Abstract
Background
The smooth muscle actin binding proteins Caldesmon and Tropomyosin (Tm) promote thin filament assembly by stabilizing actin polymerization, however, whether filament assembly affects either the stability or activation of these and other smooth muscle regulatory proteins is not known.
Methods
Measurement of smooth muscle regulatory protein levels in wild type zebrafish larvae following antisense knockdown of smooth muscle actin (Acta2) and myosin heavy chain (Myh11) proteins, and in colourless mutants that lack enteric nerves. Comparison of intestinal peristalsis in wild type and colourless larvae.
Key Results
Knockdown of Acta2 led to reduced levels of phospho-Caldesmon and Tm. Total Caldesmon and phospho-myosin light chain (p-Mlc) levels were unaffected. Knockdown of Myh11 had no effect on the levels of either of these proteins. Phospho-Caldesmon and p-Mlc levels were markedly reduced in colourless mutants that have intestinal motility comparable with wild type larvae.
Conclusions & Inferences
These in vivo findings provide new information regarding the activation and stability of smooth muscle regulatory proteins in zebrafish larvae and their role in intestinal peristalsis in this model organism.
Keywords: actin binding proteins, enteric nervous system, peristalsis, smooth muscle, zebrafish
INTRODUCTION
Contractile force in smooth muscle cells is generated by the physical interaction of filamentous actin and myosin heavy chain (Myh11) proteins. In the intestine, activation of smooth muscle muscarinic receptors at the myoenteric nerve junction leads to an increase in cytoplasmic calcium levels, which in turn, activates the myosin light chain kinase protein (Mlck)1,2. By altering the ratio of Mlck and myosin light chain phosphatase (Mlcp) activity, enteric nerve stimulation enhances phosphorylation of the regulatory myosin light chain (phospho-myosin light chain, p-Mlc), the major regulator of cross-bridge cycling in smooth muscle. While Mlck is considered to be the principal regulator of smooth muscle contraction, in vitro studies suggest that other protein kinases regulate p-Mlc levels by modulating the activity of Mlcp.1–3
In addition to actin, smooth muscle thin filaments contain two actin binding proteins, Tropomyosin (Tm) and Caldesmon, and the calcium binding protein Calmodulin. Together, these proteins function cooperatively to regulate acto-myosin interactions (and hence contraction), independently of Mlc phosphorylation.4 Normally, Tm, Caldesmon, and calmodulin inhibit activation of the myosin ATPase that occurs when actin binds myosin. Upon their own activation, the binding protein’s inhibitory function is relieved, thereby strengthening contractile force. This contractile regulatory mechanism is commonly referred to as thin filament regulation.
Although the precise mechanism of thin filament regulation is debated it is known that Caldesmon plays a central role in this process.5 Caldesmon binds both the Smooth muscle actin (Acta2) and Smooth muscle myosin (Myh11) proteins, thereby tethering thin and thick filaments to one another. Phosphorylation of Caldesmon, or calcium binding to Calmodulin disrupts one of Caldesmon’s three actin binding sites, thereby strengthening the physical interaction between thin and thick filaments and enhancing the force generated during cross-bridge cycling.6,7
Differential splicing of the Caldesmon mRNA generates low and high molecular weight isoforms that are present in non-muscle and smooth muscle cells, respectively.5 High molecular weight Caldesmon in vascular and airway smooth muscle is phosphorylated by extra-cellular regulated kinase (ERK) other cellular kinases.8–10 ERK also plays a role in phosphorylation of the non-muscle low molecular weight Caldesmon isoform.11 The precise role of Caldesmon phosphorylation by ERK and other kinases in phasic smooth muscle present in the intestine has not been conclusively determined.12,13 Indeed, relatively little is known about Caldesmon regulation or stability in these cells, or the stability of the other smooth muscle actin binding proteins in vivo. To begin to address these questions, we took advantage of the ease with which antisense techniques can be used in the zebrafish system to knockdown translation of proteins non-essential for vertebrate embryonic development. In this study, we examined the effect of actin and myosin knockdowns on levels of Caldesmon and Tm and p-Mlc in intestinal smooth muscle in vivo. We also measured the levels of regulatory phospho-proteins in zebrafish colourless mutants that lack enteric nerves14,15 and correlated these data with intestinal peristalsis and smooth muscle contraction in live mutants.
MATERIALS AND METHODS
Fish stocks
Maintenance and breeding of wild type (Tu) and colourless (sox10m241) was as previously described.16 Colourless mutants were purchased from the Zebrafish International Resource Center (Eugene, OR, USA). Transgenic colourless mutants Tg(NBT: DsRed); sox10m241 were generously provided by Dr. Michael Granato. The Tg(NBT:DsRed) line has previously been described.17 All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the animal welfare committee University of Pennsylvania School of Medicine.
Morpholino injections
Zebrafish embryos were microinjected with morpholinos targeting the translation initiation site of the zebrafish acta2 and myh11 genes using previously described methods.16 The myh11 and acta2 morpholinos were purchased from Gene-Tools (Corvalis, OR, USA) and Open Biosystems (Huntsville, AL, USA), respectively. The mhy11 morpholino sequence was previously reported.16 The acta2 morpholino (5′-GCTTTCTTCGTCGTCACACATTTTC-3″) was injected at a concentration of 100 nmol L−1.
Antibodies and immunohistochemistry
Antibodies for immunohistochemistry and Western blot analyses were anti-human beta-actin (Sigma-Aldrich, St. Louis, MO, USA); anti-human alpha-actin (Fisher Scientific, Fremont, CA, USA); anti-Caldesmon (a generous gift of Albert Wang); anti-human phospho-Caldesmon (serine 789; Upstate Biotechnology, Millipore Inc, Bellerica MA); anti-human phospho-myosin light chain (Sigma-Aldrich); and anti-chicken gizzard Tm (Sigma-Aldrich); anti-human smooth muscle myosin antibody (Biomedical Technologies, Stoughton, MA, USA); and anti-human phospho-ERK (Cell Signaling Technologies, Danvers, MA, USA). Protocols for zebrafish immunostains were previously reported.16
Confocal microscopy
Confocal images of Tg(NBT:DsRed) and Tg(NBT:DsRed); sox10m241 larvae were taken using an Olympus spinning disc microscope (Olympus, Center Valley, PA, USA). Larvae were mounted in 0.8% low melting agarose and anesthetized with tricaine until intestinal movements were not detected. Stacks of 0.2 µmol L−1 thick images (n = 30) were taken from the superior half of the intestine. Images were processed using image j software (http://rsbweb.nih.gov/ij/). Cutaneous nerve staining was erased manually and the stacks were merged using maximal intensity Z projections.
