Summary
Blood blister-like aneurysms (BLAs) are rare lesions, associated with diffuse subarachnoid hemorrhage (SAH). BLAs tend to rebleed quickly after first bleeding and must be treated as an emergency. Acute treatment is challenging using surgical and endovascular approaches due to the fragile aneurysm wall and small sac. Flow-diverter stents (FDSs) may offer a new option for the treatment of difficult small aneurysms. We describe a case of a ruptured BLA on the anterior communicating artery (AComA) treated in the acute phase of SAH by endovascular exclusion of the AComA with deployment of two FDSs in the A1/A2 junctions of both anterior cerebral arteries (ACAs). A 61-year-old man was admitted for diffuse SAH with a focal interhemispheric hematoma. Angiography revealed multiple arterial wall irregularities on the AComA and both ACAs. We performed an endovascular shunt of the AComA by deploying two FDSs in both A1/A2 junctions. Immediate control injections confirmed flow diversion in the A1/A2 segments of the ACAs with decreased blood flow in the AComA. The patient's course in hospital was uneventful. A three-month follow-up angiogram confirmed complete exclusion of the aneurysms, complete exclusion of the AComA, and patency of the two ACAs without any persistent arterial wall irregularity. Endovascular bypass using an FDS for a ruptured BLA has never been described. It establishes a new therapeutic option despite the need for antiplatelet therapy. Endovascular AComA exclusion using an FDS may be a solution when no other treatment is available for a ruptured BLA.
Keywords: blister-like aneurysm, flow-diverter stent, subarachnoid hemorrhage
Introduction
Blood blister-like aneurysms (BLAs) are rare lesions, which can sometimes be difficult to recognize and in most cases are associated with diffuse subarachnoid hemorrhage (SAH) 1,2. These aneurysms tend to rebleed quickly after first bleeding and must be treated as an emergency 3.
Acute treatment of a ruptured intracranial BLA is challenging using surgical and endovascular approaches 2-5. When occlusion of the parent vessel is not possible, choosing the right endovascular treatment is difficult because there is no consensus on the best management strategy. Coiling of BLA is a high-risk procedure 6 as the fragile aneurysm wall can easily rupture during catheterization or coil insertion 1. Furthermore, the small sac and wide-neck anatomy make coil placement difficult, enhancing the risk of thromboembolic complications 1.
Improvements in endovascular techniques, with the development of flow-diverter stents (FDSs), could offer a new option for the treatment of difficult small aneurysms, even if the safety and efficiency of these stents are based only on small retrospective series 7,8. Princiotta et al. recently described two patients treated for blood BLAs in two stages, with stent-assisted coiling followed by FDS in-stent insertion for reconstruction of the parent artery 8. When initial coiling is not possible due to unfavorable anatomy, an FDS can be the first option for reconstructing the parent vessel 9. These options with stent placement in the acute phase of SAH involve dealing with antiplatelet and anticoagulant therapies and balancing hemorrhagic and thromboembolic risks 10. If a reconstruction strategy is not suitable due to multiple aneurysms or insertion on a bifurcation, a deconstruction option must be considered. The aim of this paper is to describe a case of a ruptured BLA on the anterior communicating artery (AComA) treated in the acute phase of SAH by endovascular exclusion of the AComA with deployment of two FDSs in the A1/A2 junctions of both anterior cerebral arteries (ACAs).
