii F, Lee GA, Sanfilippo P, Stephensen DC. Pellucid marginal degeneration and scleroderma. Clin Exp Optom. 2004;87:180–4. doi: 10.1111/j.1444-0938.2004.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 11.Allanore Y, Parc C, Monnet D, Brezin A, Kahan A. Increased prevalence of ocular glaucomatous abnormalities in systemic sclerosis. Ann Rheum Dis. 2004;63:1276–8. doi: 10.1136/ard.2003.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serup L, Serup J, Hagdrup HK. Increased central cornea thickness in systemic sclerosis. Acta Ophthalmol. 1984;62:69–74. doi: 10.1111/j.1755-3768.1984.tb06758.x. [DOI] [PubMed] [Google Scholar]
- 13.Serup J, Serup L. Increased central cornea thickness in localized scleroderma (morphoea) Metab Pediatr Syst Ophthalmol. 1985;8:11–4. [PubMed] [Google Scholar]
- 14.Egerer I, Fanta D. Changes in the ocular muscles in progressive scleroderma. Klin Monatsbl Augenheilkd. 1976;168:216–20. [PubMed] [Google Scholar]
- 15.Boschi A, Snyers B, Lambert M. Bilateral optic neuropathy associated with the crest variant of scleroderma. Eur J Ophthalmol. 1993;3:219–22. doi: 10.1177/112067219300300408. [DOI] [PubMed] [Google Scholar]
- 16.Ushiyama O, Ushiyama K, Yamada T, et al. Retinal findings in systemic sclerosis: a comparison with nailfold capillaroscopic patterns. Ann Rheum Dis. 2003;62:204–7. doi: 10.1136/ard.62.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das CP, Prabhakar S, Lal V, Kharbanda PS. Scleroderma, stroke, optic neuropathy: a rare association. Neurol India. 2002;50:504–7. [PubMed] [Google Scholar]
- 18.Airo’ P, Scarsi M, Rossi M, Mondini M. Onset and enhancement of systemic sclerosis after treatments for multiple sclerosis. Rheumatol Int. 2008;28:703–7. doi: 10.1007/s00296-007-0507-2. [DOI] [PubMed] [Google Scholar]
- 19.Mabon M, Whitcher JP, Anderson R. Bilateral scleral pit associated with systemic sclerosis. Am J Ophthalmol. 1999;128:521–2. doi: 10.1016/s0002-9394(99)00191-9. [DOI] [PubMed] [Google Scholar]
- 20.Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology. 2007;46:1757–62. doi: 10.1093/rheumatology/kem173. [DOI] [PubMed] [Google Scholar]
- 21.Shahneh FZ, Babalo Z, Baradaran B, Sepehr KS. Insights into Behçet's disease. Postep Derm Alergol. 2012;29:461–6. [Google Scholar]
- 22.Pastuszka M, Kaszuba A. Status of combination drugs with betamethasone dipropionate and salicylic acid in the treatment of skin diseases. Postep Derm Alergol. 2012;29:196–204. [Google Scholar]
- 23.Dańczak-Pazdrowska A. Place of methotrexate in the treatment of psoriasis in the era of biologic agents. Postep Derm Alergol. 2012;29:182–8. [Google Scholar]
- 24.Chan AY, Liu DT. Increased prevalence of ocular glaucomatous abnormalities in systemic sclerosis. Ann Rheum Dis. 2005;64:341–2. [PMC free article] [PubMed] [Google Scholar]
- 25.Peter S, Dietrich H, Wick G. Investigations for retinopathy in an avian model for systemic sclerosis. Exp Eye Res. 2004;79:85–92. doi: 10.1016/j.exer.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Kraus A, Guerra-Bautista G, Espinoza G, et al. Defects of the retinal pigment epithelium in scleroderma. Br J Rheumatol. 1991;30:112–4. doi: 10.1093/rheumatology/30.2.112. [DOI] [PubMed] [Google Scholar]
- 27.Egerer I, Fanta D, Freyler H. Fundus changes in scleroderma. Klin Monatsbl Augenheilkd. 1975;167:571–6. [PubMed] [Google Scholar]
- 28.Serup L, Serup J, Hagdrup H. Fundus fluorescein angiography in generalized scleroderma. Ophthalmic Res. 1987;19:303–8. doi: 10.1159/000265512. [DOI] [PubMed] [Google Scholar]
- 29.Milenkovic S, Petrovic L, Risimic D, et al. Choroidal sclerosis in localized scleroderma (morphea en plaque) Ophthalmic Res. 2008;40:101–4. doi: 10.1159/000113889. [DOI] [PubMed] [Google Scholar]
- 30.Saari KM, Rudenberg HA, Laitinen O. Bilateral central retinal vein occlusion in a patient with scleroderma. Ophthalmologica. 1981;182:7–12. doi: 10.1159/000309083. [DOI] [PubMed] [Google Scholar]
- 31.Berndt K, Hoffmann A. Closure of the central artery of the retina in progressive scleroderma. Klin Monatsbl Augenheilkd. 1977;171:597–600. [PubMed] [Google Scholar]
- 32.Peter S, Dietrich H, Wick G. Investigations for retinopathy in an avian model for systemic sclerosis. Exp Eye Res. 2004;79:85–92. doi: 10.1016/j.exer.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh P, Bhaskar VM, Keshavamurthy R, Garg S. Proliferative vascular retinopathy in polymyositis and dermatomyositis with scleroderma (overlap syndrome) Ocul Immunol Inflamm. 2007;15:45–9. doi: 10.1080/09273940601147653. [DOI] [PubMed] [Google Scholar]