Abstract
The vagus nerve supplies low-threshold chemo- and mechanosensitive afferents to the mucosa of the proximal gastrointestinal (GI) tract. The absence of a full characterization of the morphology and distributions of these projections has hampered comprehensive functional analyses. In the present experiment, dextran (10K) conjugated with tetramethylrhodamine and biotin was injected into the nodose ganglion and used to label the terminal arbors of individual vagal afferents of both rats and mice. Series of serial 100-µm thick sections of the initial segment of the duodenum as well as the pyloric antrum were collected and processed with diaminobenzidine for permanent tracer labeling. Examination of over 400 isolated afferent fibers, more than 200 from each species, indicated that three vagal afferent specializations, each distinct in morphology and in targets, innervate the mucosa of the proximal GI tract. One population of fibers, the villus afferents, supplies plates of varicose endings to the apical tips of intestinal villi, immediately subjacent to the epithelial wall. A second type of afferent, the crypt afferent, forms subepithelial rings of varicose processes encircling the intestinal glands or crypts, immediately below the cryptvillus junction. Statistical assessment of the isolated fibers indicated that the villus arbors and the crypt endings are independent, issued by different vagal afferents. A third vagal afferent specialization, the antral gland afferent, arborizes along the gastric antral glands and forms terminal concentrations immediately below the luminal epithelial wall. The terminal locations, morphological features, and regional distributions of these three specializations provide inferences about the sensitivities of the afferents.
INDEXING TERMS: antrum, crypt, duodenum, intestines, nodose, stomach, vagus, villus, visceral afferent
The vagus nerve supplies most of the extrinsic low-threshold chemosensitive (e.g., Mei, 1985; Grundy and Scratcherd, 1989) and mechanosensitive (e.g., Clarke and Davison, 1978; Grundy, 1988) afferents innervating the mucosa of the proximal gastrointestinal (GI) tract. Both modalities, which have been described electrophysiologically, have been implicated as important in controlling the digestion and absorption of nutrients and in generating feedback that influences ingestion (Mei, 1985; Jänig, 1996; Bates et al., 1998; Raybould, 1999; Beyak et al., 2006). The vagal chemosensitive afferents appear to be broadly tuned, responding to a variety of nutritive (e.g., Randich et al., 2000; Cuche et al., 2001), osmotic (e.g., Cottrell and Iggo, 1984c; Mei and Garnier, 1986), and other chemical (e.g., Mei, 1985; Page et al., 2002) properties of chyme. The mechanosensitive afferents innervating the mucosa are typically characterized as low-threshold, often fast-adapting, fibers (e.g., Clarke and Davison, 1978; Page et al., 2002) that presumably monitor grinding, movement, and emptying of material from the stomach into the lumen of the intestine. At least some vagal afferents innervating the mucosa appear to be polymodal, responding to both chemical and mechanical events (e.g., Cottrell and Iggo, 1984a,c; Grundy, 1988) and, though less studied, apparently thermal stimuli as well (cf. Mei and Garnier, 1986; Jänig, 1996).
Little is known about the morphologies of these visceral afferents. Conventional histological analyses have documented a variety of neural elements in the mucosa and submucosa (e.g., Berkley, 1893; Hill, 1927; Palay and Karlin, 1959; Stach, 1973; Keast, 1987), but these early observations were typically unable to distinguish fibers based on their source (e.g., vagal or dorsal root, intrinsic or extrinsic, efferent or afferent, etc.).
More recently, limited observations on vagal afferents in the mucosa and submucosa of the duodenum have been made with tracers, including autoradiographic markers (Sato and Koyano, 1987) and the carbocyanine label “Dil” (Powley et al., 1994; Berthoud et al., 1995; Berthoud and Patterson, 1996). These initial analyses are instructive, but incomplete. They focused more on the population of afferents than on the morphology of the individual neuron, and they were based on tracer protocols that did not routinely yield the highest definition of terminal fields.
Thus, the present experiment was designed to extend previous descriptions of primary vagal afferents in the submucosa and mucosa of the GI tract. The experiment employed the marker dextran conjugated with tetramethylrhodamine and biotin to circumvent the low resolution and other limitations of early autoradiographic analyses and in vivo DiI protocols (cf. Powley et al., 1994; Berthoud et al., 1995; Powley and Phillips, 2005). By employing injection parameters yielding strong labeling of only a few fibers in any one specimen, combined with permanentlabeling histochemistry, the experimental protocol made it practical to examine and characterize the terminal fields of individual fibers. Serial series of thick sections of the gut wall were collected to facilitate following and reconstructing the labeled afferents.
MATERIALS AND METHODS
Animals
Young adult male rats (Sprague-Dawley, Harlan, Indianapolis, IN; n = 35) weighing 277 ± 11 g at surgery and a smaller group of male mice (S129, Jackson Laboratories, Bar Harbor, ME; n = 9) weighing 27 ± 2 g at tracer injection were examined. Animals were housed individually in an AAALAC-approved facility with ad libitum access to food and water in colony rooms maintained on 12:12 LD schedules, at 22–24°C and a relative humidity of 45–50%. All protocols were conducted as suggested in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised in 1996) and approved by the Purdue University Animal Care and Use Committee. Every effort was made to minimize the number of animals used and to ameliorate any discomfort.
Tracer injections
After being fasted (but with access to water) overnight, animals were anesthetized (rats: sodium pentobarbital, 60 mg/kg, intraperitoneally [i.p.]; mice: ketamine hydrochloride 75 mg/kg, xylazine, 10 mg/kg, i.p.). The nodose ganglion was exposed with an incision of the skin of the ventral neck and blunt dissection of the muscle and fascia overlying the ganglion. Dextran-conjugated tetramethylrhodamine-biotin (10K, “Mini-Ruby,” 10% in distilled water; rats: 1.5 µL; mice: 0.5 µL; Molecular Probes, Eugene, OR) was pressure injected (Picospritzer II; General Valve, Fairfield, NJ) into the nodose (bilaterally in rats; unilaterally, in left nodose, in mice) with a glass micropipette (ID = 25 µm). After surgery, once their righting reflexes had recovered animals were treated with an analgesic (Buprenex; rats: 0.01 mg/kg, subcutaneously [s.c.]; mice: 0.05 mg/kg, s.c.) and returned to their home cages.
Tissue processing
Following a survival interval for transport of the tracer into the periphery (rats: 14 days; mice: 8–9 days), animals were euthanized with an overdose of sodium pentobarbital (180 mg/kg, i.p.). When completely unresponsive to nociceptive stimuli, each animal was perfused through the left ventricle of the heart with 0.01 M sodium phosphate buffer (PBS; pH = 7.4; 38°C; rats: 200 mL; mice: 50 mL), followed by 4% paraformaldehyde (PF) in 0.1 M PBS (pH = 7.4; 4°C; rats: 500 mL; mice: 100 mL). The GI tissue specimens were removed, separated, cleaned, and postfixed as described elsewhere (Powley and Phillips, 2005).
Multiple separate blocks of gut encompassing the initial 1.5 cm segment of the duodenum and a block including the pyloric antrum (the ≈300 µm of antrum immediately proximal to the torus) were embedded in 15% gelatin. Specimens were then transversely (in a plane parallel to the pyloric ring) sectioned as 100-µm serial sections with a Vibratome. Sections were collected into phosphate buffer in well trays, and neurites labeled with the biotinylated tracer were processed in the ABC reaction (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) using diaminobenzidine (DAB) as the chromagen, per directions provided with the kit (see also Powley and Phillips, 2005). To minimize any interference from background that can mask finely labeled elements, a factor that becomes particularly significant for thick sections, no counterstaining or other processing was performed. Sections were rinsed, dehydrated, cleared, and coverslipped with DPX.
Microscopy
Nomarski differential interference contrast (DIC) optics (100× to 1,000×) were used to examine the unstained mucosa and to evaluate which tissue elements were accessory to the afferent endings.
Analyses proceeded in two stages. Initially, all specimens were examined systematically at 100× in brightfield illumination with a Leica Orthoplan II or Leica DMRE microscope, and each labeled fiber in the submucosa or mucosa was identified and inventoried. For this step, neurites were inventoried even if they were incomplete, weakly labeled, apparently spanned beyond the limits of the serial section series, etc.
In a second stage, however, all those labeled afferents in the rat cases that met a set of explicit criteria of completeness were identified and used for a more formal, quantitative assessment. (Mice were similarly screened and their fibers were used for the chi-square tests of independence described below, but, because of the relatively limited number of mouse cases based on only left unilateral injections, we did not attempt to quantify the size of the terminal fields in the mouse tissue.) Afferents used for the full second-stage analysis had to be 1) well stained; 2) located in a complete, uninterrupted series of serial sections; 3) fully contained within such a series, without extending to either the oral or aboral limit of the series of sections from an individual tissue block; 4) located in specimens with little background staining, folding, or section damage that might complicate following individual neurites; and 5) continuous enough that the fiber could be followed through the smooth muscle and the submucosa to its mucosal target sites. All labeled fibers satisfying the five criteria (i.e., roughly one out of every four fibers) were evaluated for target independence with a chi-square analysis. For rats, a total of 103 afferents innervating villi, 87 cases innervating crypts, and 21 fibers projecting to pyloric antral mucosa were evaluated; for mice, 238 afferents projecting to villi, 10 innervating crypts, and seven projecting to the antral mucosa were assessed.