Western blots
Dissected intestines from 3 to 6 dpf larvae were homogenized in 1× sample buffer and the protein samples were denatured on boiling water bath for 15 min. Total protein recovered from 30 embryos was loaded into each well and then separated in a 10% SDS–polyacrylamide gel. The blot was then electrophoretically transferred to a nitrocellulose membrane using Bio-Rad protein mini gel apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked overnight at 4_C in tris-buffered saline containing 0.1% Tween 20 and 5% skimmed milk. The blots were probed with anti-human beta-actin (Sigma-Aldrich); anti-human alpha-actin (NeoMarkers); anti-Caldesmon (a generous gift of Albert Wang); anti-human phospho-Caldesmon (serine 789; Upstate Biotechnology); anti-human phospho-myosin light chain (Cell Signaling Technologies); anti-chicken gizzard Tm (Sigma-Aldrich). Following washes, the blots were incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-rabbit IgG at a 1 : 5000 dilution for 2 h. Detection of immunoreactive proteins was performed with an ECL system (Amersham Biosciences Corp., Piscataway, NJ, USA). All blots are representative of at least triplicate experiments. To quantify Western blot bands, films were digitally scanned at a resolution of 300 dpi using Adobe Photoshop and the resulting image inverted. Band relative intensity was calculated by multiplying the mean value derived from the band histogram by the band’s pixel number.
Peristalsis assay
Wild type, morpholino injected, and colourless larvae (5 and 6 dpf) were fed paramecia in media containing fluorescent latex beads (Fluoresbrite YG2.0; Polysciences, Warrington, PA, USA) for 3 h, or in some experiments, 12 h (as indicated in the manuscript). Larvae were then placed in media containing only paramecia and expulsion of beads was monitored using a fluorescent dissecting microscope (Olympus, MVX-10). For time lapse movies, larvae were anesthetized in tricaine (64 mg L−1) and mounted in methylcelluose. Images were obtained at 1 s intervals for 2 min using an Olympus BX61 compound microscope, Hamamatsu ORCA-ER digital camera, and slidebook software (Intelligent Imaging Innovations, Philadelphia, PA, USA).
RESULTS
Antisense knockdowns of Acta2 and Myh11 in zebrafish larvae
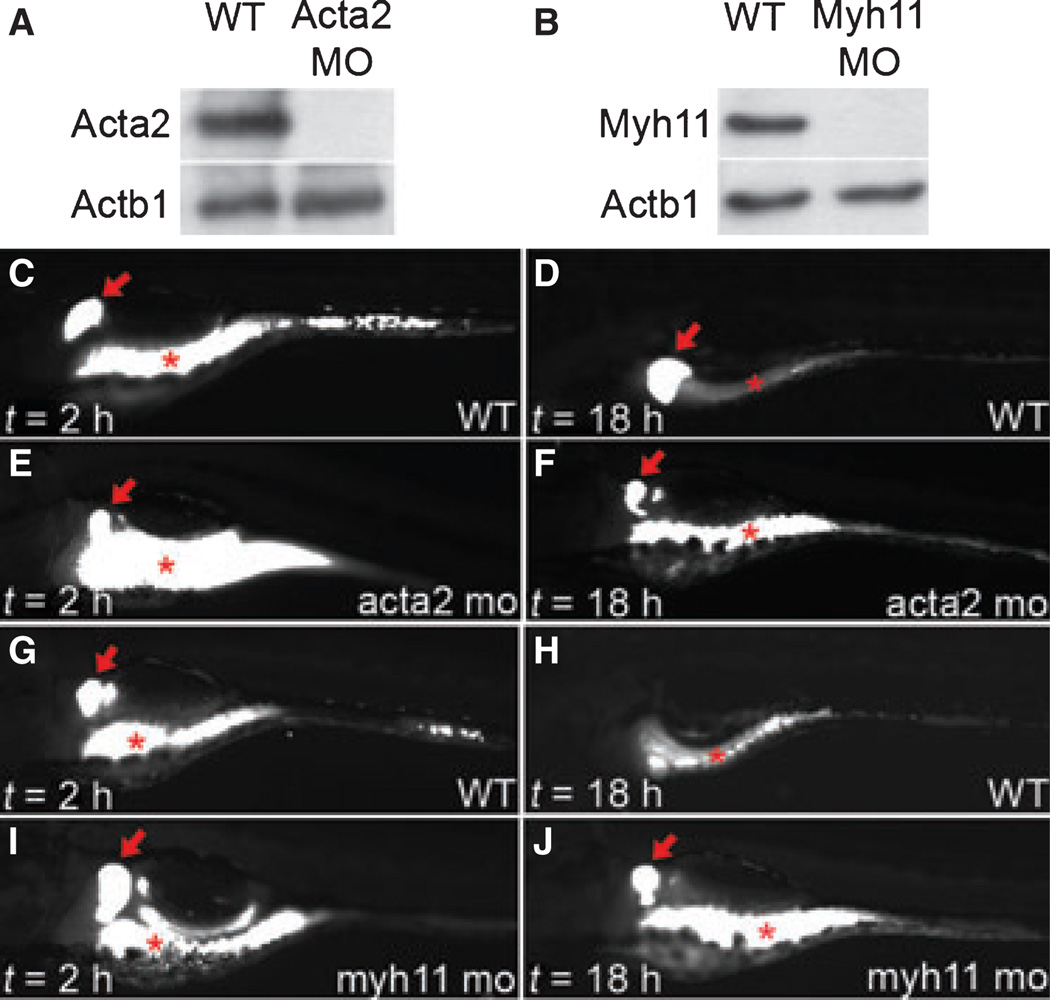
The developmental expression of acta2 and myh11 genes in the intestine of zebrafish embryos and larvae has been reported.16,18 Both genes are first detected in cells surrounding the intestinal epithelium at approximately 60 h postfertilization (hpf) and are strongly expressed at later developmental stages. Myh11 protein has been detected in the larval intestine as early as 69 hpf.19 Consistent with this, for our assays we detected intestinal Acta2 and Myh11 proteins via Western blot at 72 hpf (Fig. 1A,B).
Figure 1.
Thin and thick filament disruption in zebrafish larvae. (A, B) Western blot showing knockdown of smooth muscle actin (Acta2) and myosin heavy chain (Myh11) proteins in the intestine of 3 dpf morpholino injected larvae. (C–J) Lateral fluorescent images of live 6 dpf wild type and acta2 morpholino (C–F) and myh11 morpholino (G–J) injected larvae 2 and 18 h after ingestion of fluorescent beads. In these images, the red asterisk (*) identifies the intestinal lumen; the red arrow points to fluorescent beads adherent to the fin bud. MO, morpholino; WT, wild type control. Larvae oriented with anterior – left; posterior – right.
