Abstract
Objective:
To determine the association between age at surgical menopause and both cognitive decline and Alzheimer disease (AD) pathology in 2 longitudinal cohorts.
Methods:
Female subjects from 2 longitudinal studies of cognitive decline (Religious Orders Study and Rush Memory and Aging Project) were included (total n = 1,884). The primary analysis examined the association between age at surgical menopause and decline in a global cognition score. Secondary analyses examined additional outcomes: 1) decline in 5 cognitive subdomains and 2) a global measure of the burden of AD pathology. In exploratory analyses, we examined the effect of hormone replacement therapy (HRT). We adjusted all models for age, education, smoking, and cohort and stratified by surgical vs natural menopause.
Results:
For the 32% of subjects with surgical menopause, earlier age at menopause was associated with faster decline in global cognition (p = 0.0007), specifically episodic memory (p = 0.0003) and semantic memory (p = 0.002). Earlier age at menopause was also associated with increased AD neuropathology (p = 0.038), in particular neuritic plaques (p = 0.013). HRT use for at least 10 years, when administered within a 5-year perimenopausal window, was associated with decreased decline in global cognition. No associations were seen in women who had natural menopause.
Conclusions:
Early age at surgical menopause was associated with cognitive decline and AD neuropathology. Ongoing studies should clarify the potential effect of HRT on this relationship.
As the general population ages, cognitive decline is a major public health concern.1 Gonadal hormones may play an important modulatory function in this process. In fact, in a series of epidemiologic observations, interventional studies, and animal models, a neuroprotective role of estrogen, among other hormones, has emerged.2–4
There has been strong interest in the potential influence of menopause and the associated decrease in ovarian production of estradiol on subsequent cognitive function. An earlier age at menopause,5,6 particularly surgical menopause,7–9 has been associated with increased risk of dementia and cognitive decline in some but not all10,11 studies.12–14 In general, prior studies of surgical menopause examined the risk of developing Alzheimer disease (AD) or dementia but did not assess intermediate phenotypes, such as change in performance on detailed cognitive testing or development of the neuropathologic features associated with AD.
We leveraged data from 2 prospective cohort studies of aging and dementia that include organ donation at death to test the hypothesis that earlier age at surgical menopause increases the rate of cognitive decline. In addition, we examined the relation of surgical menopause to the neuropathologic changes associated with AD.
METHODS
Subjects.
Subjects were women from 2 longitudinal studies of cognitive decline. The Religious Orders Study (ROS), started in 1994, enrolled older Catholic priests, nuns, and brothers from about 40 groups in 12 states. Participants were free of known dementia at enrollment. Participants agreed to annual clinical evaluations, and signed both an informed consent and an Anatomic Gift Act form donating their brains at time of death.15
The Memory and Aging Project (MAP), started in 1997, enrolled older men and women in the Chicago area free of known dementia at enrollment. Participants also agreed to annual clinical evaluations and signed both an informed consent and an Anatomic Gift Act form.15,16 The follow-up rate of ROS and MAP survivors exceeds 90%, and the autopsy rate exceeds 80%. Both cohorts, previously described in detail,17,18 share a large core of identical phenotypic data, allowing efficient merging for joint analyses.19,20
Analyses are based on 1,884 female participants who completed the baseline evaluation between January 1994 and August 2012 and for whom reproductive data were available. The clinical evaluation was repeated annually for up to 18 years with examiners blinded to previously collected data. It included a medical history, neurologic examination, and cognitive function assessment.
Standard protocol approvals, registrations, and patient consents.
ROS and MAP were approved by the institutional review board of Rush University Medical Center. Additionally, retrospective analysis of the data was approved by the Partners Healthcare Institutional Review Board.
Hormonal variables.
At baseline, subjects were asked about exogenous hormone use, dates of use, age at menarche and menopause, and whether menopause had occurred naturally or been induced surgically (table e-1 on the Neurology® Web site at www.neurology.org). Data on type of surgery (hysterectomy or unilateral or bilateral oophorectomy) were not available. Ten subjects were excluded because they provided highly unlikely ages at menopause (<20 or >60 years), and 4 due to unlikely ages at menarche (>30 years). We calculated the duration of reproductive period by subtracting age at menarche from age at menopause.10 We verified current hormone replacement therapy (HRT) use by inventory of prescription bottles during participant interviews, with an agreement of 93%. We calculated total duration of HRT use; in current HRT users, this was censored at study entry.
Measures of cognitive function.