Photomicrography
Sections were photographed in brightfield (100× to 1,000×), usually with DIC, with a Leica DMRE (Leitz, Wetzlar, Germany) widefield microscope equipped with a Spot RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI) controlled by Image-Pro Plus Software, v. 5.1 (Media Cybernetics, Silver Spring, MD).
Typically, z-series stacks of images were collected and projected as extended-depth-of-field composites. For these z-series, multiple image planes were collected at fixed intervals (0.5–2.0-µm steps, depending on the series) using a motorized stage controlled by the Image-Pro software. The z-stacks (from 2–30-µm blocks) were then converted to single in-focus projection images. For each stack, an all-in-focus image was generated with the Image-Pro “Extended Depth of Field” algorithm, and a similar projection was produced with Photoshop CS4 (v. 11.0; Adobe Systems, San Jose, CA) “Auto-Align Layers” and “Auto-Blend Layers” algorithms. Of the two alternatives, the projection giving the more veridical representation of the image as seen in the microscope was used for illustration.
For both single-plane images and the composite projected image planes (such in-focus projections were produced before any other figure processing), Adobe Photoshop was used to apply text and scale bars, make minor adjustments to the color, brightness, contrast, and sharpness of the images to match as closely as possible to the appearance of the original material viewed with the microscope, and to organize the final layout of the figures.
RESULTS
Dextran labeling of the vagal afferents provided high-definition, Golgi-like delineation of fibers and their terminal fields, and the restricted labeling achieved with the injection protocol employed made it practical to examine individual fibers coursing from the smooth muscle coat, through the submucosa, and into the mucosa without the ambiguities that result when multiple fibers course in close proximity and intertwine. Typically, with the injection strategy used, the initial 1.5 cm of proximal duodenum examined might have a total of 5–20, usually widely separated, well-labeled fibers in the mucosa and submucosa. Although similar numbers of fibers terminating in the smooth muscle wall of the organ, including the myenteric plexus and pyloric ring, were also labeled, those without terminals in the mucosa were not included in the analyses of the present experiment. The lack of background interference achieved by not using a counterstain facilitated examinations and unambiguous verification of whether one or more fibers occupied the same region.
Examination of the labeled vagal fibers identified three distinct types of afferents terminating in three different tissues within the mucosa of the proximal duodenum and most distal stomach. Of the 211 afferents in the rat that satisfied the criteria for full assessment (see Materials and Methods), all the mucosal afferents appeared to be one or another of these three types. The same three afferent profiles also occurred in the fibers (n = 255) meeting the survey criteria in the mice. The samples of duodenal tissue surveyed were more extensive than the segments of antrum and, hence, yielded a larger sample of fibers. Thus, the afferent innervation of the duodenal mucosa is considered first, followed by an examination of the gastric mucosa.
Vagal villus afferents
One type of vagal afferent in the intestinal mucosa selectively innervated the apical or luminal pole of one villus (e.g., Fig. 1A) or, more commonly, the apical poles of two or more contiguous villi. These villus afferents passed from the mesenteric attachment through the longitudinal muscle into the myenteric plexus, where they coursed for short distances before turning to pass again “vertically” through the circular muscle layer into the submucosa. Within the submucosa, villus afferents might course a limited distance circularly and longitudinally, usually aborally, before then traveling to a location at the level of the muscularis mucosa deep to the submucosal poles of the crypts or intestinal glands (e.g., Fig. 2A,B). Immediately subjacent to the basal poles of the crypts, the villus fibers commonly divided into arbors of several collaterals oriented parallel to the long axes of the villi. These collaterals coursed between neighboring crypts, sometimes curving around the wall of a gland in the process, to enter and continue within their target villus (or villi). These villus fibers did not typically divide or give off terminal spurs in their trajectories through smooth muscle, myenteric plexus, and submucosa.
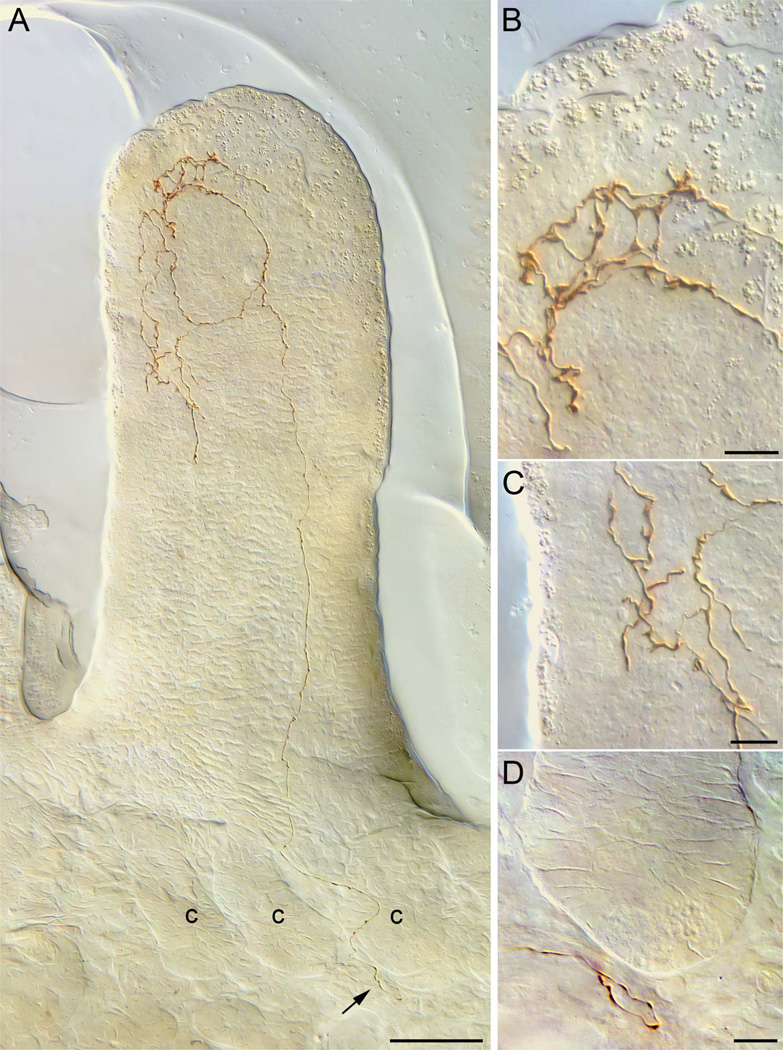
Figure 1.
A vagal villus afferent innervating a duodenal villus. A: A neurite of a villus afferent coursing through the submucosa and between intestinal crypts (lower right of panel) and then projecting to the apex of a villus (seen in transverse profile) to ramify in the apical half of the villus into a terminal plate (seen in side profile) at the basal side of the epithelial wall. B: A higher-power view of the apical terminal plate produced by the villus afferent seen in panel A. C: A higher-power view of terminal processes that the villus afferent in panel A distributes just deep to the basal pole of the epithelial wall of the villus. D: A higher-power view (area designated by arrow in panel A) of the trajectory of the villus afferent as it enters the tissue section (from left), courses near but without any tight apposition to the basal pole of crypt, and then reverses course and begins to ascend between two crypts (also labeled in panel A) on a path into the villus. c, crypt. Scale bars = 50 µm in A; 10 µm in B–D.
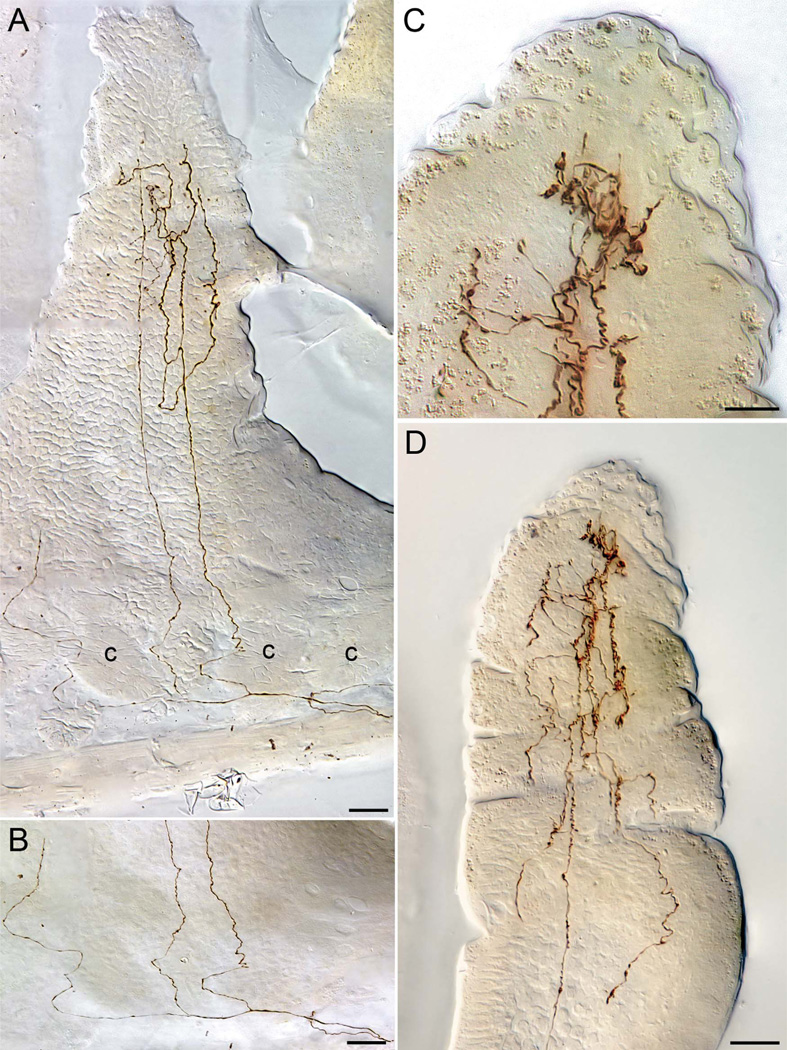
Figure 2.