To examine the role of Acta2 protein in intestinal smooth muscle contraction, we assayed peristaltic expulsion of fluorescent beads ingested by larvae that had been injected with an antisense morpholino targeting Acta2 mRNA. The assay was conducted between 5 day postfertilization (dpf) and 6 dpf, a stage when the digestive system is well developed and larvae normally ingest exogenous food.20 At the injected dose (100 nmol L−1), the acta2 morpholino-injected larvae had only a slight developmental delay at 3 dpf that was no detected when assayed at 4 and 5 dpf. Western blot analyses of intestinal protein isolated from wild type and morpholino-injected larvae at 3 dpf and later stages confirmed successful knockdown of the Acta2 protein (Fig. 1A and data not shown). Flourescent beads ingested by 5 dpf wild type larvae were expelled from the intestine 12 h after ingestion, whereas the beads were retained in all Acta2 morpholino injected larvae examined (n = 20 larvae examined; Fig. 1C–F). These data show that the Acta2 protein is required for propulsive intestinal peristalsis in zebrafish larvae. Similiar findings were found following knockdown of the Myh11 protein as shown in Fig. 1B,G–J, and as previously reported.16
Actin binding protein levels in intestinal smooth muscle are reduced in Acta2 deficient zebrafish larvae
We measured levels of the high molecular weight isoform of Caldesmon, phosphorylated Caldesmon (p-Caldesmon), smooth muscle Tm, and p-Mlc in the intestine of wild type and Acta2 deficient larvae between 3 and 5 dpf. The antibodies used for this study detect proteins on Western blots with comparable molecular weight to the orthologous proteins reported in the zebrafish genome database.21 The antibody directed against p-Caldesmon and non-phosphorylated Caldesmon detect low and high molecular weight isoforms that are predicted to be present in intestinal epithelium and smooth muscle, respectively. The antibody directed again Tm derived from chicken gizzard detects a protein of comparable molecular weight on Western blot analysis. The p-Mlc antibody recognizes a highly conserved phosphoepitope that is strongly expressed in zebrafish intestinal smooth muscle (Fig. S2). The zebrafish and host epitopes recognized by each of these antibodies are presented as supporting data online (Table S1).
Western blot analyses of smooth muscle regulatory proteins in wild type zebafish larvae are presented in Figs 2 and 4. These larvae were siblings of the Acta2 and Myh11 morpholino injected larvae and thus serve as controls for these experiments. Identical data were derived from wild type larvae in independent experiments (data not shown). The first time point we analyzed (~74 hfp) occurred before peristaltic contractions are detected in the larval intestine (at ~80 hpf). We also analyzed larvae at 4 and 5 dpf, when coordinated peristaltic smooth muscle contraction is easily detected. Western blot analyses revealed that all of the regulatory proteins were detected in the intestine at these stages, although Caldesmon phosphorylation was not always detected in larvae collected at the beginning of the third dpf (<76-h postfertilization; Fig. 4B). There was a significant increase in pMlc levels between 4 and 5 dpf, (Figs 2C and 4D – accounting for loading controls) most likely reflecting increased peristaltic activity that is detected at 5 dpf.
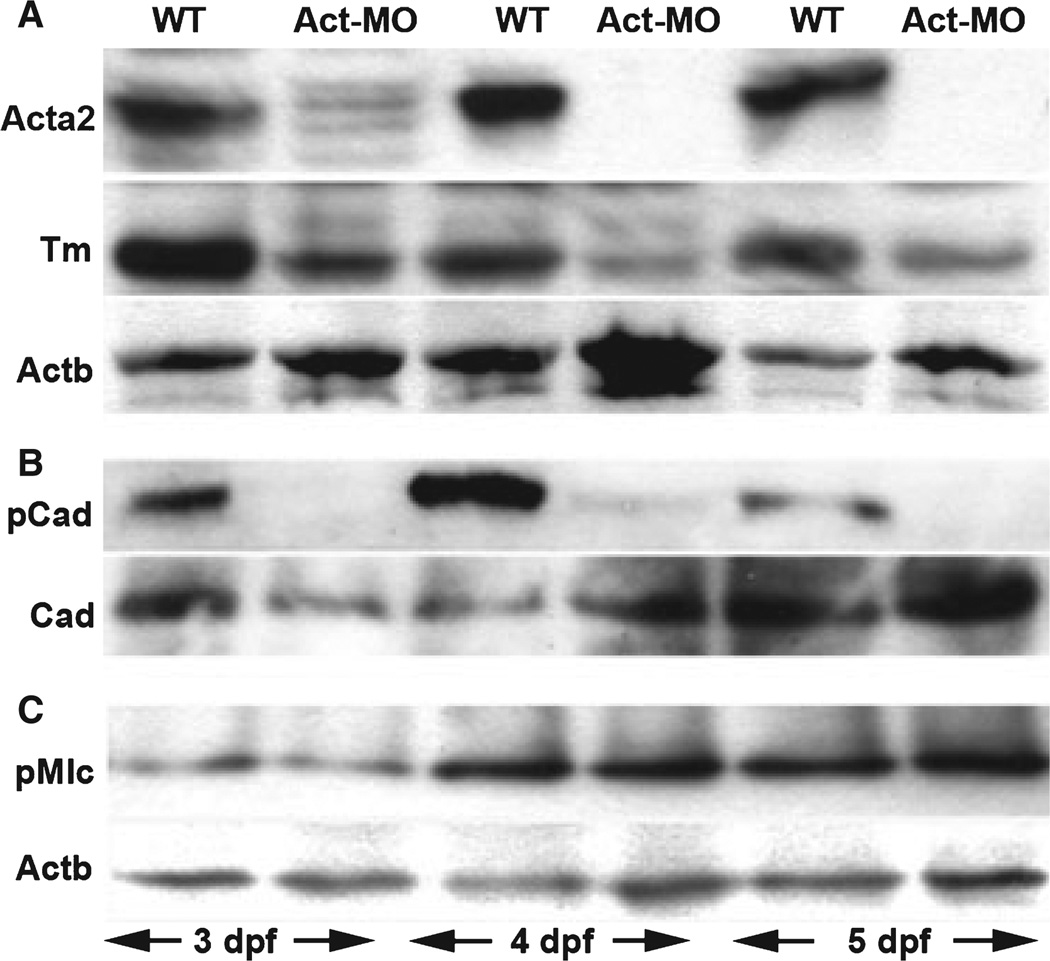
Figure 2.
Smooth muscle regulatory proteins in smooth muscle actin (Acta2) deficient larvae. Western blot of intestinal protein derived from 3 to 5 dpf wild type and Acta2 deficient larvae. Age designations are indicated at the bottom of each lane. (A) This blot shows successful knockdown of Acta2 protein in acta2 morpholino injected larvae, along with reduced levels of Tropomyosin (Tm). beta-Actin (Actb) serves as loading control. (B) This blot shows reduced p-Caldesmon (pCad) in 4 and 5 dpf Acta2 deficient larvae but stable levels of total Caldesmon (Cad). The upper bands in these blots corresponds to the high molecular weight Caldesmon isoform. (C) This blot shows stable phosphomyosin light chain (p-Mlc) levels in Acta2 deficient larvae. WT, wild type; Act-MO, acta2 morpholino injected.