Case Report
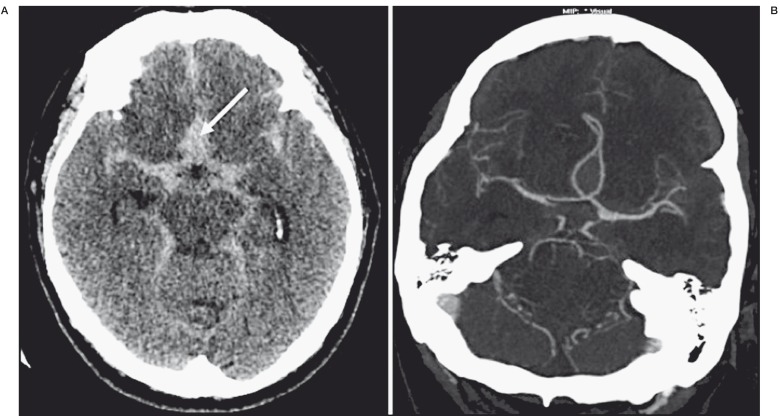
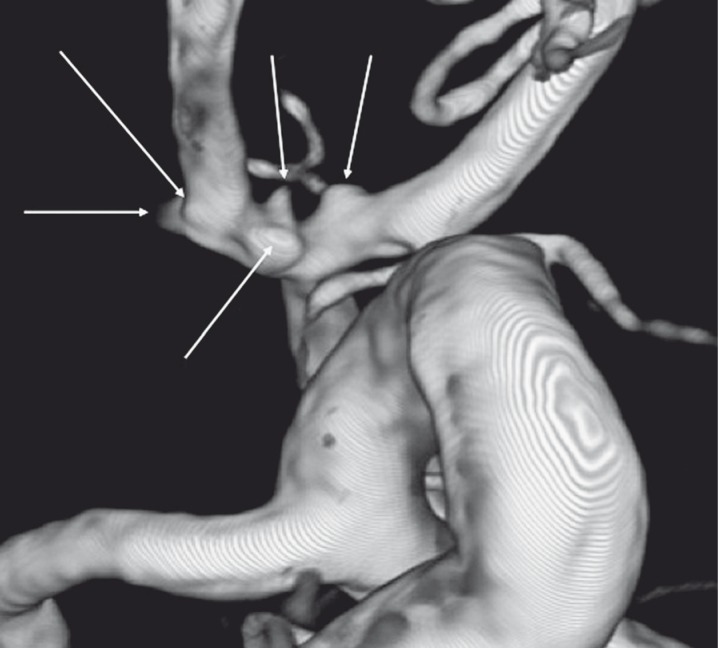
A 61-year-old man with no medical history or vascular risk factors was admitted to our institution for headache and vomiting. His Glasgow Coma Scale score was 15. A computed tomography (CT) scan showed diffuse SAH (Fisher grade III, World Federation of Neurosurgeons grade I) with a focal interhemispheric hematoma, without ventricular dilatation (Figure 1A). CT angiography did not highlight any saccular aneurysm (Figure 1B). Three-dimensional rotational angiography revealed multiple arterial wall irregularities located on the AComA and on the right and left A1/A2 junctions of the ACAs (Figure 2). All arterial wall irregularities measured < 1 mm, with no well-defined neck. These irregularities were considered to be BLAs because they were small in size and because intracranial dissection (another possible diagnosis) does not involve different arterial segments at the same time. One of these irregularities had to be considered as the ruptured BLA, but there was no means of identifying which one corresponded to the bleeding lesion.
Figure 1.
A) Initial non-contrast computed tomography (CT) scan on presentation, showing diffuse subarachnoid hemorrhage with anterior interhemispheric hematoma (arrow). B) CT angiography axial reformatted image showing no aneurysm.
Figure 2.
Three-dimensional digital subtraction angiography of the left internal carotid artery showing multiple irregularities (arrows) on the anterior communicating artery and on the A1/A2 junctions on both right and left sides.
Selective neurosurgical or endovascular exclusion of each aneurysm was not possible because they were too small and had unfavorable anatomy. Occlusion of the AComA with clipping or coiling was not considered a good option because the target BLAs were located not only on the AComA but also on the right and left A1/A2 segments, and such a procedure could not protect irregularities located on the ACAs. We were facing a challenge: we had to exclude all these arterial irregularities without shunting the ACA territories.