Vagal villus afferents illustrating details of typical trajectories followed by these afferents. A: A low-power montage of a fiber coursing through the smooth muscle wall (extreme lower right of image), dividing at the point, and then issuing a secondary neurite that breaks into three collaterals directed into a cone-shaped villus. Two of the collaterals can be seen ascending to roughly the midpoint of the villus, where they begin to ramify into a terminal arbor. This terminal arbor is somewhat truncated in the present case, because the apical tip of this wedge- or cone-shaped villus is located in adjacent section, and the arbor that can be seen is located in a “shoulder” of the villus. (The third collateral, at the far left, has been interrupted, but continued into the apex of the villus on an adjacent tissue section. The fourth collateral, which separates even as it enters the submucosa, at the lower far right, courses out of plane to innervate a second villus on a neighboring section.) B: The base of the villus illustrated in panel A. In this illustration the DIC interference has been attenuated in order to give a clearer view of the pattern of division and the continuity of the collaterals of the villus afferent in the submucosal and crypt region of the mucosa. C,D: A villus afferent in the apical tip of a villus illustrating how individual afferent neurites course, without branching or with minimal branching (see bottom of lower power panel D), into the apical pole of a villus where they then ramify extensively to end in plates of varicosities as well as numerous distributed terminals (higher-power panel C). c, crypt. Scale bars = 25 µm in A,B; 10 µm in C; 20 µm in D.
From one to five or six of these collaterals from a parent neurite situated below the villi would course into a villus and travel towards its apical pole (e.g., Fig. 2A,B). As they passed through the submucosa, between crypts and into the base of a villus, the fibers tended to remain relatively smooth, with few dilations or varicosities or spurs. The major collaterals of the afferents also tended to remain relatively unbranched and simple (occasionally giving off minor spurs) in the basal third or half of the villus, the region sometimes referred to as the villus shaft (Figs. 1A, 2A,D). As the collaterals continued towards the intestinal lumen and into the apical half of the villus, however, the neurites began ramifying repeatedly so that the terminals produced an arbor of afferent terminals (e.g., Figs. 1A, 2D). In these arbors, the neurites typically formed numerous varicosities and swellings.
In transverse sections of the intestine that displayed villi in side profile, some fibers clearly traveled apically by coursing up along the epithelial walls in tight proximity to basal poles of the epithelial cells (e.g., Fig. 3B). Other afferent collaterals appeared to be situated more centrally, between the two side walls, in the lamina propria of the villus (e.g., Figs. 1A, 2B). Typically, and in fact in the examples illustrated in Figures 1A and 2B, although not always, when these collaterals that appeared to be located more centrally in the villus were examined systematically by focusing up and down through the z-dimensions of the tissue section, the seemingly central collaterals proved to be applied just subepithelially to either an out-of-focus villus wall located high in the section or a villus wall located deep within the specimen (for example, in both Figs. 1A, 2A, the “cobblestone” pattern in the villus wall seen with DIC reflects the basal poles of epithelial cells, illustrating the proximity of the ascending collaterals to the epithelium).
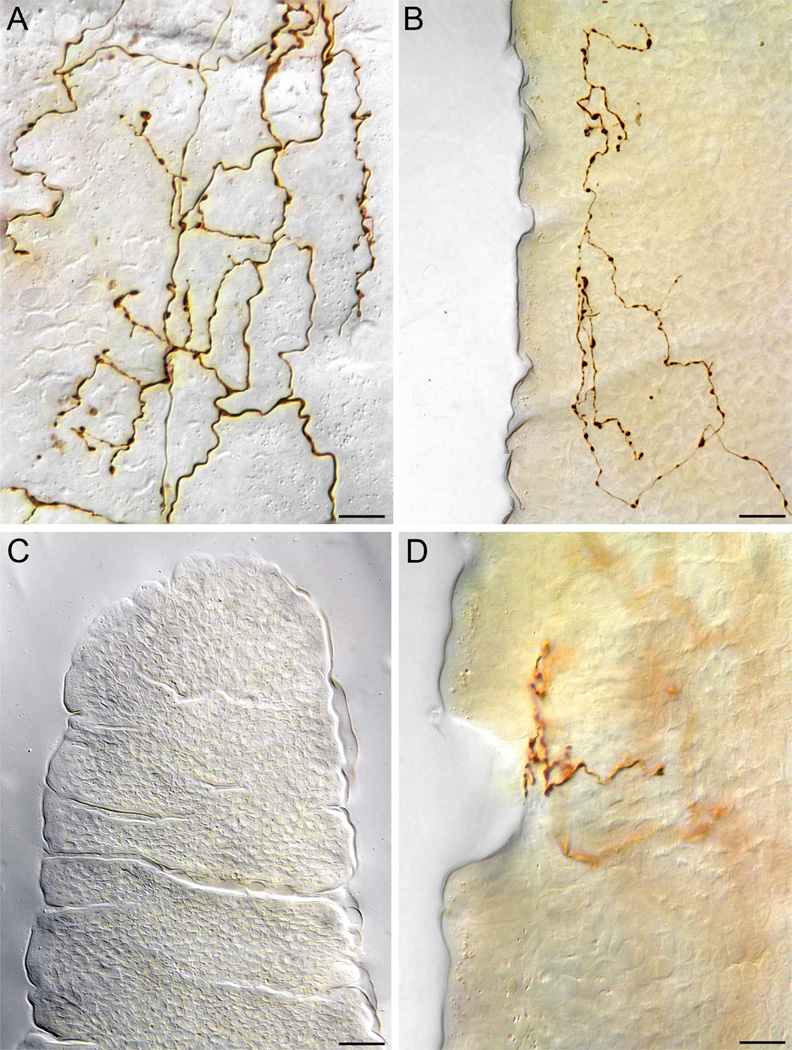
Figure 3.
Villus afferent termination patterns at high power. A: At higher power, from a perspective looking at the villus wall formed by the basal pole of epithelial cells (the “honeycomb” or “cobblestone” or “waffle” pattern that can be seen faintly in the DIC image is the typical pattern formed by the basal epithelium) the terminal plates of villus afferents consist of webs of varicosities and terminals. In side profile (i.e., parallel to the epithelial wall), depending on the magnification and depth of field as well as the density of the terminal plate, these endings can appear as relatively simply profiles (such as the one illustrated in panel B) or more tightly packed mats of terminals (such as the examples in Figs. 1B, 2C). B: A terminal of a villus afferent seen, in side perspective, coursing up from the lower right to terminate along the epithelial wall of a villus. C.D: Illustration of one pattern of villus afferent termination with potential functional implications. Panel C is a luminal surface view of a villus tip scanned in the plane of the brush border. As the image indicates, the epithelial wall is scored with trenches that have been discussed in terms of movement and mechanical forces which play on the villus. These trenches can also be seen in side profile in panel B. As panel D, a side view (same perspective as in panel B) of the villus wall, illustrates vagal villus afferent processes in some cases terminate at the trenches, thus potentially exposing the afferent to mechanical forces. To eliminate ambiguities that might be introduced by an extended depth-of-field projection, this image in panel D is a single optical plane. Scale bars = 10 µm in A,D; 25 µm in B,C.
In the apical tips of villi, the afferents formed loose webs of terminals that appeared to be in contact with the basal wall of the epithelium (Fig. 1B). Seen from a perspective perpendicular to the epithelium, these webs were created by repeated branching of the neurites as they reached the target site (Fig. 3A). In side profile, the webs appeared as plates or aggregates of terminals distributed along the epithelial wall (Figs. 1B, 2C,D, 3B). Within these webs or plates, after the last bifurcations of the neurites, the fibers frequently terminated in varicose dilations or end-bulbs (Figs. 1A, 2C, 3B). These dilations were typically distributed immediately subjacent to basal poles of epithelial cells. Notably, however, some of the terminal processes could also be seen extending especially close to the lumen in the clefts or furrows created by a shortening or thinning of the cells of the epithelium (cf. Fig. 3C,D).