A battery of 19 tests was administered annually to each participant by trained examiners. We used the Mini-Mental State Examination only for descriptive purposes,21 and excluded another test because of an extremely skewed distribution. We combined the remaining 17 tests to form a global cognition score,19 and categorized them into 5 cognitive domains: 1) episodic memory (7 tests), 2) semantic memory (3 tests), 3) working memory (3 tests), 4) perceptual speed (2 tests), and 5) visuospatial ability (2 tests). Summary measures were created for each domain and for the global cognition score, using averaged sums of z scores (detailed in table e-2 and prior publications22–24).
Classification of dementia and AD.
The clinical diagnosis of dementia and AD was made by a clinician with expertise in the evaluation of older persons for dementia based on the criteria of the Joint Working Group of the National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) following a detailed clinical evaluation.25 These criteria require a history of cognitive decline and evidence of impairment in memory and cognition. Clinical diagnoses were implemented in a 2-step process as previously detailed.26 The diagnosis of clinical AD was confirmed pathologically in 90% of autopsied participants.26 Participants meeting criteria for dementia at the baseline clinical evaluation were excluded from analyses.
Neuropathologic measures.
Brain autopsies were available for 600 women. Brains were removed at 12 predetermined sites across the United States, using standardized autopsy procedures and postmortem data collection.15,27,28 We obtained counts for the following markers of AD pathology: neuritic plaques, diffuse plaques, and neurofibrillary tangles. We standardized the raw counts from 5 brain regions by dividing each person's count by the SD for that count and formed a global pathology summary score by averaging the standardized scores. We used the square root of the score to minimize the skewed distribution. A pathologic diagnosis of AD was also made, based on National Institute on Aging-Reagan criteria.29
Statistical analysis.
Demographic and reproductive characteristics of women undergoing natural and surgical menopause are described using means and SDs for continuous variables and frequency and proportions for categorical variables. We compared these variables across studies using 2 independent sample t tests, χ2 tests, and Fisher exact test, when warranted.
Our primary analysis examined the association between age at menopause and longitudinal decline in the global cognition composite score. For all longitudinally collected cognitive variables, we performed mixed models of cognitive decline, adjusting for age at enrollment, years of education, study (ROS vs MAP), and smoking (pack-years at study baseline). Similar analyses examined change in 5 cognitive domains. Menopausal type (natural vs surgical) was an effect modifier in the relationship between age at menopause and decline in global cognition (p = 0.016); therefore, all analyses were stratified by menopausal type.
We next examined the association between age at menopause and AD-related neuropathologic outcomes using multivariate linear regression adjusted for age at death, years of education, smoking, and study.
Finally, in exploratory analyses of the association between other reproductive variables and cognitive decline, we performed the analyses detailed above, including as independent variables age at menarche, duration of reproductive period, and HRT use (ever/never, and duration). In order to evaluate the effects of HRT use within the perimenopausal “window of opportunity,”12 we categorized ever-use of HRT as whether it occurred within 5 years of menopause, or 5 or more years after menopause.30 We categorized the duration of HRT use as lasting 10 or more years vs fewer than 10 years, similar to prior studies.30
We performed all tests using SAS software, version 9.3 (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics.
Cohort characteristics are listed in table 1. The 1,884 female subjects considered in these analyses had a mean age of 78 years at enrollment (range 53–100 years), and one-third of women reported having undergone surgical menopause. These women were less likely to be Caucasian, had a higher body mass index, and reported both a younger mean menopausal age (42 vs 49 years) and a greater use of HRT (53% vs 27%) than women who had undergone natural menopause. More women in MAP (35%) than in ROS (28%) reported surgical menopause (p = 0.003). Among all HRT users (n = 632), 71% reported HRT use within 5 years of menopause, with a mean duration of more than 13 years. As more than 96% of HRT users reported oral use, all HRT types were collapsed together.