We decided to perform endovascular exclusion of the AComA by the deployment of two 2.5 × 20 mm FDSs (Pipeline Embolization Device; ev3 Endovascular Inc., Plymouth, MN, USA) in both right and left A1/A2 junctions. This option could not only exclude the AComA but also reconstruct the arterial wall of both ACAs and protect all the BLAs (Figure 3). The procedure was performed under general anesthesia with systemic heparinization (8000 IU during the procedure). The patient was loaded with 250 mg aspirin just before the first stent deployment and abciximab (7.2 mg/h) was started just after the deployment of the first stent and continued for 12 hours, followed by dual antiplatelet therapy for three months and aspirin for one year after the procedure. Antiplatelet therapy is challenging in case of acute intracranial hemorrhage because of the possible need for external ventricular derivation (EVD), but in this case there was no ventricular dilatation, so EVD was not required and there was no contraindication for antiplatelet treatment.
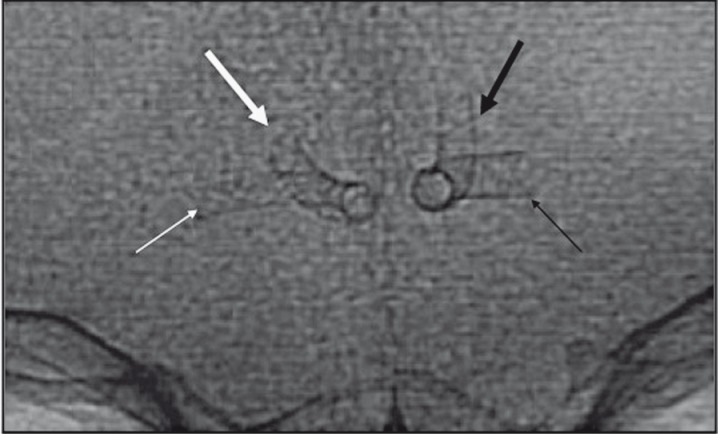
Figure 3.
A) Postprocedural flat-panel CT scan showing the implantation of the two flow diverter stents (FDSs) in the A1/A2 segments of both right and left anterior cerebral arteries. B) High dose flat panel CT scan showing both FDSs. C) X-ray single shot showing both FDSs.
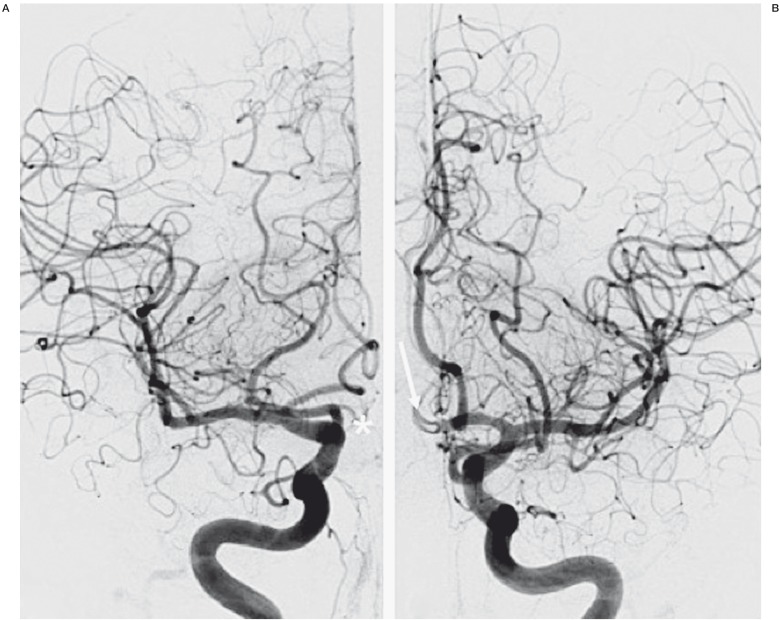
Immediate control injections confirmed flow diversion in the A1/A2 segments of the ACAs with decreased blood flow in the AComA. Flow competition was confirmed by the washing flow coming from the contralateral side (Figure 4). The patient's course in hospital was uneventful with no rebleeding, vasospasm, or ischemic lesions in the shunted territories. A three-month follow-up catheter angiogram confirmed complete exclusion of the aneurysms, complete exclusion of the AComA, and patency of the two ACAs (Figures 5 and 6) without any persistent arterial wall irregularity.
Figure 4.