The majority of individual villus afferents supplied collaterals into two or more neighboring villi. Of the 103 complete, intact, and strongly labeled villus fibers that were evaluated fully, the individual afferents innervated an average of 2.09 ± 0.14 villi (range: 1–7 villi). Most commonly, such afferents innervated contiguous villi, although 21% (i.e., 22 of 103 cases) of the afferents innervated one villus and then a second villus, one villus or more removed from the first. In straight-line distances, in the rat specimens the villus arbors spanned an average longitudinal, or oral-to-aboral, distance of 277.2 ± 26.3 lm (range: 50–1300 µm) and an average circular distance of 558.7 ± 53.9 µm (range: 30–3,360 µm). Because of the extensive increase in surface area provided by the evaginated villi, such linear or simple radial measures, however, considerably underestimate the receptive field of these endings.
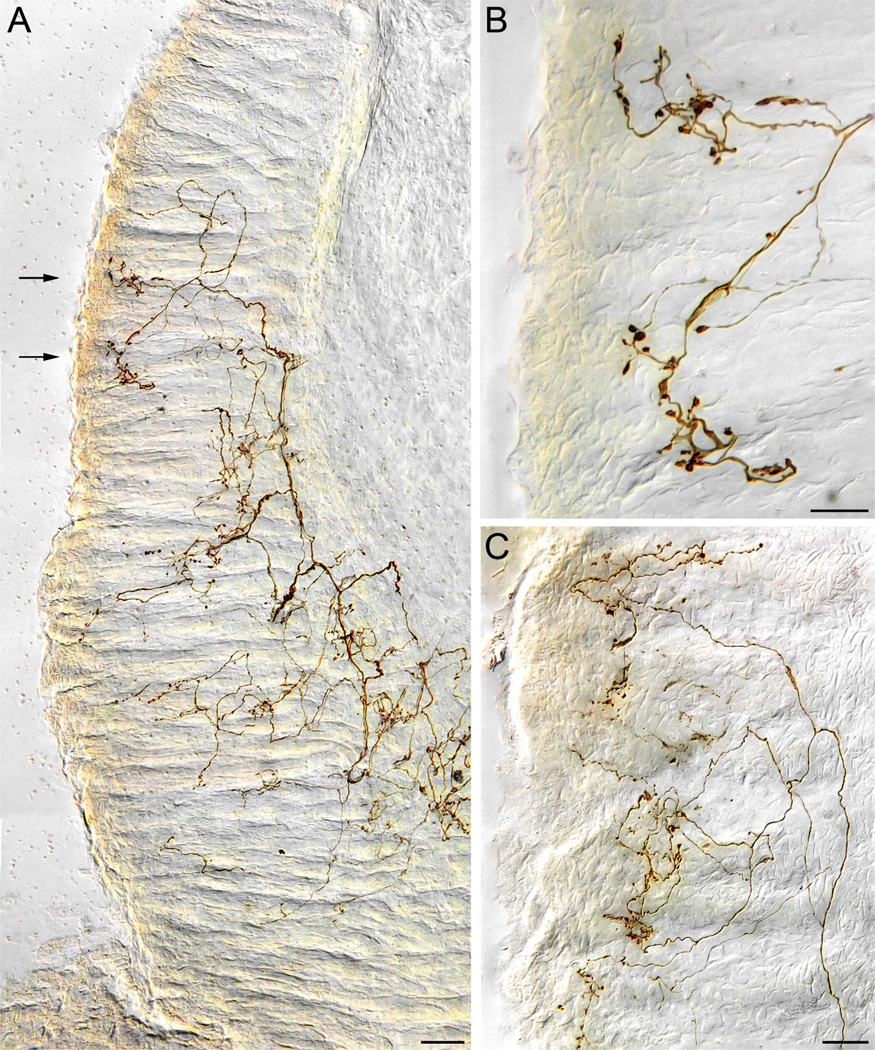
Vagal crypt afferents
A second population of vagal afferents to the duodenal mucosa projected selectively to the glands or crypts. Like villus fibers, these crypt-innervating afferents entered from the mesenteric attachment, penetrated the longitudinal muscle, traveled a short distance in the myenteric plexus, then traversed the circular muscle. The crypt fibers continued on through the submucosa without projecting to the submucosal plexus or to the other tissues in the region.
As the afferents approximated the basal poles of the crypts, however, the fibers divided into several collaterals that continued “upwards” (in the direction of the intestinal lumen) along the subepithelial side of the epithelial wall of a crypt. The individual collaterals then encircled a crypt, usually multiple times (Fig. 4). An individual collateral might repeatedly encircle a single crypt or two or more neighboring crypts. Through its collaterals, an individual vagal crypt fiber innervated an average of 3.16 ± 0.34 crypts (see Fig. 4A), with a range of 1–12 crypts.
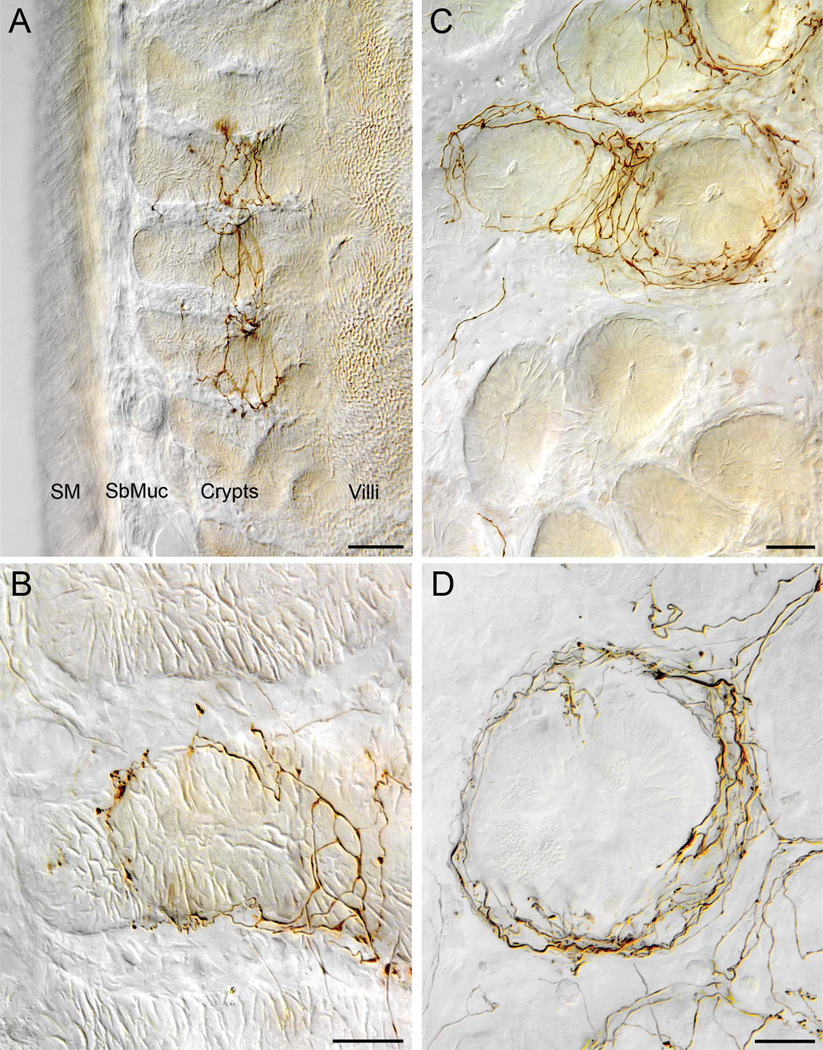
Figure 4.
Vagal crypt afferents. A: A low-power transverse section of the duodenal wall in which a crypt afferent ends encircling the necks (region immediately below the crypt-villus transition) of three adjacent crypts. B: A higher-power view, with the same orientation as that in panel A, of a crypt afferent innervating a single crypt. The fiber coils around the more basal region of the crypt, making some apparent contacts at that level, and then continues to the more luminal neck region of the crypt where it terminates more profusely. C: A crypt afferent innervating four adjacent crypts (upper part of panel) while ignoring other neighboring crypts (bottom of panel). In this view, a plane of view tangent to the circular muscle wall or at right angles with the view in panel A, the image is taken through the “neck” or region immediately below the crypt. The labeled crypt afferent can be seen repeatedly encircling the target crypts. D: A higher-power view of a crypt afferent encircling a single crypt (same orientation as the crypts in panel C). Additional elements of the neurite can be seen running on crypt-villus transitional floor (e.g., lower right) and forming rings around additional crypts (upper right; partially cropped and out of field). SbMuc, submucosa; SM, smooth muscle wall. Scale bars = 50 µm in A; 20 µm in B,D; 25 µm in C.