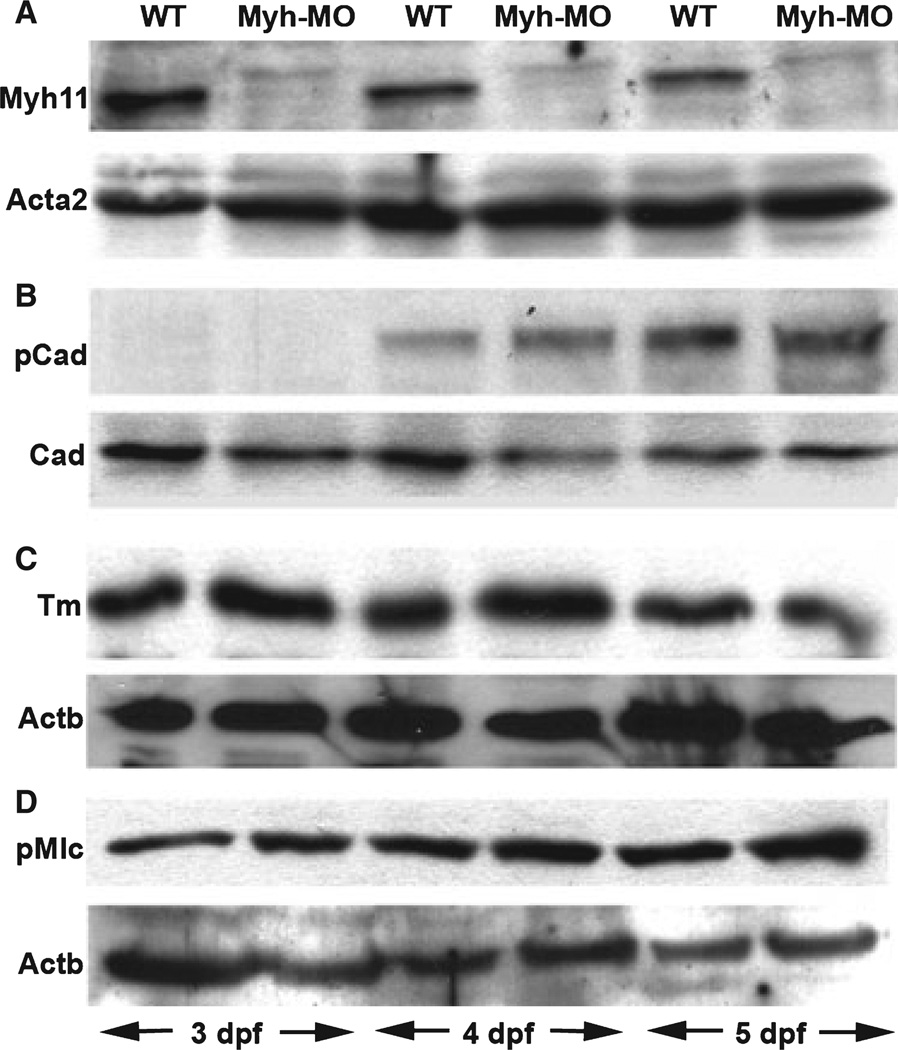
Figure 4.
Smooth mucle regulatory protein levels are unchanged in myosin heavy chain (Myh11) deficient larvae. (A–D) Western blots of intestinal proteins derived from 3 to 5 dpf wild type and Myh11 morpholino (MO) injected larvae. (A) Knockdown of Myh11 protein in morpholino injected larvae. Acat2 levels are unchanged by the knockdown and serve as a loading control. (B) p-Caldesmon levels are unchanged with respect to total Caldesmon levels in Myh11 deficient larvae. (C, D) Tropomyosin (Tm) and phospho-myosin light chain (p-Mlc) levels are unchanged with respect to beta-actin (Actb) levels in Myh11 deficient larvae.
We next measured the levels of the regulatory proteins in the Acta2 deficient larvae. Levels of p-Caldesmon and Tm were significantly reduced compared with wild type larvae at all stages analyzed (Fig. 2B,C). Total Caldesmon and p-Mlc levels were comparable with wild types at all stages other than a reduction in total Caldesmon at 3 dpf (Fig. 2A,E).
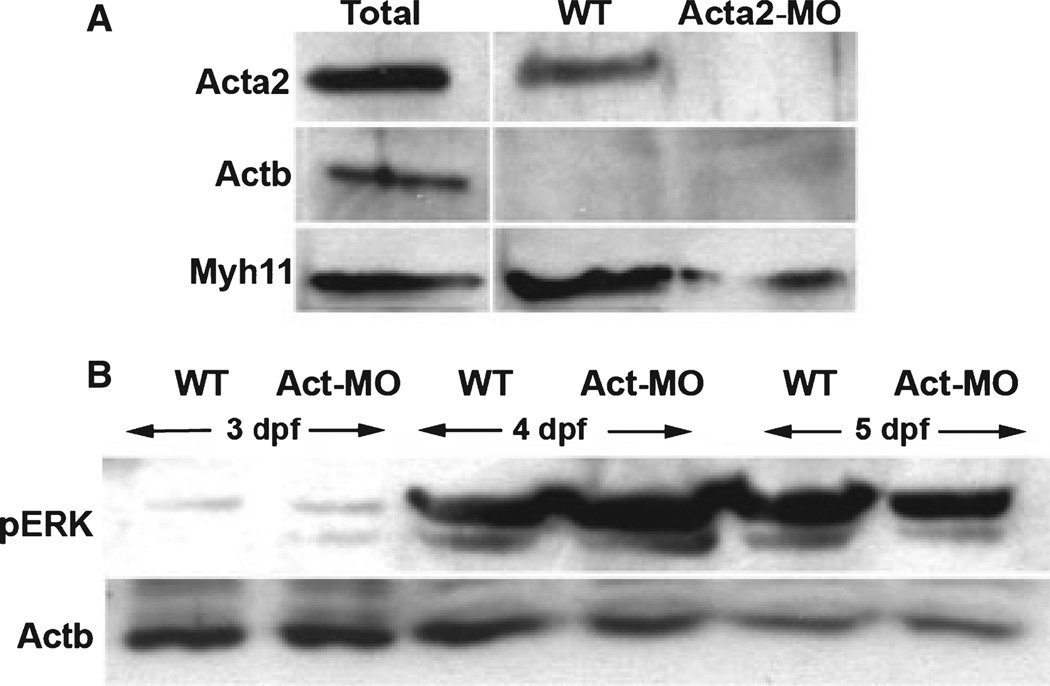
As both Caldesmon and Tm bind actin, we sought to explain why Tm levels were reduced in Acta2 deficient larvae while total Caldesmon levels were unchanged. One possible explanation we considered was binding of Caldesmon to beta-actin (Actb), the other actin isoform normally expressed in smooth muscle. However, immunoprecipitation experiments did not detect Actb bound to Caldesmon in either Acta2 deficient or wild type larvae (Fig. 3A). By contrast, Caldesmon remained bound to Myh11 in the absence of Acta2 protein, whereas no interaction between Tm and Myh11 was detected (data not shown). In addition, we found equivalent levels of activated ERK kinase in Acta-2 deficient and wild type larvae (Fig. 3B), thus arguing against the idea that Acta-2 knockdown interfered with upstream signaling pathways required for Caldesmon phosphorylation.