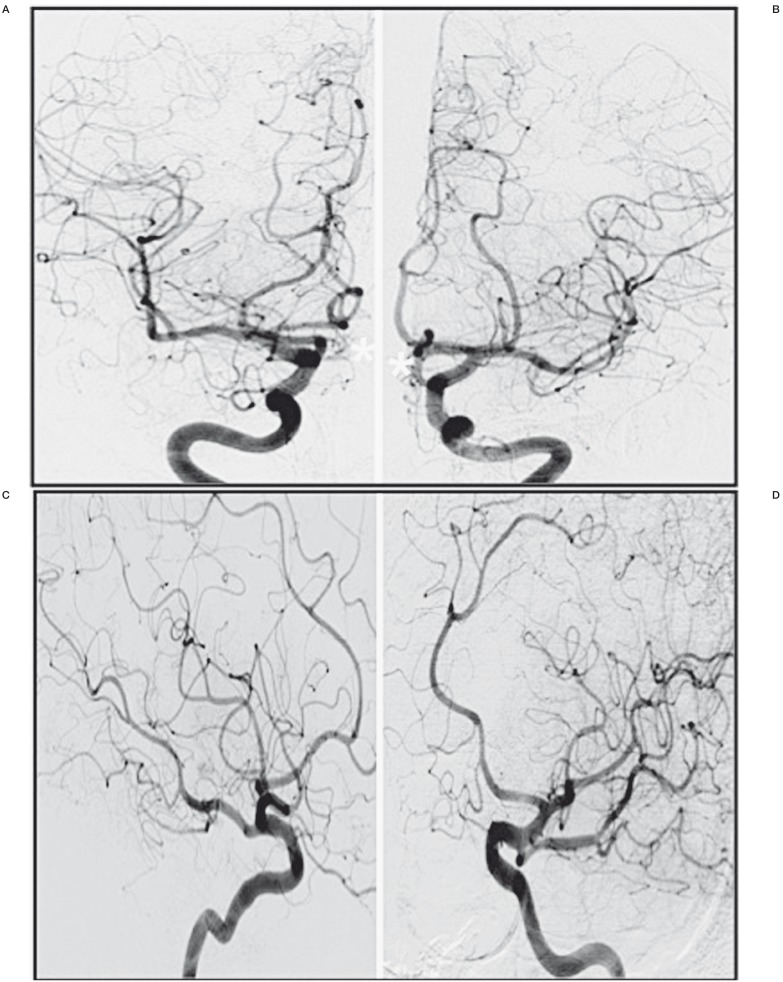
Postoperative digital subtraction angiography in the anteroposterior projection showing the flow competition in the anterior communicating artery (AComA). A) No filling of the AComA by the right internal carotid artery (*). B) Persistence of low flow in the AComA by the left internal carotid artery (arrow).
Figure 5.
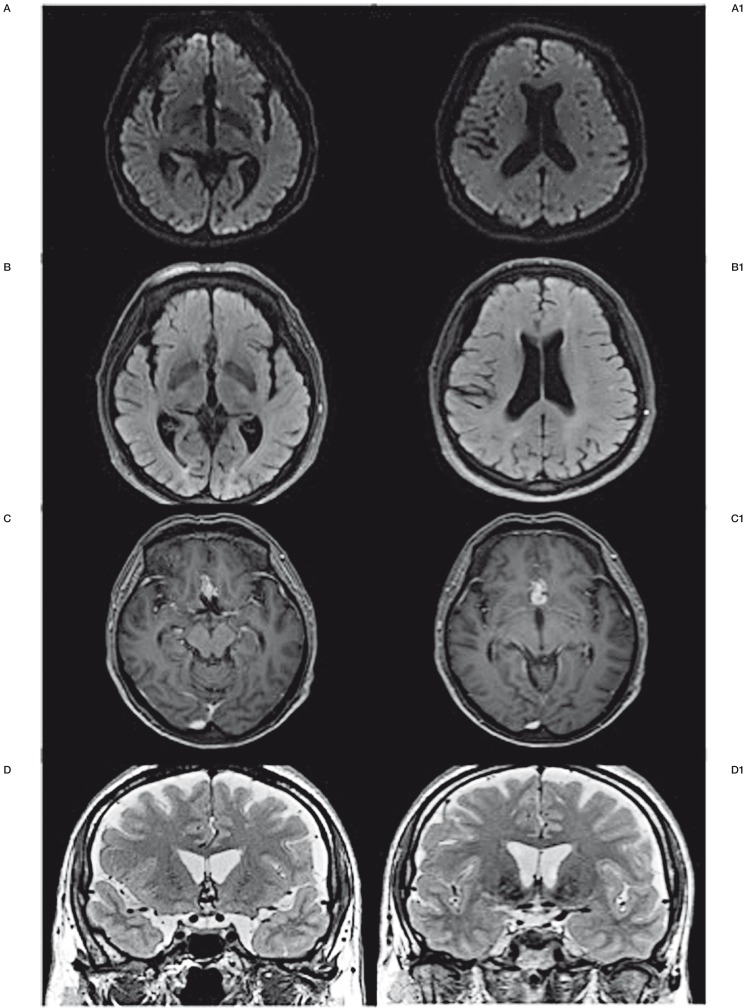
Postoperative magnetic resonance imaging scan showing no ischemic lesion in the shunted territories of the anterior communicating artery. A) DWI. B) FLAIR. C) Gadolinium-enhanced T1. D) T2.
Figure 6.
Six-month control digital subtraction angiography demonstrating total exclusion of the anterior communicating artery (*) and persistent flow in both anterior cerebral arteries. A) Anteroposterior projection of the right internal carotid artery. B) Anteroposterior projection of the left internal carotid artery. C) Oblique projection of the right internal carotid artery. D) Oblique projection of the left internal carotid artery.
Discussion
Acutely ruptured intracranial BLAs are unstable and have a high tendency to rebleed in the acute phase 3 – more so than other types of aneurysms. Protecting the patient against further bleeding has to be the goal of treatment. Due to their small size and fragile wall, selective treatment of these lesions is very difficult to perform using endovascular or surgical approaches. Most previous studies have reported BLAs on the non-branching supraclinoid portion of the internal carotid artery – the most common location for these lesions 2. These rare lesions are challenging in terms of treatment but also diagnosis, as previously reported by Gaughen et al. 11. BLA remains an elusive diagnosis, particularly using CT angiography, which does not have 100% sensitivity 11. Thus, in the face of SAH and otherwise negative CT angiogram findings, a catheter cerebral angiogram should be obtained to absolutely exclude a BLA.