The terminal distributions of the crypt afferents were concentrated in the rings of processes around the necks or isthmuses of crypts just below the floor formed by the epithelium at the crypt-villus junction (Fig. 4A,B). The rings were comprised of relatively smooth neurites containing modest numbers of swellings or varicosities. The swellings, which had the appearance of en passage contacts, occurred in neurites throughout their circular paths and, perhaps most frequently, were situated in the furcations formed between the necks of neighboring crypts. Although neurites and their varicosities were most densely concentrated in the rings encircling the necks of crypts, approximations to the basal wall of the epithelium and potential contacts in the form of varicose swellings were also observed throughout the crypt region. To reach the necks of the crypts, the afferents typically spiraled up along the basal epithelial wall of the crypt (Fig. 4B), potentially making contacts—certainly forming varicosities—or appositions as they coursed apically. Also, at the crypt-villus junction, spurs, and collaterals sometimes left the tightly encircled rings of neurites at the crypt neck to travel along the basal side of the epithelial wall or the “floor” formed in the crypt-villus transition zone. Although the crypt afferents issued such extensions or spurs to other regions of the crypts, to other crypts, and to the epithelial floor of the crypt-villus junctions, the afferents did not collateralize into villi.
Independence of vagal villus afferents and crypt afferents
Given that the innervation pattern has implications for analyses of afferent function (see Discussion) and given that some investigators have assumed that individual fibers may innervate polytopically both villi and crypts, the possible independence of villus afferents and crypt afferents was evaluated two ways. First, as already described, we inventoried all the labeled fibers in our sample. We then identified from the inventory, for subsequent analysis, all isolated fibers that met a set of five criteria of labeling and completeness (see Materials and Methods). This qualitative survey suggested, as described immediately above, that the villus fibers and crypt fibers were distinctively different independent types of endings.
In addition, however, a formal analysis of the independence of the two categories was performed with a chi-square analysis. We evaluated all apparent villus fibers as to whether or not they also had a collateral(s) that encircled crypt(s) at the base of the villi, and we evaluated all apparent crypt ring fibers as to whether they also issued collaterals extending into an overlying villus. More specifically, we considered an afferent that innervated a villus to also innervate a crypt if the fiber or one of its collaterals encircled or wrapped fully (360°) one or more times around a crypt, and we judged an afferent that innervated a crypt to also innervate a villus if the fiber or one of its collaterals extended into the apical half of a villus.
Of the 103 afferents categorized as villus fibers, one afferent appeared to have a labeled neurite around an “underlying” crypt, and, of the 87 presumptive crypt fibers in the rat sample, six were possibly associated with collaterals entering an “overlying” villus. A chi-square of the contingency table demonstrated that the two types of fibers were reliably and strongly independent (χ2 [df = 1] = 171.3; P < 0.00001).
Similar results were obtained when we relaxed the five criteria for complete fibers to only four requirements (by dropping the criterion that fibers found at the oral and aboral limits of the tissue block would be excluded, even when those fibers otherwise appeared to be complete) and thus increased the neurite sample by about 25%. Of the enlarged sample, 127 villus fibers included one case possibly associated with a collateral to a crypt, and 111 crypt fibers included nine instances potentially associated with a villus (χ2 [df = 1] = 210.38; P < 0.00001).
The sample of mouse fibers reinforced the same conclusions. Of 238 villus afferents, two fibers also appeared to encircle a crypt as well, whereas of the 10 crypt fibers, one fiber appeared to issue a collateral into a villus. By a chi-square analysis, the two types of afferents were independent (χ2 [df = 1] = 187.46; P < 0.00001).
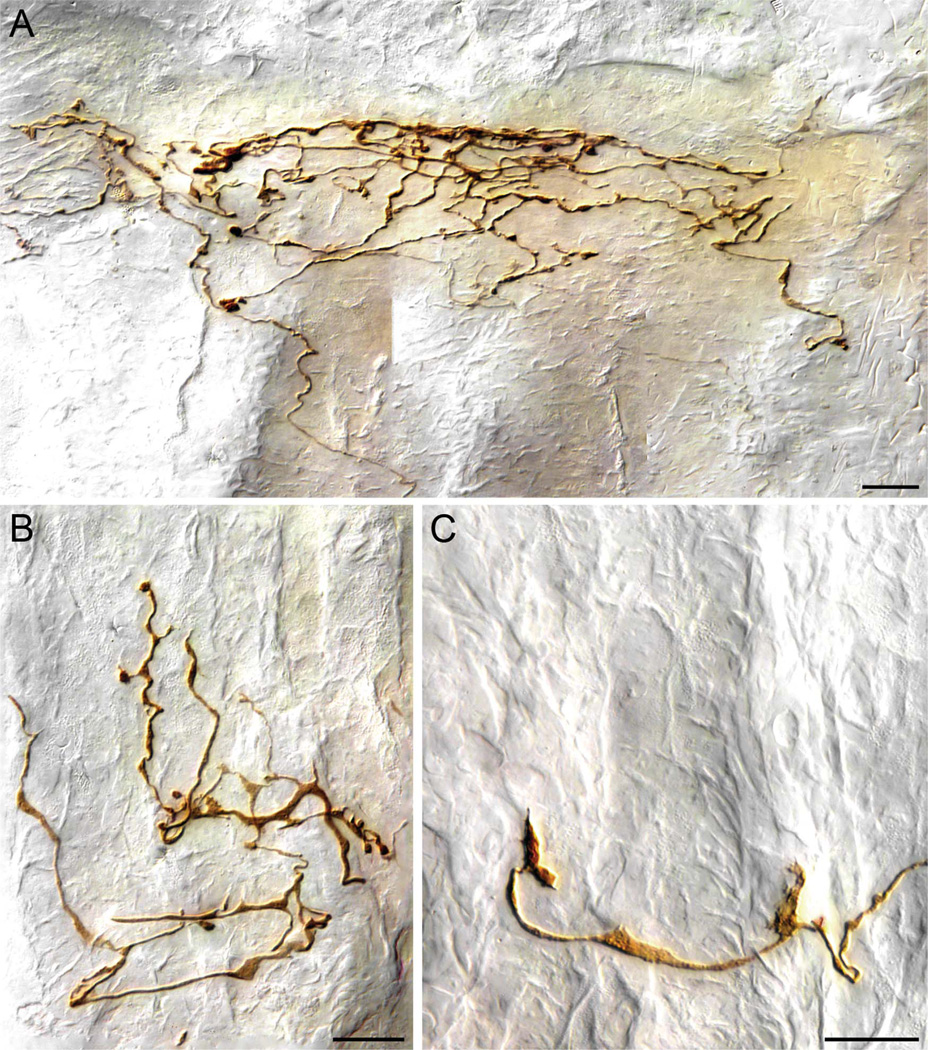
Vagal Antral Gland Afferent
The vagus also supplied a characteristic and distinctive type of afferent terminal to the gastric glands in the pyloric antral mucosa. These afferents passed through the smooth muscle wall of the antrum and then continued through the submucosa without collateralizing or ramifying. At about the level of the lamina muscularis mucosae, these fibers began to divide (Fig. 5A,C). The secondary processes traveled along the basal surface of the epithelial wall of the gastric glands, producing some apparent appositions on the gland walls as they coursed towards the gastric lumen. As the secondary fibers traveled along the epithelium, they continued to bifurcate repeatedly, creating “bushy” arbors that spread laterally or horizontally as they neared the luminal surface of the gastric gland mucosa. In transverse sections, many of the gastric gland arbors appeared to form plates or aggregates of terminals just below the epithelial surface of the antrum (see Figs. 5, 6A).
Figure 5.
Vagal antral gland afferents. A: A low-power view of part of the terminal arbor of an afferent innervating the gastric mucosa. One of two second-order collaterals of a vagal afferent fiber (entering from lower right corner of plate) distributes to form an arbor of higher order processes that travel along the glandular epithelial walls of the antral mucosa immediately adjacent to the antral lumen (far left of image). The collaterals tend to form varicose terminals and in some cases lamellar processes along the basal surfaces of the epithelial walls of the antral glands. On reaching the epithelium that constitutes the luminal surface, the collaterals often form aggregates of terminal varicosities and swellings. B: A higher-power image of two luminal aggregates of varicosities taken from the case illustrated in panel A (the area of the aggregates is designated with arrows in panel A). C: A lower-power image illustrating part of the arbor of terminals of a collateral of an antral afferent. In this case, the afferent collateral can be seen bifurcating in the lamina propria before coursing into the glandular mucosa to arborize. Scale bars = 50 µm in A; 20 µm in B; 25 µm in C.
Figure 6.
Vagal antral afferent terminals in the gastric mucosa. A: An antral afferent neurite travels (from bottom of panel) to arborize into a plate of terminals immediately below the luminal epithelium of the antrum (in this case, the lumen is just immediately above the top of the image, and the gastric glands are running vertically to secrete into the lumen). B: An aggregate of terminals of an antral afferent located within the glandular wall. In this example, and in all panels of this figure, the glands are oriented in a vertical direction, and the afferent terminals are distributed among the necks of the glands, not at the gastric lumen. As illustrated by this terminal, many of the deeper terminal endings of the antral afferents–particularly those elements of the neurite that coursed laterally or at right angles to the length of the gastric glands–were commonly lamelliform or flattened in appearance. C: An example of the lamellar or growth-cone shaped terminals often formed by the antral afferents. Scale bars = 20 µm in A; 15 µm in B,C.