Figure 3.
Caldesmon binding in smooth muscle actin (Acta2) deficient larvae. (A) Western blots of intestinal protein from wild type and Acta2 deficient larvae following immuno-precipitation using total Caldesmon antibody followed by blotting with anti-beta-Actin (Actb) and anti-myosin heavy chain (Myh11) antibodies. Caldesmon binds Acta2 and Myh11 in wild type intestine. Caldesmon remains bound to Myh11 in Acta2 deficient larvae. Total refers to intestinal protein prior to Caldesmon immunoprecipitation. (B) Western blots of intestinal protein from wild type and Acta2 deficient larvae assayed for levels of phospho-ERK (pERK) at the indicated developmental stages. Beta-actin (Actb) serves as loading control.
Levels of p-Mlc and actin binding proteins are not reduced in intestinal smooth muscle of Myh11 deficient zebrafish larvae
We next examined the effect of Myh11 knockdown on total Caldesmon, p-Caldesmon, Tm, and p-Mlc levels in 3–5 dpf larvae (Fig. 4). In contrast to the Acta2 knockdown, the Myh11 knockdown did not decrease total or p-Caldesmon levels or levels of Tm at these time points. Phospho-myosin light chain levels at these stages were also unchanged in the Myh11 morpholino injected larvae.
Reduced p-Mlc and p-Caldesmon levels in the intestine of zebrafish colourless mutants that lack enteric nerves
Mutation of sox10 in zebrafish colourless mutants disrupts development of neural crest derivatives such as pigment cells, and a subset of glia and neurons.14,15 Previous studies have shown that colourless mutants have few if any enteric nerves,14,15 however, whether phosphorylation of Mlc or other smooth muscle regulatory proteins is disrupted in colourless mutants is not known. Studies conducted in developing mice and zebrafish suggest that intestinal smooth muscle contraction can occur in the absence of enteric nervous stimulation.22,23 Thus, it is conceivable that actin and myosin regulatory proteins are phosphorylated in colourless mutants.
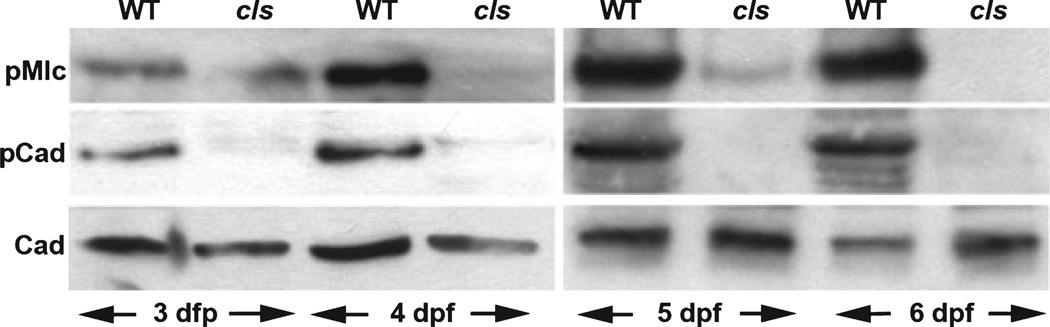
To examine this question, we used Western blot analyses to measure intestinal p-Mlc and p-Caldesmon levels in colourless mutants and their wild type siblings (Fig. 5). Low levels of p-Mlc were detected in 3 dpf colourless mutants and wild type siblings. p-Mlc levels increased in wild types between 3 and 6 dpf, whereas in colourless mutants, p-Mlc levels declined to undetectable levels over this period. The difference in p-Caldesmon levels in colourless and wild type siblings was similar to the differences in p-Mlc: p-Caldesmon was not detected in 3–6 dpf colourless mutants, whereas high levels of p-Caldesmon were detected in wild types between 4 and 6 dpf, with lower levels at 3 dpf. Total Caldesmon levels in colourless and wild type siblings were similar at all stages analyzed.
Figure 5.
Reduced levels of activated smooth muscle regulatory proteins in colourless (cls) larvae. Western blots of phospho-myosin light chain (p-Mlc), p-Caldesmon and total Calesmond in intestinal extracts from 3 to 6 dpf wild type (WT) and cls larvae. Phospho-myosin light chain (p-Mlc) and p-Cad levels are markedly reduced in cls.
The colourless mutants used for this study are hemizgyous for a dsRed transgene that is expressed within enteric neurons.17 Confocal fluorescence microscopy confirmed the absence of enteric nerves in colourless mutants (Fig. 6A,B). Rare axons from cutaneous or trunk neurons projected toward the intestine but did not appear to innervate smooth muscle. In a small number of mutants, examined the vagus nerve was detected adjacent to the anterior intestine (data not shown). However, in comparison with the appearance of the vagus nerve in wild type larvae,24 it showed little if any axonal branching and made no obvious contacts with intestinal smooth muscle.
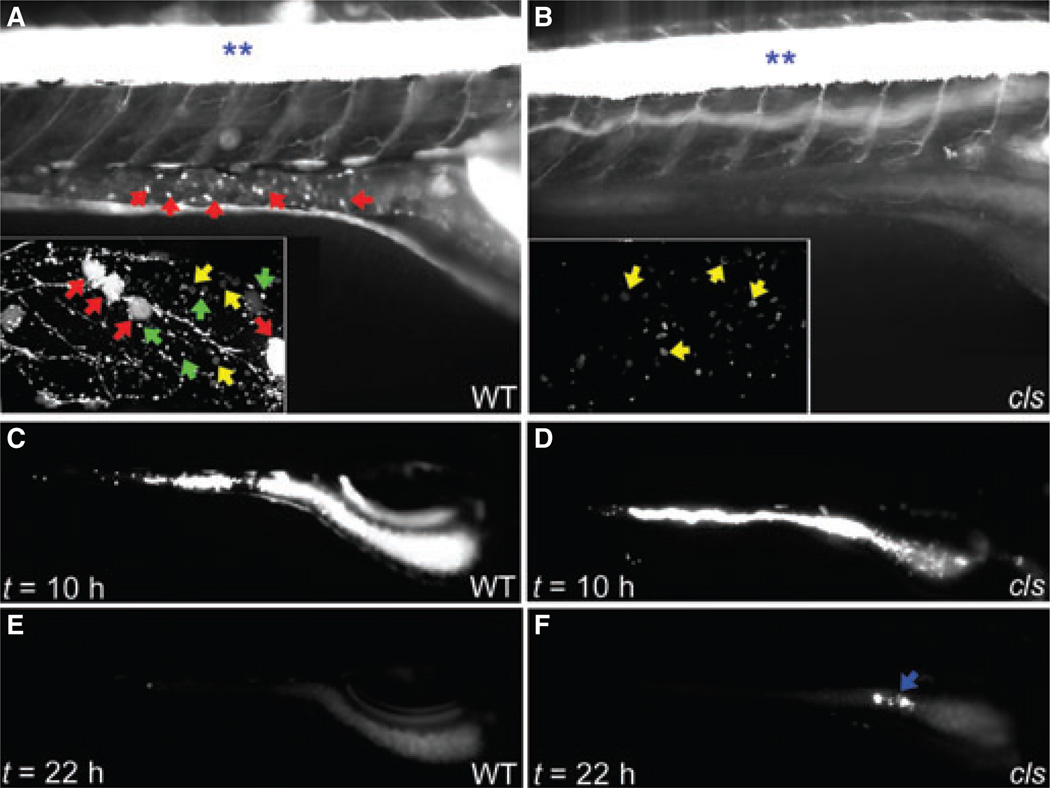
Figure 6.
