Abstract
Introduction
To facilitate the clinical translation of 18F-fluoroacetate (18F-FACE), the pharmacokinetics, biodistribution, radiolabeled metabolites, radiation dosimetry, and pharmacological safety of diagnostic doses of 18F-FACE were determined in non-human primates.
Methods
18F-FACE was synthesized using a custom-built automated synthesis module. Six rhesus monkeys (three of each sex) were injected intravenously with 18F-FACE (165.4± 28.5 MBq), followed by dynamic positron emission tomography (PET) imaging of the thoracoabdominal area during 0–30 min post-injection and static whole-body PET imaging at 40, 100, and 170 min. Serial blood samples and a urine sample were obtained from each animal to determine the time course of 18F-FACE and its radiolabeled metabolites. Electrocardiograms and hematology analyses were obtained to evaluate the acute and delayed toxicity of diagnostic dosages of 18F-FACE. The time-integrated activity coefficients for individual source organs and the whole body after administration of 18F-FACE were obtained using quantitative analyses of dynamic and static PET images and were extrapolated to humans.
Results
The blood clearance of 18F-FACE exhibited bi-exponential kinetics with half-times of 4 and 250 min for the fast and slow phases, respectively. A rapid accumulation of 18F-FACE-derived radioactivity was observed in the liver and kidneys, followed by clearance of the radioactivity into the intestine and the urinary bladder. Radio-HPLC analyses of blood and urine samples demonstrated that 18F-fluoride was the only detectable radiolabeled metabolite at the level of less than 9% of total radioactivity in blood at 180 min after the 18F-FACE injection. The uptake of free 18F-fluoride in the bones was insignificant during the course of the imaging studies. No significant changes in ECG, CBC, liver enzymes, or renal function were observed. The estimated effective dose for an adult human is 3.90–7.81 mSv from the administration of 185–370 MBq of 18F-FACE.
Conclusions
The effective dose and individual organ radiation absorbed doses from administration of a diagnostic dosage of 18F-FACE are acceptable. From a pharmacologic perspective, diagnostic dosages of 18F-FACE are non-toxic in primates and, therefore, could be safely administered to human patients for PET imaging.
Keywords: 18F-Fluoroacetate, Positron emission tomography, Radiation dosimetry, Toxicology, Non-human primate
Introduction
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) has become a widely used molecular imaging method for diagnosis and staging of several tumor types and for monitoring the efficacy of different anti-cancer therapeutic interventions [1–4]. Examples of 18F-FDG-avid tumor types include cancers of the head and neck, lung, breast, and malignant lymphoma. For these, 18F-FDG PET has been accepted as a standard method of imaging and approved for procedure reimbursement. Increased uptake of 18F-FDG in many types of tumors is due to the so-called Warburg effect [5], resulting from the oncogenic signaling-induced shift of tumor metabolism toward anaerobic glycolysis even under normoxic conditions [6, 7].
However, several tumor types do not accumulate 18F-FDG at higher levels than the surrounding normal tissues, which makes the application of 18F-FDG PET imaging for their diagnosis, staging, and monitoring of therapeutic interventions futile. Examples of such “18F-FDG-nonavid” tumor types include prostate carcinoma [8], renal cell carcinoma [9, 10], hepatocellular carcinoma [11], and gastric cancers [12]. Furthermore, diagnostic PET imaging of some 18F-FDG-avid tumors can be challenging because of their locations in tissues (organs) having high intrinsic glycolytic activity, such as brain gliomas [13]. 18F-FDG-nonavid tumors represent a large fraction of malignancies for which the development and clinical translation of alternative PET imaging agents is urgently needed [14].
One such alternative agent is 11C-labeled acetate (11C-ACE). In the past two decades, 11C-ACE has been used mainly to study myocardial metabolism [15–18]. In this application, the correlation of tracer washout from the myocardium to the rate of myocardial oxygen consumption has been established. It was shown that 11C-CO2 is the main radiolabeled metabolite of 11C-ACE leaving the myocardium, indicating clearly that the tricarboxylic acid cycle (TCA) in myocardium is the main metabolic pathway of 11C-ACE. More recently, 11C-ACE has been evaluated in prostate cancer for differential diagnosis, staging, and follow-up of its recurrence after surgery or treatment [19–24]. Upon uptake into the cells by the mono-carboxylate transporter, the 11C-ACE is utilized in multiple metabolic pathways including TCA, for generation of energy and for de novo fatty acid synthesis catalyzed by fatty acid synthase (FAS). FAS has a very low expression and activity in normal cells, but it is upregulated in many cancer types, including prostate, breast, and ovary. Such differential FAS over-expression and upregulation of lipogeneic activity in malignant cells as compared to normal cells is believed to be the mechanism for entrapment of 11C-ACE-derived radioactivity in cancer cells, thereby enabling the detection of tumors with PET. However, the clinical utilization of 11C-ACE is not widespread because of the relatively short physical half-life of 11C (t1/2=20.5 min), which precludes commercial distribution and dictates the need for an on-site cyclotron and radiochemistry facility. Therefore, we and others have been developing and validating an 18F-labeled analog of acetate, 18F-fluoroacetate (18F-FACE), as a clinically useful PET imaging agent. Compared to 11C, the much longer half-life of 18F (t1/2=110 min) should offer not only a more manageable manufacturing and delivery process for 18F-FACE but also the advantage of delayed imaging, with the potential for greater tumor-to-background ratios.