Like both the villus afferents and the crypt afferents already described, the terminals comprising gastric gland arbors consisted of neurites that contained varicose swellings or dilations. The terminal arbors of antral afferents did, however, differ in appearance from those observed in association with either villi or crypts. Most distinctively, antral afferent terminal arbors tended to have multiple lamellar flattenings along the terminal segments of the neurites. These lamellar elements of the antral afferents were particularly prevalent along the length or the necks of the gastric glands (Fig. 6B,C). The neurites typically ended in prominent terminal varicose bulbs, and both lamella-like flattenings and varicosities intermingled in the terminal apparatus of the antral afferents.
Vagal afferents in antral mucosa were independent of both villus afferents and crypt afferents
As might be inferred from the fact that antral endings are for the most part found proximal to the intestinal mucosa, it seemed likely that the antral gland afferents were independent of both the crypt afferents and the villus afferents, and the conclusion was buttressed by the initial qualitative survey. However, because there is a transitional zone in the most proximal duodenum in which antral and intestinal mucosa abut and intermingle and also because vagal fibers can be found running from antrum to pylorus, a quantitative analysis of independence was also computed. Of 103 rat villus afferents analyzed, six potentially issued collaterals to antral mucosa. Of 87 crypt afferents examined, one appeared to be associated with an antral afferent arbor. Chi-square tests of the contingency tables indicated that the antral arbors examined were statistically independent of both villus afferents (χ2 [df = 1] = 71.04; P < 0.00001) and crypt afferents (χ2 [df = 1] = 97.86; P < 0.00001). The pattern was once again corroborated by the observations on the mouse vagal afferents.
Comparisons of rat and mouse afferents
Although full analyses of the patterns in mice and then quantitative comparisons across the two rodent species would require material from a larger sample of mice—with sampling of the right nodose afferents too—in the present survey we did not observe any qualitative differences in innervation patterns between the two afferent types in the two rodent species examined. The same three distinct types of afferents were observed in both rats and mice, and in both species the three types appeared to comprise the entire population of mucosal terminals.
Two minor apparent differences appeared between the rat and mouse vagal afferent samples, although the differences may well reflect somewhat different sampling biases. First, somewhat more vagal afferent fibers were observed in the smaller sample of mice than in the larger group of rats. This may reflect in whole or in part some combination of the facts that 1) there was no simple means of making strictly equivalent injections (in either location or in volume) in the nodose ganglia of rats and mice; and 2) the 1.5-cm block of duodenum sampled in the two species represents a proportionately greater fraction of the full length of mouse duodenum compared to the rat duodenum. Alternatively, of course, a species difference in the innervations densities cannot be excluded. Second, although both species had the same three—and only three—categories of afferent fibers in the mucosa, the ratio of villus afferents to crypt afferents was greater for the rats than the mice. This apparent difference might signal either i) that more crypt fibers originate in the right nodose ganglion, which was sampled in rats but not in mice; or ii) a quantitative species difference.
One practical difference between the rat and mouse samples is worth noting, however. The present analyses of the two species were done with relatively thick (100 lm) sections in order to facilitate following the labeled afferent fibers through the three dimensions of the gut wall. Given the more compact dimensions of the mouse GI tract, this meant that proportionately more of an individual villus and villus arbor might appear in a single section of the mouse GI tract than of a rat GI tract. For example, entire villi would often appear in a single section of mouse material (cf. Fig. 1), but a villus would routinely span multiple 100-µm sections in the rat. Similar condensations of the crypt endings and antral mucosal endings also occurred in the mice. Such differences of scale were to be expected, but nonetheless the more complete terminals seen in a single 100-µm section of mouse GI tract proved useful for surveying and photographing different aspects of the afferent innervation.
DISCUSSION
The present observations indicate that the vagus supplies three separate and independent types of afferent endings to the mucosa of the proximal GI tract. Additionally, the observations delineate features of these three types of terminals that should help focus questions related to the physiology of vagal afferents to the gut.
Before discussing different implications in terms of the three types of afferents, it is appropriate to consider two related sets of issues. The first is whether the three classes of endings are independent. Such independence is a prior issue since the answer to that question determines the number of types of afferent specializations and also affects considerations of how the electrophysiological and other functional analyses might be mapped onto the architectures of the different afferents. The second issues concern an assessment of the limitations of the present protocol and afferent survey.
Independence of villus, crypt, and antral afferent projections
In the present analysis, which is the first structural study of vagal projections to the mucosa to use both specific as well as limited labeling and a statistical assessment of independence, different tissues were found to be innervated by different afferents, not by collaterals from common fibers. Not surprisingly, given their distinct regional distributions, the afferents to the antral mucosa were independent of the two types of afferents to the proximal intestinal mucosa. More controversially, however, the two types of endings in intestinal mucosa were also essentially independent and were not issued by the same afferents.
The issue of this independence is a long-standing question. The ambiguity likely stems, in part, from the proximity and physical arrangements of crypts and villi. Since afferent collaterals projecting into villi pass between crypts, discriminating whether villus and crypt fibers are separate and independent will, perforce, be difficult. As early as 1893, Berkley suggested, although his Golgi staining was not limited to the vagus, that nerve networks around crypts (his “Lieberkuhnian sub-plexus”) were independent of the networks in the villi (his “villous subplexus”). Other investigators, generally relying on techniques that indiscriminately label many or all neurites, however, have assumed that some plexuses associated with crypts interconnected with those in the villi (see, for example, Stach and Hung, 1979; Keast, 1987, for historical review; Li, 2007).
More recently, though, Berthoud et al. (1995) used DiI to selectively label vagal afferents. While these investigators did not attempt to reconstruct individual afferents in the mucosa, they hypothesized, in a summary schematic, that single afferents innervate both villi and crypts (cf. their fig. 5). The present observations suggest that such an inference is likely incorrect and that, at least in the case of vagal afferents, Berkley’s suggestion that crypts and villi are innervated independently is correct.
The possibility that a small percentage of hybrid polytopic fibers also occur cannot be completely excluded, however. Indeed, roughly 4% of the afferents we surveyed met our criteria of having collaterals both encircling crypts and innervating villi. Given that we had adopted a priori and arbitrary criteria to classify endings, some inaccuracies or imprecisions might be expected, and the small minority of fibers we classified as “hybrid” may only be false-positive cases. Alternatively, one might argue that the subset of fibers were in fact hybrids and that our criteria may have produced a complementary set of false-negative assignments.
Sampling and survey limitations
Although the present survey revealed three—and only three—types of vagal afferents in the mucosa of the antrum and proximal duodenum, the labeling protocol and the tissue samples analyzed could have failed to reveal other vagal endings in the mucosa. We are unaware of any demonstration that a class of nodose fibers is refractory to labeling with dextran, but it is fully conceivable that one or more species of vagal afferent fails to incorporate the dextran we used. In addition, more comprehensive characterizations of the three types of afferent endings that we observed might always distinguish subcategories, and thus a larger number of afferent types, based on their specific neurochemical phenotypes or on their accessory tissues. Importantly too, our survey was limited to the distal antrum and proximal duodenum, hence the mucosa of the more rostral GI tract and/or the more distal bowel might be innervated by types of afferents that are not observed in the proximal gut tissues we examined. With such provisos in mind, it is still instructive that, in the entire sample of rat and mouse afferents that met the criteria for tracing individual dextran-labeled fibers from the muscle layers to their mucosal targets (n > 400), all neurites were one (or very rarely hybrids of two) of the three types of terminals described in the present experiment. It is also worth noting that all of the less optimally labeled afferents that we observed in the initial screening, i.e., those that did not individually satisfy all five of the sampling criteria for complete and well labeled endings (n ∼1,200), appeared to sort into the same three terminal specializations we have described. It could be argued, however, that a particularly rare type of afferent to the mucosa was missed, even with the sample of several hundred fibers we traced and analyzed thoroughly or the still larger sample of endings that we surveyed more qualitatively.
Vagal villus afferents
The vagal afferent innervation of the duodenal villi consists of individual fibers that divide into several collaterals near the submucosal/mucosal transition. As the collaterals course towards the villi, they climb and curve between crypts. Within a villus, these collaterals then course from the base to the apex, typically running along the interior wall formed by the basal poles of epithelial cells. The villus afferents arborize repeatedly in the apical half of the villus to create terminal aggregates of puncta immediately subjacent to the epithelium of the villus tip. One afferent villus fiber typically innervates a cluster of two or more neighboring villi. Presumably, the receptive field of the fiber would correspond to the patch of adjacent villi into which its collaterals course.