Peristalsis is preserved in colourless (cls). (A, B) Lateral confocal projections of live 6 dpf wild type [WT: sox10+/+; Tg(NBT:dsRed)] and cls (sox10m148/m148; Tg(NBT:dsRed) larvae. Red arrows point to cell bodies of enteric neurons in wild type larvae that are absent in cls. Inset shows high magnification image of intestine with enteric neuron cell bodies (red arrows), axon vesicles containing dsRed protein (green arrows) and overlying skin neurons (yellow arrows). (C–F) Lateral fluorescent images of live 6 dpf wild type and cls larvae 10 and 22 h after ingestion of fluorescent beads. At 22 h (E, F), there is low level residual fluorescence from bile in WT and cls. A small number of residual beads are present in this cls larvae (blue arrow). Blue asterisk (**) denotes spinal cord in A, B. Orientation of larvae in all panels is anterior – right; posterior – left.
Intestinal peristalsis is preserved in colourless mutants that lack enteric nerves
A recent study reported that intestinal smooth muscle contraction in zebrafish larvae is reduced but not eliminated by tetrodotoxin, a potent neurotoxin.23 This finding suggests that in the absence of neural stimuli, intrinsic acto-myosin interactions could be sufficient to activate cross-bridge cycling and hence, smooth muscle contraction. To examine this question in a physiologically relevant assay, we quantified peristaltic contractile waves in colourless mutants and their wild type siblings and then correlated these findings with the ingestion and expulsion of fluorescent beads (Fig. 6).
Peristaltic contractions in colourless and wild type siblings were recorded using video microscopy. Contractions in both colourless and wild type larvae were present in the anterior intestinal bulb and mid and posterior intestine (Movies S1 and S2). The anterior contractions were largely directed orally (mixing peristalsis), while the posterior contractions were responsible for moving luminal contents in the aboral direction (propulsive peristalsis). To quantify these observations, we counted the number of contractile waves in the anterior intestinal bulb and the posterior intestine. This showed that colourless and wild type larvae had the same frequency of contractions over 2 min in the anterior intestine (4.5 contractions per minute; n = 5 wild type and n = 5 colourless larva. Range wild type 8–10 contractions per 2 minutes; colourless 8.5–10 contractions per 2 minutes), and the posterior intestine (1.9 and 1.8 contractions per minute respectively, in n = 5 colourless and, n = 5 wild type larvae. Range for both groups of larvae, 3–4 contractions per 2 minutes).
We next determined how quickly colourless and wild type larvae expelled fluorescent beads ingested from their aqueous media. Larvae were fed with paramecia and beads for 12 h and afterwards were kept in media containing only paramecia. Feeding was essential to ensure consistent uptake of the fluoresecent beads. At 10 h, both colourless and wild type larvae had a large amount of beads in the intestinal bulb and the mid and posterior intestine (Fig. 6C,D). When examined 12 h after their removal from beads (t = 22 h; Fig. 6E,F), 25% colourless larvae (n = 5 of 20 larvae), and 50% wild type larvae (n = 12 of 25 larvae) had cleared the ingested beads from the anterior intestinal bulb and had only a small number of beads if any in the mid and posterior intestine. In the remaining colourless larvae and wild type larvae (75% and 50%, respectively), beads were present throughout the intestine. In a separate experiment, 68% of colourless larvae (n = 15 of 22) and 96% of wild type larvae (n = 22 of 23 larvae examined) had cleared all beads from the intestine at 24 h after removal from media containing the beads (data not shown).
DISCUSSION
In this study, we examined the influence of actin and myosin filament assembly on the activation and levels of smooth muscle regulatory proteins. We took advantage of the ease with which smooth muscle proteins non-essential for vertebrate development can be knocked down using zebrafish system. Our data are novel in that they not only reveal different effects of Act2a and Myh11 knockdowns on Caldesmon, Tm, and p-Mlc but also because these data were derived in vivo.
Mammalian smooth cells express multiple muscle actin isoforms.25 Expression of Acta2 predominates in vascular smooth muscle cells, whereas Actg2 is the predominant isoform in phasic visceral smooth muscle. Gene structure analyses suggest that Actg2 evolved from Acta2.25 Although a comprehensive analysis of zebrafish actin isoforms has not been performed, ortholgs of Actb, Actc1, and Acta1, but not Actg2 have been reported in the genome sequencing project.18,21 These data argue that in zebrafish, acta2 is the functional ortholog of the mammalian Actg2 gene, most likely because Actg2 evolved after divergence of the teleost lineage. Given these considerations, Acta2 knockdown in zebrafish is predicted to abolish thin filament assembly in zebrafish intestinal smooth muscle. Consistent with this prediction, we found that intestinal peristalsis was markedly inhibited by antisense knockdown of zebrafish Acta2.
The peristaltic defects in Acta2 deficient larvae on their own do not preclude complementation of thin filament assembly by another actin isoform. Indeed, studies in mice have shown that Actg2 weakly complements cardiac contractility in the absence of Actc1.26 Thus, we considered the possibility of Acta2 complementation by Actb, the widely expressed cytoplasmic actin isoform, or the skeletal or cardiac muscle actins, Acta1 and Actc1. Western blot analyses, however, did not support thin filament complementation by any of these actin isoforms. We found no evidence of Actb binding to high molecular weight Caldesmon via immunoprecipitation experiments, and the anti-mammalian Acta2 antibody, which also recognizes mammalian Acta1 and Actc1, did not detect either of these highly conserved proteins in the intestine of Acta2 deficient larvae.