Regarding treatment, parent vessel occlusion remains the simplest treatment, using either the endovascular or surgical approach when possible 12. Surgical trapping 13-15 and stent-assisted endovascular coiling 16-19 have also been reported.
In the present case, several sidewall irregularities were detected on the AComA complex, including the AComA and both ACAs at the A1/A2 junctions. One of these irregularities had to be considered the culprit ruptured BLA, but it was impossible to determine which lesion had bled (Figure 2). Exclusion of all the lesions from the blood circulation was the only way to guarantee that the ruptured BLA had been excluded.
Surgical treatment was discussed. The advantage of the surgical approach was that it might have enabled the BLA that had bled to be identified, but it would not have been possible to achieve selective treatment of each aneurysm and clipping of the AComA would not have been sufficient, as it would not have protected BLAs located on both sides of the A1/A2 segments. In addition, endovascular occlusion of the AComA with coiling would not have protected irregularities located on the ACAs and selective coiling was impossible to perform.
Recently, FDSs have been employed in the acute phase of SAH to reconstruct the vessel wall and cure the aneurysms, with favorable results reported in small series or case reports 8,9,20-24. However, given the location of the multiple lesions in our patient, using a unique FDS in the AComA would not have covered all the lesions and one of the ACAs would have been shunted. Endovascular treatment of aneurysms located on the AComA using X-configured cross-kissing stents has been reported 19,25, but this alternative was not suitable in our conformation as FDS meshes are too dense to allow cross-kissing. Stent implantation involves antiplatelet therapy, which constitutes a challenge in case of acute intracranial hemorrhage. The main risk of complication secondary to antiplatelet therapy in the acute phase of intracranial hemorrhage is due to EVD. If EVD is required for ventricular dilatation, it should be done before antiplatelet therapy is started and hence before stent deployment.