The distribution of the vagal afferent collaterals within a villus provides suggestions as to the factors that may control their organization and the stimuli to which they may be sensitive. The fact that the afferent collaterals tend to course along the epithelial walls and to ramify and distribute primarily in the apical end of the villus means that their terminals are distributed in proximity to the basal poles of enterocytes and other epithelial cells. Epithelial tight junctions and the composition of the lamina propria block, of course, simple diffusion from the intestinal lumen, but neural sensitivity to paracrine factors released by epithelial events might provide mechanisms through which the afferents could indirectly detect chemical or mechanical information about the nutrient handling of the intestines. The physiological evidence that serotonin (5-HT), histamine, cholecystokinin (CCK), and various other neuropeptides and hormones are involved in the sensory transduction of at least mucosal chemoreceptors (Raybould, 1999; Li et al., 2001; Kreis et al., 2002; Glatzle et al., 2003; deFonseka and Kaunitz, 2009) is consistent with such a conclusion. Also, the expression of the taste receptor molecules and transduction peptides in the epithelial wall (e.g., Sbarbati and Osculati, 2005; Margolskee et al., 2007) provide candidate mechanisms for transducing nutrient signals and potentially indirectly activating the villus afferents. Given the concentrations of afferent terminals in the apex of the villus, transduction possibilities might also be facilitated by the fact that the basal lamina of the epithelial layer in the apical two-thirds of the villi is characterized by many fine fenestrations (Komuro and Hashimoto, 1990).
Additionally, our observation that vagal villus afferents often terminate in the floor of the trenches or clefts in the epithelial wall (see Fig. 3C,D) underscores the potential of the afferents to access mechanical as well as paracrine signals. Mechanically, the clefts are thought to serve as sites of flexure facilitating changing villous length (cf. Krstic, 1997), and thus should be strategic loci for monitoring displacements or movements of villi.
Vagal crypt or gland afferents
Of the three afferent specializations observed in the present experiments, crypt afferents are the most problematic to correlate, even provisionally, with previous electrophysiological observations. In fact, it is arguable that these endings may not have been described electrophysiologically. Limited batteries of mechanical and chemical stimuli have been used in past electrophysiological analyses of proximal duodenal afferents, and it is conceivable that the batteries may have failed to include adequate stimuli for activation of the vagal afferents encircling crypts. The earlier neurophysiology typically reported a percentage of “unresponsive” and/or “silent” units (e.g., Cottrell and Iggo, 1984a,c), and such fibers could, conceivably, be crypt afferents that had not been effectively activated by the stimuli employed.
The architecture and location of crypt afferent terminals may offer significant clues as to their functional roles (cf. Merchant, 2007). Some observations would suggest that the afferents might monitor and perhaps contribute to the regulation of secretory activity of the crypts (i.e., intestinal glands). One such feature is the finding that the vagal crypt afferents selectively encircle the most luminal end of the crypt where secretion occurs. Along its length, from base to apex, the epithelial wall of the crypt consists of different maturational and functional domains. At its base, close to the submucosa, the wall is populated with immature stem cells and Paneth cells. The mid-region of the crypt wall consists of a proliferative zone in which additional cells forming the mucosal wall are produced and begin to differentiate. The “apical” or luminal third of the crypt wall near the crypt-villus transition zone that the vagal crypt afferents preferentially target is formed of mature postmitotic epithelium and secretory enterocytes.
This distinctive gradient raises the possibility that the vagal afferents play a role in relaying paracrine or neurocrine signaling. Many of the gut peptides and hormones are elaborated by enterocytes in the epithelial wall and appear to have paracrine as well as conventional endocrine effects. For example, CCK-producing enterocytes (I-cells) are found predominately in this region near the orifice of the crypt (Buffa et al., 1976) and seem to have, inter alia, paracrine effects (e.g., Cottrell and Iggo, 1984b; Randich et al., 2000). Furthermore, vagal afferents express receptors for many of the gut peptides, including CCK (Moran and Ladenheim, 1998; Li, 2007). In relevant experiments, Richards et al. (1996) and Blackshaw and Grundy (1990) concluded that a specific subset of vagal afferents projecting to the intestinal mucosa were directly sensitive to close-arterial infusion of CCK, whereas other intestinal fibers as well as gastric vagal afferents responded only indirectly to CCK. Although there is not direct evidence as to whether those afferents that we have observed innervating the intestinal glands specifically express receptors for CCK (and other relevant gut peptides and hormones) as well as whether these vagal crypt fibers constitute the subpopulation distinguished by Richards et al. and by Blackshaw and Grundy, it is a plausible possibility.
The observation that some Paneth cells and possibly other epithelial cells in the crypts express molecular taste receptors and signaling molecules (Mace et al., 2009; rat jejunum) underscores the possibility that crypt afferents might operate as chemoreceptors. If the afferents innervating the crypts are chemosensitive fibers able to indirectly monitor the contents of the GI tract, their locations below the epithelial floor at the base of the villi, plus the limited space between villi, plus the still more limited spaces in the long (≈500–800 µm) narrow (≈20 µm diameter) lumens of the glands (and the fact that any chemical stimulus would need to diffuse against the flow of the succus entericus), would all suggest that the crypt endings would have long latency responses or would integrate over protracted events. If the afferents are mechanosensitive fibers affected by the contents of the GI tract (cf., for example, Cottrell and Iggo, 1984a,c), their location and organization would suggest that they may have higher thresholds than any mechanoreceptors situated at the tips of the villi.
Although the biophysics of the crypt and villus are incompletely understood (cf. Hosoyamada and Sakai, 2005), the distribution of the crypt afferents’ terminals situated around the glands suggests other possibilities as well. For example, since the afferents are located near fibers of the muscularis mucosa, they may well be deformed in the process of villous contractions. Or, for another example, Paneth cell defensin secretion and other secretory processes of the intestinal glands are affected by cholinergic stimuli, so it is conceivable that crypt afferents participate in the mobilization of vagovagal secretory reflexes. Finally, it is recognized that intestinal adaptation to diets varying in nutrients involves a reprogramming of crypt cells to express appropriate numbers of the different transporter systems and that vagal afferents have been implicated in this crypt reprogramming (e.g., Bates et al., 1998).
Vagal antral mucosal afferents
The vagal afferent innervation of the antral mucosa consists of fibers that pass through the submucosa to the level of the muscularis mucosa, where the neurites then arborize extensively. Starting from the parent neurite, a “bushy” arbor is formed through the repeated division and collateralization as the higher-order collaterals travel along the basal side of the epithelium towards the luminal surface of the mucosal layer. As the terminal arbors arrive at the apical epithelium of the antral mucosal wall, they form mats or plates of endings and puncta immediate below the epithelium.
Identification of this type of ending with electrophysiologically characterized afferent profiles must remain provisional, but it is worth noting 1) that the morphological features observed in the present experimental series appear to map readily onto the initial electrophysiological descriptions of gastric mucosal afferents; and 2) the fact that only one type of afferent structure has been routinely observed in the mucosa would suggest that it must be the feature responsible for the recorded sensibilities.
Comprehensive neurophysiological analyses of the sensitivities of these afferents remain to be done, but the available electrophysiological characterizations seem consistent with the morphology. In one analysis, Clarke and Davison (1978) described 34 vagal afferents projecting to the gastric mucosa of the rat. These fibers were sensitive to both chemical stimulation of varying pH and light mechanical probing—but not the deeper tension and stretch stimulations that activated afferents in the smooth muscle wall. The same fibers appeared to operate as rapidly adapting mechanoreceptors presumably organized to detect the presence and passage of material in the lumen of the organ and slowly adapting chemoreceptors that would be able to detect acid-base (and possibly osmotic) features of chyme.
Vagal afferents to gastric mucosa with similar functional characteristics have also been described in cats (Iggo, 1957) and sheep (Harding and Leek, 1972). Other details of the electrophysiology in these species are also consistent with the morphological features of the afferents labeled in the present experiment. In a striking convergence with the “bushy” arbor patterns the vagal mucosal afferents formed in the present rodent series (see Figs. 5A,C, 6A), Iggo (1957), from his dissections and scrapings of the mucosal wall during recording experiments, concluded with prescience that a “single axon innervates” its receptive field “by sending collateral fibres into the superficial mucosa from a main trunk which ramifies in the deeper layers of the mucosa.” Finally, the collections of afferent terminals into plates immediately below the gastric mucosal surface epithelium in immediate contact with the contents of the organ are situated so that diffusion and transport times would be minimal, thus perhaps explaining the very short latencies observed for chemoreceptor responses (e.g., ≈1 second in the rat: Clarke and Davison, 1978).
Developing an inventory of vagal afferents
The prospect of a comprehensive inventory of vagal afferents to the GI tract has only recently begun to take form (e.g., Costa et al., 2004; Powley and Phillips, 2005), and such a complete survey is not yet available. Surveys of the smooth muscle wall of the gut, from the esophagus to colon (e.g., Wang and Powley, 2000) have commonly recognized two classes of vagal afferents in the tunica muscularis. One, the intraganglionic laminar ending, is associated with myenteric ganglia, is found from esophagus to colon, and appears to operate as mechanoreceptor detecting tension (Phillips and Powley, 2000; Zagorodnyuk et al., 2001). The second smooth muscle ending, the intramuscular array, is found running in smooth muscle in association with interstitial cells of Cajal; although it is uncertain whether these afferents have been adequately sampled in electrophysiological analyses, they are presumed to be mechanoreceptors and have been hypothesized to serve as stretch receptors (see discussions in Phillips and Powley, 2000; Zagorodnyuk et al., 2001).