Having established that the Acta2 knockdown disrupts thin filament assembly, it is interesting that the knockdown caused a profound reduction in level of smooth muscle Tm, but had no effect on the total level of the high molecular weight isoform of Caldesmon. This was unexpected, because both proteins rapidly dissociate from thin filaments in vitro.27 A simple explanation for that could account for these findings is the stabilization of Caldesmon through its interaction with Myh11 in the absence of Acta2 protein. Alternatively, the rate of Tm gene transcription, translation, or mRNA stability could be reduced in the setting of the Acta2 knockdown.
Although the Acta2 knockdown had no effect on total Caldesmon levels, levels of p-Caldesmon were significantly reduced. The simplest interpretation of these findings posits a conformational requirement for Caldesmon phosphorylation that is dependent upon its physical interaction with Acta2. Reduced Tm in Acta2 deficient larvae, on its own, is an unlikely cause of reduced p-Caldesmon levels because the two proteins physically interact only when bound to actin.28 We also considered the possibility that reduced p-Caldesmon arises from reduced activity of ERK, the kinase that phosphorylates the serine residue recognized by the p-Caldesmon antibody used for this study. However, ERK is activated downstream of muscarinic receptor signaling that also activates myosin light chain kinase.29 Unchanged p-Mlc levels in the Acta2 deficient larvae argue that this signaling pathway is activated normally in the absence of Acta2 protein. Indeed, pErk levels (as determined by Western blot analyses) were comparable in Acta2-deficient and wild type larvae.
In contrast to Acta2-deficient larvae, levels of Tm, or total or p-Caldesmon were not decreased in Myh11 deficient larvae. These data argue that thin filaments are stable in vivo in the absence of Myh11. An attractive alternative explanation for these findings is that Myh11 is complemented by one of the non-muscle Myh11 proteins that are normally present in smooth muscle (Myh9; Myh10). Indeed, non-muscle myosins have been shown to interact with thin filaments in Myh11 deficient mammalian smooth muscle cells.30 However, because there are lower levels of the non-muscle Mlc isoform in digestive tract smooth muscle we speculate that the quantity of non-muscle Myh11 protein normally present in smooth muscle is not sufficient to complement Myh11.31
In addition to having no effect on actin binding protein levels, Myh11 knockdown had no effect on levels of p-Mlc. These findings were unexpected because mammalian Mlck phosphorylates Mlc when bound to Myh11.32 As p-Mlc levels were also normal in Acta2 deficient larvae, our data argue that Mlck activity in vivo is not dependent upon its interaction with either thin or thick filaments, as suggested by in vitro assays.33 Normal p-Mlc levels in Myh11 and Acta2 deficient larvae also argue that neither Mlck nor Mlcp in smooth muscle is subject to feedback inhibition by contractile force.
Having measured smooth muscle regulatory protein levels in larvae that were deficient in the predominant smooth muscle actin and myosin isoforms, we next examined role of enteric nerve stimulation on the activation of these proteins. Given the well conserved role of neural activation of smooth muscle Mlck, the finding that p-Mlc levels were reduced in the intestine of colourless mutants was not surprising. Reduced p-Caldesmon levels in colourless mutants were also expected given prior studies showing that phosphorylation by the ERK kinase is mediated by neuronal activation of the smooth muscle M3 muscarinic receptor.29 Together, these findings argue that cross-bridge cycling can be initiated by intrinsic actomyosin interactions that are not dependent upon activation of either Mlc or Caldesmon. However, it is also possible that Caldesmon is activated in colourless mutants by another cellular kinase, as has been suggested by previous studies of mammalian smooth muscle.9,10,12,13
Intestinal peristalsis in mammals derives from the coordinated contraction of circular and longitudinal smooth muscle that is controlled by the enteric nervous system. Effective propulsive peristalsis in colourless larvae was therefore unexpected. In addition, propulsive peristaltic muscular contractions were not reported in the study that performed video imaging of live colourless larvae.34 A possible explanation for this is the simple architecture of the zebrafish larval intestine, which is comprised of an anterior bulb, and a tube-like mid and posterior intestine. The junction of the two regions narrows but is not folded. We speculate that intrinsic interactions between contiguous circular and longitudinal smooth muscle cells are sufficient for coordinated radial and axial contraction at this developmental stage. Activation of this peristaltic mechanism could occur in colourless larvae because they are likely to retain interstitial cells of cajal, the gastrointestinal pacemaker cells that originate from mesenchymal rather than neuroectodermal progenitors.35–37 Other factors that influence smooth muscle, such as humoral signals from enteroendocrine cells also are predicted to not be altered in colourless larvae.38 Thus, it is conceivable that they too facilitate peristalsis.
Colourless mutants do not survive beyond larval stages, thus it is not known whether intrinsic activation of intestinal peristalsis would be sufficient for their nutritional needs. In mammals, initiation of intestinal smooth muscle contraction by intrinsic acto-myosin interactions is not sufficient for intestinal peristalsis, as conditional disruption of Mlck in adult smooth muscle is lethal.39 Whether lethality of colourless mutants resulted from the enteric nervous system defect cannot be determined because the sox10 mutation also affects development of sensory and sympathetic neurons, satellite glia and Schwann cells.10,11 As the genetic programs that specify these various neural crest derived lineages are discovered in future studies, it may be possible to complement sox10 activity in non-enteric neurons of colourless mutants, thereby enabling studies that evaluate the role intrinsic acto-myosin interactions during intestinal peristalsis in both larval and juvenile zebrafish.
Supplementary Material
ACKNOWLEDGMENTS
DISCLOSURES
The authors thank Mani Muthumani for technical assistance, David Cobb and the UPenn zebrafish facility technical staff for assistance with the maintenance of fish stocks. This work was supported by R01 DK54942 (MP).
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
GD, CS, JA, and AJS performed research. GD, CS, and MP designed the research and analyzed data. MP wrote the paper.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Figure S1. Conserved zebrafish epitope recognized by the anti-human phospho-myosin light chain (p-Mlc) antibody.
Table S1. Comparison of epitopes recognized by regulatory protein antibodies.
Movie S1. Intestinal peristalsis in a 6 dpf wild type larva with fluorescent microspheres in the intestinal lumen.