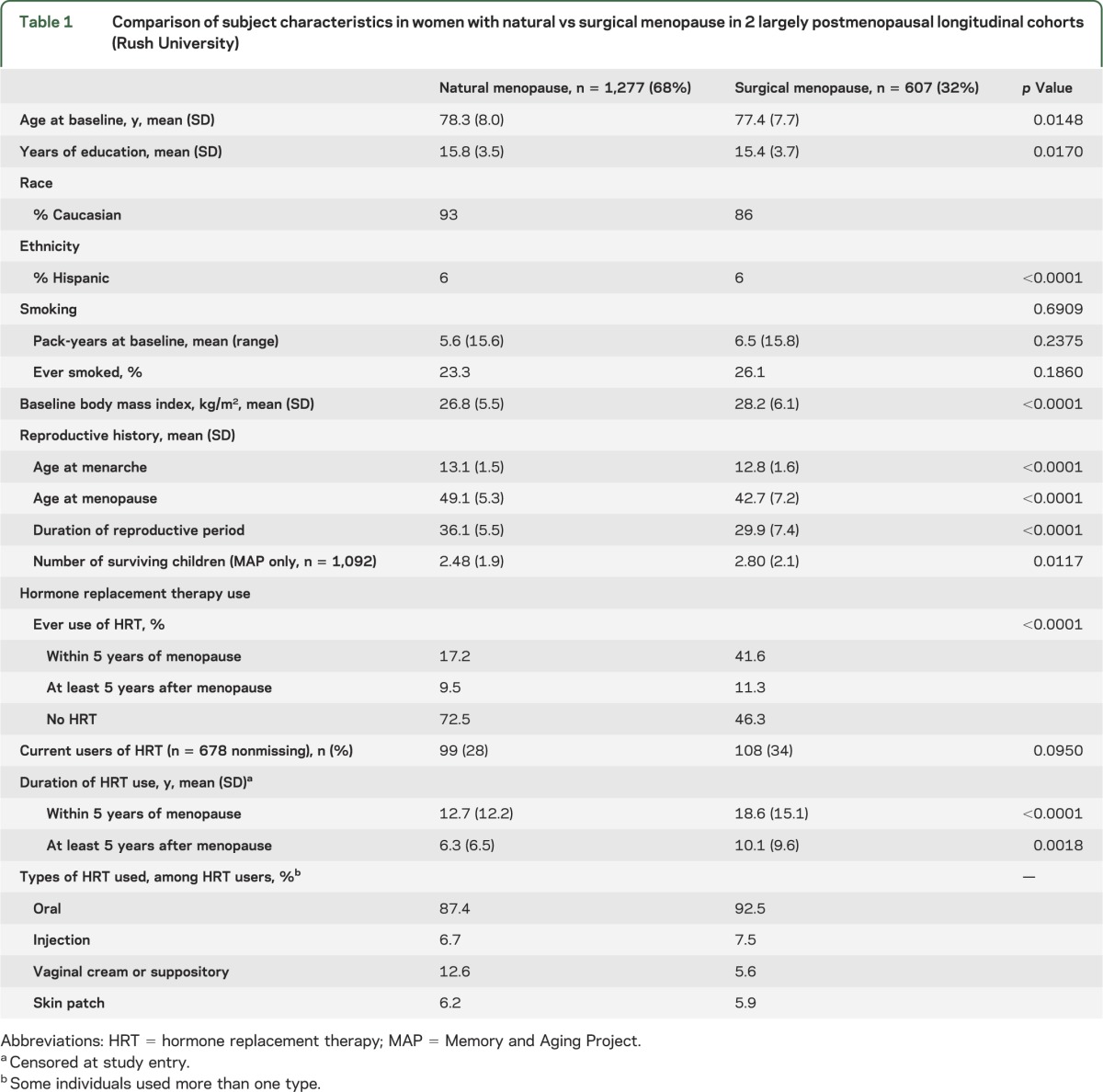
Table 1.
Comparison of subject characteristics in women with natural vs surgical menopause in 2 largely postmenopausal longitudinal cohorts (Rush University)
Age at menopause and cognitive decline.
In our primary analysis examining the association of menopause with cognitive decline, earlier age at surgical menopause was associated with a steeper slope of global cognitive decline (p = 0.0007) (table 2). Their coefficients indicate that the effect of each year of earlier surgical menopause on the rate of cognitive decline was equivalent to the effect associated with 6 months of aging (age at menopause × time: 0.0024 × 10.5 = age × time: −0.00503 × 5). When age at surgical menopause was categorized by quartiles, this association was also significant (p = 0.008). These divergent estimated slopes are represented in the figure, centered on a woman with median age at study entry (78 years) and level of education (16 years). The figure illustrates the finding that women who were younger at the time of surgical menopause have a more rapid rate (steeper slope) of cognitive decline than women who were older at the time of surgery or than women undergoing natural menopause. Further, because the women who were younger at the time of surgery have a lower intercept at study entry, their cognitive decline may, on average, have started many years before, yielding a lower level of cognitive performance at study entry.
Table 2.