Less information and consensus has been available for mucosal afferents. However, the present survey of the mucosa, in conjunction with early preliminary assessments of neurites in the region (e.g., Berkley, 1893; Hill, 1927; Stach and Hung, 1979; Powley et al., 1994; Berthoud et al., 1995), suggests that three distinctly different afferent specializations (i.e., the vagal villus afferent, the vagal crypt afferent, and the vagal antral gland afferent) can be found in the mucosa of the GI tract. When combined with the previously described smooth muscle afferents, these three classes of mucosal afferents would bring the number of different vagal afferent types in the GI tract to at least five.
Since both the present survey of the proximal duodenum and pyloric antrum and the earlier preliminary assessments of mucosa were also done with limited segments of the GI tract, it is as yet unclear whether the three specializations constitute a complete set of types of vagal afferents in the mucosa, or, alternatively, whether additional specializations in the gut await identification through examinations of the GI tract either oral or aboral to the antrum and initial segment of the duodenum (see also the consideration of our sampling strategy earlier in the discussion). To this point, it is instructive that Berkley’s Golgi observations (dog, mouse; Berkley, 1893) which would seem consistent with the present observations were obtained more distally, in the ileum. At any rate, however, the present experiment and other recent tracer studies of the mucosa, as well as similar examinations of vagal afferents to GI smooth muscle, indicate the practicality of producing more definitive and complete inventories of the vagal—as well as other visceral afferent—specializations in the gut.
CONCLUSION
The present series of observations, designed to extend earlier experiments identifying tracer-labeled vagal afferents in the mucosa of the proximal GI tract, characterizes three distinctive types of vagal afferents in the mucosa, each distinguished by a specialized terminal architecture. In conjunction with the two previously described and widely recognized afferent specializations found in the smooth muscle coat of the proximal GI tract, the mucosal endings described in the present report bring the inventory of distinct vagal afferent specializations in the gut to at least five.
ACKNOWLEDGMENTS
We thank Elizabeth Baronowsky (dextran injections) and Dr. Nazim Anwar (embedding and sectioning) for their exceptional and skilled help. In addition, we thank Dr. Robert Phillips for expert assistance with photomicrography and figure production. The article was prepared by the first two authors, owing to the untimely death of S.A.H.; his commitment to and tireless work on the project, including the development of the protocol, dictated that he be considered a coauthor.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: DK27627, DK61317, HD05112.
LITERATURE CITED
- Bates SL, Sharkey KA, Meddings JB. Vagal involvement in dietary regulation of nutrient transport. Am J Physiol. 1998;274:G552–G560. doi: 10.1152/ajpgi.1998.274.3.G552. [DOI] [PubMed] [Google Scholar]
- Berkley HJ. The nerves and nerve endings of the mucous layer of the ileum, as shown by the rapid Golgi method. Anat Anz. 1893;8:12–19. [Google Scholar]
- Berthoud H-R, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive enteroendocrine cells in the rat small intestinal mucosa. Acta Anat (Basal) 1996;156:123–131. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 4th ed. vol. 1. Burlington, MA: Elsevier Academic Press; 2006. pp. 685–725. [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Buffa R, Solcia E, Go VL. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology. 1976;70:528–532. [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol. 1978;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Brookes SH, Zagorodnyuk V. How many kinds of visceral afferents? Gut. 2004;53(Suppl 2):ii1–ii4. doi: 10.1136/gut.2003.033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984a;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. The responses of duodenal tension receptors in sheep to pentagastrin, cholecystokinin and some other drugs. J Physiol. 1984b;354:477–495. doi: 10.1113/jphysiol.1984.sp015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J Physiol. 1984c;354:497–522. doi: 10.1113/jphysiol.1984.sp015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuche G, Blat S, Malbert CH. Desensitization of ileal vagal receptors by short-chain fatty acids in pigs. Am J Physiol. 2001;280:G1013–G1021. doi: 10.1152/ajpgi.2001.280.5.G1013. [DOI] [PubMed] [Google Scholar]
- deFonseka A, Kaunitz J. Gut sensing mechanisms. Curr Gastroenterol Rep. 2009;11:442–447. doi: 10.1007/s11894-009-0068-5. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittle TT, Tso P, Wei J, Raybould HE. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550(2):657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J Auton Nerv Syst. 1988;22:175–180. doi: 10.1016/0165-1838(88)90104-x. [DOI] [PubMed] [Google Scholar]
- Grundy D, Scratcherd T. Sensory afferents from the gastrointestinal tract. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of physiology Sect 6: The gastrointestinal system. Vol. I: Motility and circulation, Part 1. Bethesda, MD: American Physiological Society; 1989. pp. 593–620. [Google Scholar]
- Harding R, Leek BF. Gastro-duodenal receptor responses to chemical and mechanical stimuli, investigated by a ‘single fibre’ technique. J Physiol. 1972;222 139P–140P. [PubMed] [Google Scholar]
- Hill CJ. A contribution to our knowledge of the enteric plexuses. Philos Trans R Soc Lond B Biol Sci. 1927;215:355–387. [Google Scholar]
- Hosoyamada Y, Sakai T. Structural and mechanical architecture of the intestinal villi and crypts in the rat intestine: integrative reevaluation from ultrastructural analysis. Anat Embryol (Berl) 2005;210:1–12. doi: 10.1007/s00429-005-0011-y. [DOI] [PubMed] [Google Scholar]
- Iggo A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957;42:398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Keast JR. Mucosal innervation and control of water and ion transport in the intestine. Rev Physiol Biochem Pharmacol. 1987;109:1–59. doi: 10.1007/BFb0031024. [DOI] [PubMed] [Google Scholar]
- Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa) Arch Histol Cytol. 1990;53:1–21. doi: 10.1679/aohc.53.1. [DOI] [PubMed] [Google Scholar]
- Kreis ME, Jiang W, Kirkup AJ, Grundy D. Cosensitivity of vagal mucosal afferents to histamine and 5-HT in the rat jejunum. Am J Physiol. 2002;283:G612–G617. doi: 10.1152/ajpgi.00206.2001. [DOI] [PubMed] [Google Scholar]
- Krstic RV. Human microscopic anatomy, corr. 3rd printing. Berlin: Springer; 1997. pp. 206–207. [Google Scholar]
- Li Y. Sensory signal transduction in the vagal primary afferent neurons. Curr Med Chem. 2007;14:2554–2563. doi: 10.2174/092986707782023334. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, Foley D. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587:195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N. Intestinal chemosensitivity. Physiol Rev. 1985;65:211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst. 1986;16:159–170. doi: 10.1016/0165-1838(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Merchant JL. Tales from the crypts: regulatory peptides and cytokines in gastrointestinal homeostasis and disease. J Clin Invest. 2007;117:6–12. doi: 10.1172/JCI30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Ladenheim EE. Identification of receptor populations mediating the satiating actions of brain and gut peptides. In: Smith GP, editor. Satiation: from gut to brain. New York: Oxford University Press; 1998. pp. 126–163. [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Palay SL, Karlin LJ. An electron microscopic study of the intestinal villus. I. The fasting animal. J Biophys Biochem Cytol. 1959;5:363–372. doi: 10.1083/jcb.5.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Advances in vagal afferent neurobiology. Boca Raton, FL: CRC Press; 2005. pp. 123–145. [Google Scholar]
- Powley TL, Holst M-C, Boyd DB, Kelly JB. Three-dimensional reconstructions of autonomic projections to the gastrointestinal tract. Microsc Res Tech. 1994;29:297–309. doi: 10.1002/jemt.1070290407. [DOI] [PubMed] [Google Scholar]
- Randich A, Tyler WJ, Cox JE, Meller ST, Kelm GR, Bharaj SS. Responses of celiac and cervical vagal afferents to infusions of lipids in the jejunum or ileum of the rat. Am J Physiol. 2000;278:R34–R43. doi: 10.1152/ajpregu.2000.278.1.R34. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol. 1999;277:G751–G755. doi: 10.1152/ajpgi.1999.277.4.G751. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J Physiol. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Koyano H. Autoradiographic study on the distribution of vagal afferent nerve fibers in the gastroduodenal wall of the rabbit. Brain Res. 1987;400:101–109. doi: 10.1016/0006-8993(87)90657-3. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Stach W. Über die Nervengeflechte der Duodenalzotten. Acta Anat (Basel) 1973;85:216–231. [PubMed] [Google Scholar]
- Stach W, Hung N. Zur Innervation der Dünndarmschleimhaut von Laboratoriumstieren. I. Architektur, lichtmikroskopische Struktur und histochemische Differenzierung. Z Mikrosk Anat Forsch. 1979;93:876–887. [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SH. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]