Movie S2. Intestinal peristalsis in a 6 dpf colourless larva with fluorescent microspheres in the intestinal lumen.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Ding HL, Ryder JW, Stull JT, Kamm KE. Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem. 2009;284:15541–15548. doi: 10.1074/jbc.M900888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008;295:C358–C364. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 4.Ansari S, Alahyan M, Marston SB, El-Mezgueldi M. Role of caldesmon in the Ca2+ regulation of smooth muscle thin filaments: evidence for a cooperative switching mechanism. J Biol Chem. 2008;283:47–56. doi: 10.1074/jbc.M706771200. [DOI] [PubMed] [Google Scholar]
- 5.Kordowska J, Huang R, Wang CL. Phosphorylation of caldesmon during smooth muscle contraction and cell migration or proliferation. J Biomed Sci. 2006;13:159–172. doi: 10.1007/s11373-005-9060-8. [DOI] [PubMed] [Google Scholar]
- 6.Foster DB, Huang R, Hatch V, et al. Modes of caldesmon binding to actin: sites of caldesmon contact and modulation of interactions by phosphorylation. J Biol Chem. 2004;279:53387–53394. doi: 10.1074/jbc.M410109200. [DOI] [PubMed] [Google Scholar]
- 7.Huang R, Li L, Guo H, Wang CL. Caldesmon binding to actin is regulated by calmodulin and phosphorylation via different mechanisms. Biochemistry. 2003;42:2513–2523. doi: 10.1021/bi0268605. [DOI] [PubMed] [Google Scholar]
- 8.Gorenne I, Su X, Moreland RS. Caldesmon phosphorylation is catalyzed by two kinases in permeabilized and intact vascular smooth muscle. J Cell Physiol. 2004;198:461–469. doi: 10.1002/jcp.10440. [DOI] [PubMed] [Google Scholar]
- 9.Lee YR, Lee CK, Park HJ, et al. c-Jun N-terminal kinase contributes to norepinephrine-induced contraction through phosphorylation of caldesmon in rat aortic smooth muscle. J Pharmacol Sci. 2006;100:119–125. doi: 10.1254/jphs.fp0050777. [DOI] [PubMed] [Google Scholar]
- 10.Smolock EM, Wang T, Nolt JK, Moreland RS. siRNA knock down of casein kinase 2 increases force and cross-bridge cycling rates in vascular smooth muscle. Am J Physiol Cell Physiol. 2007;292:C876–C885. doi: 10.1152/ajpcell.00343.2006. [DOI] [PubMed] [Google Scholar]
- 11.Hai CM, Gu Z. Caldesmon phosphorylation in actin cytoskeletal remodeling. Eur J Cell Biol. 2006;85:305–309. doi: 10.1016/j.ejcb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Krymsky MA, Chibalina MV, Shirinsky VP, Marston SB, Vorotnikov AV. Evidence against the regulation of caldesmon inhibitory activity by p42/p44erk mitogen-activated protein kinase in vitro and demonstration of another caldesmon kinase in intact gizzard smooth muscle. FEBS Lett. 1999;452:254–258. doi: 10.1016/s0014-5793(99)00641-9. [DOI] [PubMed] [Google Scholar]
- 13.Nixon GF, Iizuka K, Haystead CM, Haystead TA, Somlyo AP, Somlyo AV. Phosphorylation of caldesmon by mitogen-activated protein kinase with no effect on Ca2+ sensitivity in rabbit smooth muscle. J Physiol. 1995;487:283–289. doi: 10.1113/jphysiol.1995.sp020879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutton KA, Pauliny A, Lopes SS, et al. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- 15.Kelsh RN, Eisen JS. The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development. 2000;127:515–525. doi: 10.1242/dev.127.3.515. [DOI] [PubMed] [Google Scholar]
- 16.Wallace KN, Dolan AC, Seiler C, et al. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell. 2005;8:717–726. doi: 10.1016/j.devcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Georgijevic S, Subramanian Y, Rollins EL, Starovic-Subota O, Tang AC, Childs SJ. Spatiotemporal expression of smooth muscle markers in developing zebrafish gut. Dev Dyn. 2007;236:1623–1632. doi: 10.1002/dvdy.21165. [DOI] [PubMed] [Google Scholar]
- 19.Olden T, Akhtar T, Beckman SA, Wallace KN. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis. 2008;46:484–498. doi: 10.1002/dvg.20429. [DOI] [PubMed] [Google Scholar]
- 20.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21. http://www.sanger.ac.uk/Projects/D_rerio/. [Google Scholar]
- 22.Anderson RB, Enomoto H, Bornstein JC, Young HM. The enteric nervous system is not essential for the propulsion of gut contents in fetal mice. Gut. 2004;53:1546–1547. [PMC free article] [PubMed] [Google Scholar]
- 23.Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol. 2007;210:1084–1091. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- 24.Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neuorol. 2008;508:756–770. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- 25.Miwa T, Manabe Y, Kurokawa K, et al. Structure, chromosome location, and expression of the human smooth muscle (enteric type) gamma-actin gene: evolution of six human actin genes. Mol Cell Biol. 1991;11:3296–3306. doi: 10.1128/mcb.11.6.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Crawford K, Close L, et al. Rescue of cardiac alpha-actin-deficient mice by enteric smooth muscle gamma-actin. PNAS. 1997;94:4406–4411. doi: 10.1073/pnas.94.9.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marston S. Stoichiometry and stability of caldesmon in native thin filaments from sheep aorta smooth muscle. Biochem J. 1990;272:305–310. doi: 10.1042/bj2720305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marston D, El-Mezgueldi M. Role of tropomyosin in the regulation of contraction in smooth muscle. Adv Exp Med Biol. 2008;644:110–123. doi: 10.1007/978-0-387-85766-4_9. [DOI] [PubMed] [Google Scholar]
- 29.Cook AK, Carty M, Singer CA, Yamboliev IA, Gerthoffer WT. Coupling of M(2) muscarinic receptors to ERK MAP kinases and caldesmon phosphorylation in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2000;278:G429–G437. doi: 10.1152/ajpgi.2000.278.3.G429. [DOI] [PubMed] [Google Scholar]
- 30.Morano I, Chai GX, Baltas LG, et al. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- 31.Yuen SL, Ogut O, Brozovich FV. Nonmuscle myosin is regulated during smooth muscle contraction. Am J Physiol Cell Physiol. 2009;297:H191–H199. doi: 10.1152/ajpheart.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatch V, Zhi G, Smith L, Stull JT, Craig R, Lehman W. Myosin light chain kinase binding to a unique site on F-actin revealed by three-dimensional image reconstruction. J Cell Biol. 2001;154:611–617. doi: 10.1083/jcb.200105079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobieszek A. Regulation of smooth muscle myosin light chain kinase. Allosteric effects and co-operative activation by calmodulin. J Mol Biol. 1991;220:947–957. doi: 10.1016/0022-2836(91)90365-d. [DOI] [PubMed] [Google Scholar]
- 34.Kuhlman J, Eisen JS. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev Dyn. 2007;236:118–127. doi: 10.1002/dvdy.21033. [DOI] [PubMed] [Google Scholar]
- 35.Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:245–252. doi: 10.1111/j.1365-2982.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 36.Klüppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Rich A, Leddon SA, Hess SL, et al. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev Dyn. 2007;236:903–911. doi: 10.1002/dvdy.21086. [DOI] [PubMed] [Google Scholar]
- 38.Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- 39.He W-Q, Peng Y-J, Zhang W-C, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.