Inverse association between age at surgical menopause and risk of neurologic outcomes
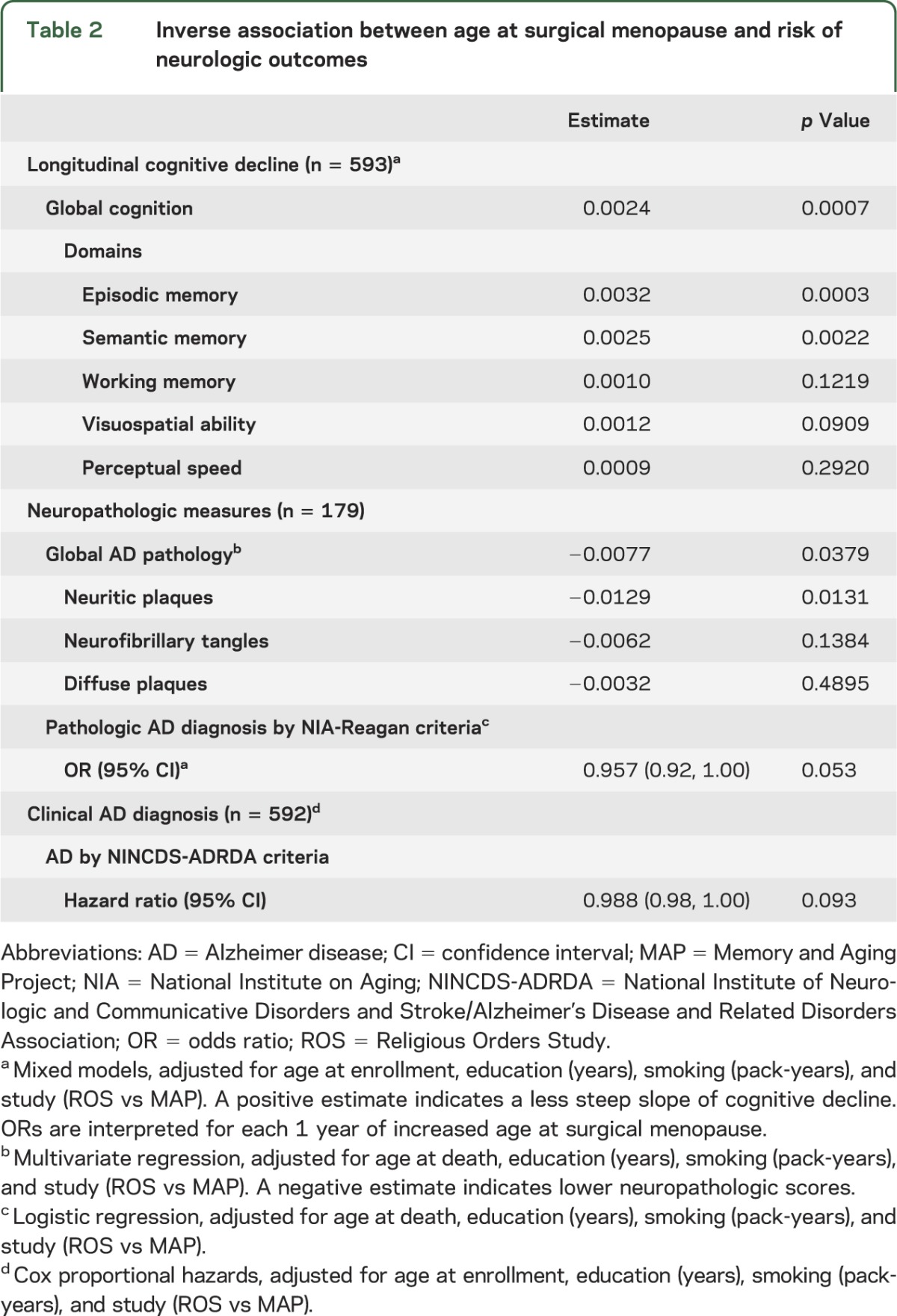
Figure. Earlier surgical menopause is associated with worse slope of cognitive decline.
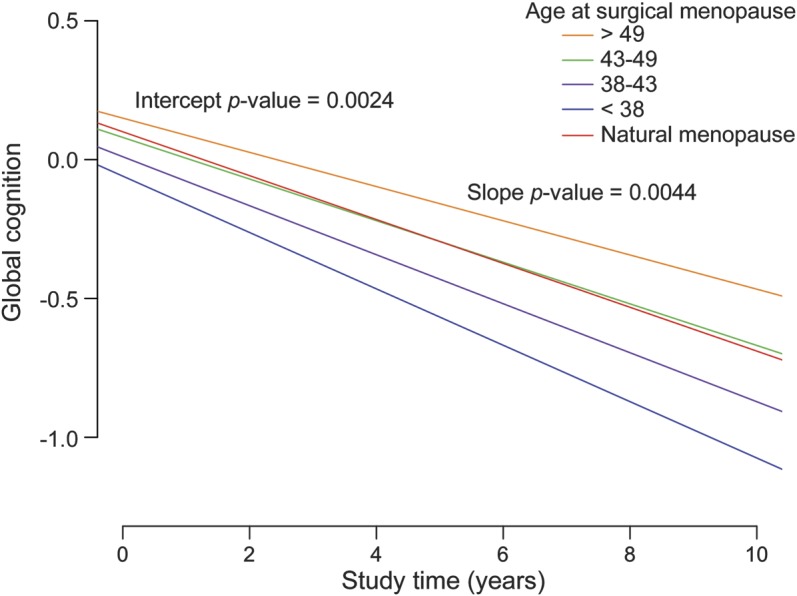
Estimated slope of decline in global cognition according to age at surgical menopause, categorized by quantile, for a woman with the median age at enrollment (78 years) and educational level (16 years) of the Religious Orders Study and Memory and Aging Project cohorts. The x-axis is study time, and the y-axis is the change in global cognitive performance from the baseline at study entry. An earlier age at surgical menopause is associated with both a steeper slope of decline during the study and a lower intercept, which suggests that a substantial amount of decline has occurred prior to study enrollment. The slope of cognitive decline for women undergoing natural menopause at any age is included as a reference.