Our therapeutic option used the principle of the FDS, according to Fiorella et al. 26. When a vessel shunted by the FDS does not supply a parenchymal territory it will be occluded as well as the covered aneurysm. We decided to perform an endovascular AComA endovascular exclusion using two FDSs positioned in both ACAs at the A1/A2 junction level. As the AComA was totally shunted, all the lesions were either covered or shunted and the flow in both ACAs was preserved, whereas there was a drastic reduction of the flow in the AComA (Figures 3 and 4). The AComA occlusion was an expected phenomenon related to the flow competition induced in both ACAs by FDS use. In order to ensure that the endovascular exclusion of the AComA was not responsible for ischemic lesions, a postoperative magnetic resonance imaging scan with a diffusion-weighted imaging sequence was performed. Despite occlusion of the AComA, no ischemic lesion in the shunted territories (i.e. Heubner's artery, the medial lenticulostriate artery territory and the subcallosal area) was observed (Figure 5). The three-month follow-up control confirmed our hypothesis, showing the total angiographic disappearance of the AComA and the regular arterial wall of both ACAs (Figure 6).
To the best of our knowledge, endovascular bypass with FDSs for a ruptured BLA has never been described and establishes a new therapeutic option despite the need for anti-platelet therapy, which remains a concern in patients presenting with acute SAH.
Conclusions
Endovascular communicating artery exclusion using FDSs is a new and original therapeutic option, which can be a solution in cases when no other treatment is available for ruptured BLA.
References
- 1.Ezaki Y, Takahata H, Kamada K, et al. Aneurysmal embolization of a blisterlike aneurysm of the internal carotid artery: a case report and review of the literature. Surg Neurol. 2006;65(6):628–630. doi: 10.1016/j.surneu.2005.09.030. discussion 630. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Park IS, Han DH, et al. Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2007;106(5):812–819. doi: 10.3171/jns.2007.106.5.812. [DOI] [PubMed] [Google Scholar]
- 3.Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108(4):662–671. doi: 10.3171/JNS/2008/108/4/0662. [DOI] [PubMed] [Google Scholar]
- 4.Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;11(3):431–436. doi: 10.3171/2008.7.JNS08257. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Choi HG, Jung JY, et al. Surgical strategies for ruptured blister-like aneurysms arising from the internal carotid artery: a clinical analysis of 18 consecutive patients. Acta Neurochir (Wien) 2009;151(2):125–130. doi: 10.1007/s00701-008-0165-5. [DOI] [PubMed] [Google Scholar]
- 6.Morris TC, Brophy BP. Blister-like aneurysm of the anterior communicating artery. J Clin Neurosci. 2009;16(8):1098–1100. doi: 10.1016/j.jocn.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Nakiri GS, Al-Khawaldeh M, Parente B, et al. Treatment of ruptured intra-cranial internal carotid artery dissection using a flow-diverter stent. J Neuroradiol. 2012;39(4):271–275. doi: 10.1016/j.neurad.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Princiotta C, Dall’Olio M, Cirillo L, et al. Staged treatment of a blood blister-like aneurysm with stent-assisted coiling followed by flow diverter in-stent insertion. A case report. Interv Neuroradiol. 2011;17(3):365–370. doi: 10.1177/159101991101700314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consoli A, Nappini S, Renieri L, et al. Treatment of two blood blister-like aneurysms with flow diverter stenting. J Neurointerv Surg. 2012;4(3):e4. doi: 10.1136/jnis.2010.004572. [DOI] [PubMed] [Google Scholar]