In secondary analyses, we examined the association of menopause with decline in 5 cognitive systems. Earlier age at surgical menopause was associated with a steeper slope of decline in episodic memory (p = 0.0003) and semantic memory (p = 0.002).
To address potential confounders, we added body mass index at study entry and race in the model and noted no decrease in the significance of the association between age at surgical menopause and outcome measures. We also included an interaction term for menopausal age and APOE ε4 haplotype31 and found no significant interaction with any cognitive outcome. Further, there was no association between age at surgical menopause and incident clinical AD by NINCDS-ADRDA criteria (p = 0.093). Finally, there was no significant association between age at natural menopause and any clinical outcome (p > 0.058) (table e-3).
Age at menopause and AD neuropathology.
We next examined the relation of menopause with AD pathology. Earlier age at surgical menopause was associated with a higher burden of the global measure of AD neuropathology (p = 0.038). In secondary analyses of the 3 individual markers of AD pathology (neuritic amyloid plaques, neurofibrillary tangles, and diffuse amyloid plaques), the strongest association was with neuritic amyloid plaques (p = 0.013). There was no association between age at natural menopause and any neuropathologic outcome (table e-3).
HRT use and cognitive decline.
We next assessed the role of HRT, when used within a perimenopausal 5-year “window of opportunity,” for women who had experienced surgical menopause (table 3). HRT use for at least 10 years was associated with decreased slope of decline in global cognition (p = 0.023). Additionally, it was associated with less decline in episodic memory (p = 0.026), semantic memory (p = 0.033), and visuospatial ability (p = 0.021). We further examined duration of HRT as a continuous variable, and it was not associated with decline in global cognition (p = 0.055) but was associated with more gentle slopes of cognitive decline in episodic memory, visuospatial memory, and perceptual speed (p < 0.020 for all).
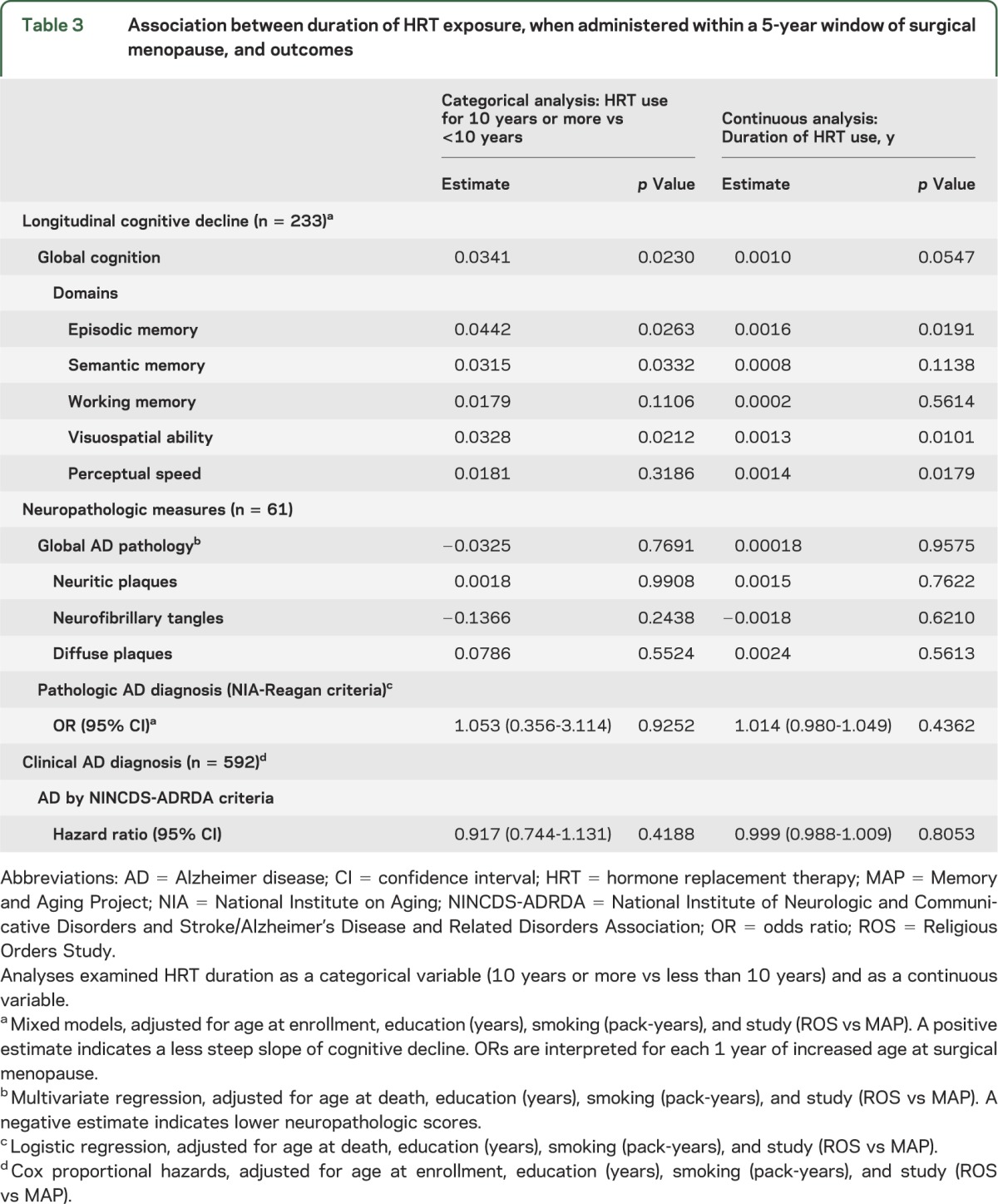
Table 3.
Association between duration of HRT exposure, when administered within a 5-year window of surgical menopause, and outcomes
Given these findings, we assessed whether duration of HRT use, when taken within 5 years of menopause, modulated some of the association between earlier surgical menopause on the slope of cognitive decline using a mediation analysis. When we included duration of HRT use in the statistical model, there was a 13.5% change in the estimate of the association between age at surgical menopause and decline in global cognition, suggesting that longer HRT use did attenuate some of the detrimental effects of early surgical menopause (table e-4).
When HRT was initiated beyond 5 years from surgical menopause, there was no association between duration of HRT use and any cognitive outcome. There were also no associations between HRT use and any cognitive outcome in the natural menopause group. Finally, there were no associations between any HRT use and AD pathology (p > 0.05).
Duration of reproductive period and cognitive decline.
In women who had undergone surgical menopause, a shorter reproductive period was associated with increased risk for decline in global cognition (p = 0.0003). When we considered cognitive domains, shorter duration of reproductive period was not only associated with greater declines in episodic (p = 0.0002) and semantic memory (p = 0.0020), but also in visuospatial ability (p = 0.048). Additionally, a shorter reproductive period was associated with increased risk of global AD-related pathology (p = 0.023) and neuritic plaques (p = 0.007) (table e-3). Interestingly, this association was not seen in persons with natural menopause despite an overlap in the duration of reproductive period (surgical menopause: mean 42.7 years, SD 7.2; natural menopause: mean 49.1 years, SD 5.3). We found no association between age at menarche or number of surviving children (MAP study only) with any of the outcome measures.
DISCUSSION
In this study of 1,884 women followed longitudinally for up to 18 years, earlier age at surgical menopause was associated with a steeper slope of decline in cognition as well as a greater level of AD neuropathology in women who survived free of dementia to a mean age of 78 years. Duration of reproductive period (years between menarche and menopause) showed similar associations. Additionally, when HRT was administered within a 5-year perimenopausal window, HRT use for 10 years or more was associated with decreased decline in cognition but not AD neuropathology. We did not find the same associations for women who had undergone natural menopause.
Prior studies have pointed to an association between surgical menopause and risk of cognitive decline. In fact, the Mayo Clinic Cohort Study of Oophorectomy and Aging reported an almost doubled risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause, as well as a trend of increasing risk of cognitive impairment and dementia with younger age at the time of oophorectomy or at the time of estrogen deficiency.7 Similarly, in a Danish cohort, earlier age at bilateral oophorectomy was associated with an increased risk of early-onset dementia,8 and, in a Chinese study, unilateral oophorectomy prior to menopause was associated with worse word recall.9 Nonetheless, not all studies have yielded the same conclusions.12 Here, we extend these prior findings by examining the relation of surgical menopause to the intermediate phenotypes of cognitive decline and to quantitative measures of AD pathology.