- 10.Fiorella D, Thiabolt L, Albuquerque FC, et al. Antiplatelet therapy in neuroendovascular therapeutics. Neurosurg Clin N Am. 2005;16(3):517–540 vi. doi: 10.1016/j.nec.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Gaughen JR Jr, Raghavan P, Jensen ME, et al. Utility of CT angiography in the identification and characterization of supraclinoid internal carotid artery blister aneurysms. Am J Neuroradiol. 2010;31(4):640–644. doi: 10.3174/ajnr.A1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CC, Hsieh TC, Wang YC, et al. Ruptured symptomatic internal carotid artery dorsal wall aneurysm with rapid configurational change. Clinical experience and management outcome: an original article. Eur J Neurol. 2010;17(10):1277–1284. doi: 10.1111/j.1468-1331.2010.03029.x. [DOI] [PubMed] [Google Scholar]
- 13.Chung JH, Shin YS, Lim YC, et al. Ideal Internal Carotid Artery Trapping Technique without Bypass in a Patient with Insufficient Collateral Flow. J Korean Neurosurg Soc. 2009;45(4):260–263. doi: 10.3340/jkns.2009.45.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamijo K, Matsui T. Acute extracranial-intracranial bypass using a radial artery graft along with trapping of a ruptured blood blister-like aneurysm of the internal carotid artery. Clinical article. J Neurosurg. 2010;113(4):781–785. doi: 10.3171/2009.10.JNS09970. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima A, Okada Y, Kawamata T, et al. Successful treatment of a blood blister-like aneurysm of the internal carotid artery by trapping with a high-flow bypass. J Clin Neurosci. 2008;15(7):797–800. doi: 10.1016/j.jocn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Ahn JY, Cho JH, Jung JY, et al. Blister-like aneurysms of the supraclinoid internal carotid artery: challenging endovascular treatment with stent-assisted coiling. J Clin Neurosci. 2008;15(9):1058–1061. doi: 10.1016/j.jocn.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Islam MS, Manabe H, Hasegawa S, et al. Successful staged treatment for ruptured blister-like dissecting aneurysm of the intracranial internal carotid artery: acute GDC embolization for the blister-like aneurysm followed by proximal occlusion with extracranial-intracranial bypass in the chronic. Minim Invasive Neurosurg. 2004;47(3):165–168. doi: 10.1055/s-2004-818490. [DOI] [PubMed] [Google Scholar]
- 18.Korja M, Rautio R, Valtonen S, et al. Primary treatment of ruptured blood blister-like aneurysms with stent-assisted coil embolization: report of two cases. Acta Radiol. 2008;49(2):180–183. doi: 10.1080/02841850701675735. [DOI] [PubMed] [Google Scholar]
- 19.Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41(1):110–115. doi: 10.1161/STROKEAHA.109.558114. [DOI] [PubMed] [Google Scholar]
- 20.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol. 2011;32(1):20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin AR, Cruz JP, Matouk CC, et al. The pipeline flow-diverting stent for exclusion of ruptured intracranial aneurysms with difficult morphologies. Neurosurgery. 2012;70(1) Suppl Operative:21–28. doi: 10.1227/NEU.0b013e3182315ee3. discussion 28. [DOI] [PubMed] [Google Scholar]
- 22.Narata AP, Yilmaz H, Schaller K, et al. Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery. 2012;70(4):982–988. doi: 10.1227/NEU.0b013e318236715e. discussion 988-989. [DOI] [PubMed] [Google Scholar]
- 23.Pistocchi S, Blanc R, Bartolini B, et al. Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke. 2012;43(4):1032–1038. doi: 10.1161/STROKEAHA.111.636019. [DOI] [PubMed] [Google Scholar]
- 24.Rasskazoff S, Silvaggio J, Brouwer PA, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interv Neuroradiol. 2010;16(3):255–258. doi: 10.1177/159101991001600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeleňák K, Zeleňáková J, DeRiggo J, et al. Flow changes after endovascular treatment of a wide-neck anterior communicating artery aneurysm by using X-configured kissing stents (cross-kissing stents) technique. Cardiovasc Intervent Radiol. 2011;34(6):1308–1311. doi: 10.1007/s00270-011-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorella D, Lylyk P, Szikora I, et al. Curative cerebrovascular reconstruction with the Pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. 2009;1(1):56–65. doi: 10.1136/jnis.2009.000083. [DOI] [PubMed] [Google Scholar]