Some caution must be taken in attributing causation to the associations noted in this study, as a confounding factor could lead to both increased risk of early gynecologic surgery as well as dementia. Nonetheless, during the years corresponding to the reproductive period of our study population, gynecologic surgeries were performed for more heterogeneous indications than they are today. Additionally, in the Mayo Clinic cohort, there was no effect of indication for gynecologic surgery on the association between surgical menopause and risk of dementia.12
The lack of association between age at natural menopause and cognitive outcomes was consistent with some,32 but not all studies.33 Indeed, several groups have found a negative effect of earlier age at natural menopause on cognitive function, specifically when menopause occurred before 47 years of age.34,35 We found no effect of natural menopause occurring before or after age 47 (data not shown). Reasons for this difference may include demographic, educational, sample size, study design, or other factors.
In the decade since the Women's Health Initiative Memory Study raised concerns for an increased risk of stroke or cognitive decline associated with HRT initiated in women of older age,32 a perimenopausal window of opportunity has been implicated, during which exogenous hormones may be protective against cognitive decline but beyond which, possibly due to estrogen receptor downregulation, treatment may be neutral or harmful.12,32,36–38 In this study, when we examined HRT used within a 5-year perimenopausal window, HRT use for 10 years or more appeared to have a small protective effect against cognitive decline. Interestingly, we did not find an association with AD neuropathology, suggesting either that we were underpowered to assess an effect of HRT use on AD pathology, or that HRT's protective effects may occur independently of neuropathologic changes. Caution must be taken in interpreting our findings in light of this window of opportunity hypothesis, however, given small effect sizes in our group, changing patterns of HRT use in the decades since this cohort's menopausal transition, the important limitations of our study, and discrepancies between observational and interventional studies.12
The study has unique strengths, including long duration of follow-up of almost 600 women with surgical menopause, which includes both detailed annual cognitive testing and neuropathologic measures for the deceased individuals, with very high rates of clinical follow-up and autopsy leading to good internal validity. The first important limitation was the retrospective and patient-reported nature of the reproductive exposures. It was not possible to stratify the surgical menopause group according to whether women had undergone unilateral or bilateral oophorectomy or hysterectomy. Physiologically, this is important because only bilateral oophorectomy is associated with abrupt cessation of all ovarian estrogenic production.12 The prevalence of surgical menopause in this study (32%) was higher than in other reports (e.g., 25% among women aged 70 to 74 years39), raising the possibility of recall biases,40 changing surgical practices, or other biases within our sample. Second, the requirement that individuals be “cognitively normal” at enrollment could have led to a selection bias, by excluding from the cohort individuals experiencing early cognitive sequelae from an early age at menopause. Thus, the inclusion into the surgical menopause group of women who may not have undergone bilateral oophorectomy, and hence rapid estrogen decline, as well the exclusion of women who did not survive free of dementia to their later years, likely led us to underestimate the association between surgical menopause and cognitive decline. Finally, we could not control for other midlife predictors of cognitive decline, such as body mass index (which was only available at study entry), or for their interaction with gonadal function.
Our findings add to an emerging literature suggesting that midlife hormonal changes may leave a lasting trace on cognition. Ongoing evaluation of the impact of current reproductive practices, including HRT, on longitudinal cognitive decline is warranted.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the clinical and research staff involved in the Rush University Religious Orders Study and Memory and Aging Project and the volunteer subjects who donated their time and participated in the Anatomical Gift Act for their contribution to the study of cognitive aging.
GLOSSARY
- AD
Alzheimer disease
- HRT
hormone replacement therapy
- MAP
Memory and Aging Project
- NINCDS-ADRDA
National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association
- ROS
Religious Orders Study
Footnotes
Editorial, page 196
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drs. Bove and De Jager contributed to the study concept and design. E. Secor and Dr. Chibnik contributed to the analysis and interpretation of data. Drs. Bennett, Barnes, and Schneider contributed to the acquisition of data and interpretation of results. Drs. Bove, Chibnik, Barnes, Schneider, Bennett, and De Jager and E. Secor contributed to drafting/revising the manuscript.
STUDY FUNDING
Supported by NIH grants R01 AG30146, R01 AG17917, R01 AG15819, P30 AG10161, K08 AG034290, and R01 NS059873. R.B. is supported by the NIH training grant 5T32AI074549. P.L.D. is a National MS Society Harry Weaver Neuroscience Scholar Award Recipient.
DISCLOSURE
R. Bove, E. Secor, L. Chibnik, and L. Barnes report no disclosures. J. Schneider has served as a consultant for AVID Radiopharmaceuticals, Eli Lilly Inc., and GE Healthcare. D. Bennett has served as consultant for Danone, Inc., Dr. Wilmar Schwabe GmbH & Co. KG Pharmaceuticals, Eli Lilly, Inc., Enzymotic Ltd., and has received research support from Danone, Inc., and GE Healthcare. P.L. De Jager has served as consultant for Merck Serono and Teva Neuroscience, received speaker fees from Biogen Idec, and received research support from Biogen Idec. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med 2013;368:1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Front Biosci 2012;4:976–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulware MI, Kent BA, Frick KM. The impact of age-related ovarian hormone loss on cognitive and neural function. Curr Top Behav Neurosci 2012;10:165–184 [DOI] [PubMed] [Google Scholar]
- 4.Spence RD, Hamby ME, Umeda E, et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci USA 2011;108:8867–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppus AM, Evenhuis HM, Verberne GJ, et al. Early age at menopause is associated with increased risk of dementia and mortality in women with Down syndrome. J Alzheimers Dis 2010;19:545–550 [DOI] [PubMed] [Google Scholar]
- 6.Corbo RM, Gambina G, Broggio E, Scacchi R. Influence of variation in the Follicle-Stimulating Hormone Receptor Gene (FSHR) and age at menopause on the development of Alzheimer’s disease in women. Dement Geriatr Cogn Disord 2011;32:63–69 [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083 [DOI] [PubMed] [Google Scholar]
- 8.Phung TK, Waltoft BL, Laursen TM, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010;30:43–50 [DOI] [PubMed] [Google Scholar]
- 9.Zhou G, Liu J, Sun F, Duan L, Yan B, Peng Q. Cognitive functioning in elderly women who underwent unilateral oophorectomy before menopause. Int J Neurosci 2011;121:196–200 [DOI] [PubMed] [Google Scholar]
- 10.Geerlings MI, Ruitenberg A, Witteman JC, et al. Reproductive period and risk of dementia in postmenopausal women. JAMA 2001;285:1475–1481 [DOI] [PubMed] [Google Scholar]
- 11.Barnes LL, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003;60:1777–1781 [DOI] [PubMed] [Google Scholar]
- 12.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 2011;1379:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vearncombe KJ, Pachana NA. Is cognitive functioning detrimentally affected after early, induced menopause? Menopause 2009;16:188–198 [DOI] [PubMed] [Google Scholar]
- 14.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause 2007;14:572–579 [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844 [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, et al. The rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–175 [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2012;9:628–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res 2012;9:646–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology 2007;68:2085–2092 [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 2012;72:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193 [PubMed] [Google Scholar]
- 23.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205 [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748 [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176 [DOI] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005;64:834–841 [DOI] [PubMed] [Google Scholar]
- 28.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004;62:1148–1155 [DOI] [PubMed] [Google Scholar]
- 29.The National Institute on Aging, and Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer's disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging 1997;18:S1–S2 [PubMed] [Google Scholar]
- 30.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 2012;79:1846–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology 2003;60:246–252 [DOI] [PubMed] [Google Scholar]
- 32.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2663–2672 [DOI] [PubMed] [Google Scholar]
- 33.McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci 2003;15:161–167 [DOI] [PubMed] [Google Scholar]
- 34.Hogervorst E, Kusdhany LS, Ismail RI, et al. Age at natural menopause and memory function: modification by education and genotype. Endocrinol Metab Syndr 2011;S7:1–7 [Google Scholar]
- 35.Hong X, Zhang X, Li H. A case-control study of endogenous estrogen and risk of Alzheimer's disease [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2001;22:379–382 [PubMed] [Google Scholar]
- 36.Henderson VW. Alzheimer's disease and other neurological disorders. Climacteric 2007;10(suppl 2):92–96 [DOI] [PubMed] [Google Scholar]
- 37.Barrett-Connor E, Laughlin GA. Endogenous and exogenous estrogen, cognitive function, and dementia in postmenopausal women: evidence from epidemiologic studies and clinical trials. Semin Reprod Med 2009;27:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig MC, Maki PM, Murphy DG. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005;4:190–194 [DOI] [PubMed] [Google Scholar]
- 39.Howe HL. Age-specific hysterectomy and oophorectomy prevalence rates and the risks for cancer of the reproductive system. Am J Public Health 1984;74:560–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