To date, there has been only one clinical case report in the literature regarding the potential efficacy of 18F-FACE for the detection of primary prostate carcinoma and pelvic lymph node metastases [25]. Recently, Ponde et al. reported a study of PET imaging with 18F-FACE in CWR22 tumor-bearing nu/nu mice and whole-body PET of a baboon [26]. The biodistribution, metabolism, and radiation dosimetry data obtained in rodents are not directly translatable into humans because the murine catabolism of 18F-FACE to 18F-fluoride greatly exceeds that in human beings. Therefore, in the same study, 18F-FACE PET imaging of a single baboon was performed for an initial assessment of the biodistribution of 18F-FACE at 1 and 2 h after intravenous injection of this radiotracer in a primate. In the baboon, almost no defluorination of 18F-FACE was apparent, as evidenced by the lack of discernible radioactivity uptake in the skeletal structures. This limited study did not include dynamic PET imaging to obtain time–activity profiles of 18F-FACE in different organs and tissues of the baboon or an analysis of radiolabeled metabolites in plasma and urine. It did not estimate the radiation dosimetry for human patients, which is necessary for translation of 18F-FACE into the clinic.
In another recent study, Lindhe et al. [27] have performed a head-to-head comparison of the biodistribution, metabolism, and excretion of 11C-ACE and 18F-FACE in three cynomolgus monkeys and one domestic pig. The 11C-ACE pharmacokinetics and organ distribution in both species were similar to those previously established in man. In contrast to 11C-ACE, 18F-FACE exhibited prolonged blood retention, no detectable trapping in the myocardium or salivary glands, rapid clearance from the liver, and extensive excretion to the bile and urine. Massive defluorination was seen in the pig, resulting in intense skeletal activity.
To facilitate translation of 18F-FACE into the clinic and in preparation to a phase I clinical study, we have conducted studies aimed at assessing the pharmacokinetics, biodistribution, metabolism, radiation dosimetry, and acute toxicity of diagnostic dosages of 18F-FACE in rhesus macaques. We demonstrate that diagnostic dosages of 18F-FACE for PET imaging studies are similar to other 18F-labeled agents.
Materials and Methods
Radiosynthesis of 18F-Fluoroacetate
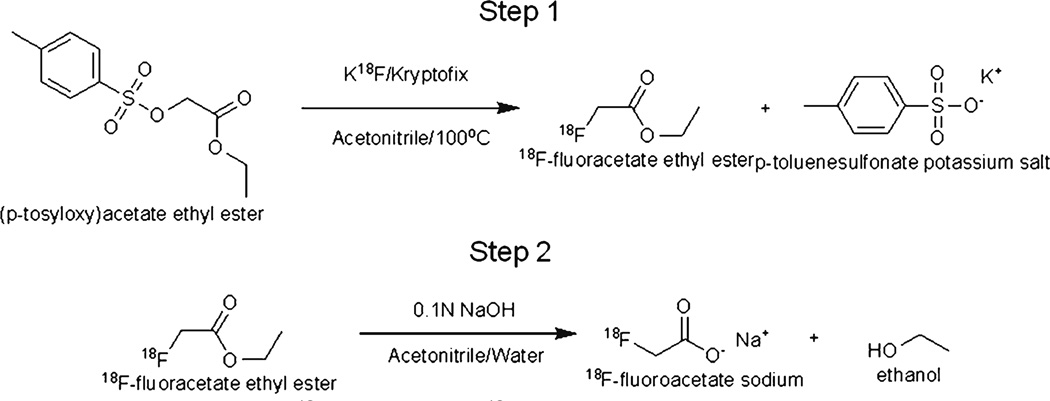
A simple, fully automated method was developed for the synthesis of no carrier added sodium 18F-FACE (Fig. 1). This method of 18F-FACE radiosynthesis differs in some of its steps from the previously reported radiosynthesis methods [26, 28–30].
Fig. 1.
Scheme for radiosynthesis of 18F-Fluoroacetate (18F-FACE). Step 1: radiolabel ETA to form the intermediate, 18F-fluoroacetate ethyl ester. Step 2: hydrolysis of EFA to form sodium 18F-FACE.
Prior to each synthesis, all reagent reservoirs and transfer lines in the automated box were rinsed either with acetonitrile or sterile water for injection. A new set of disposable glass reactors, crimp top septa, and sterile needles were used for each synthesis. 18F-fluoride was produced by irradiating 18O-water (97%+ enrichment) with 18 MeV protons from a TR19/9 cyclotron (Advanced Cyclotron Systems, Richmond, BC, Canada). The irradiated 18O-water was transferred through a Chromafix 30-PS-HCO3 (Macherey-Nagel, Germany) ion exchange cartridge. 18F was trapped with the Chromafix 30-PS-HCO3 resin and then eluted using standard K2CO3/Kryptofix222 in an acetonitrile/water solution. Solvents were removed during the two-step evaporation processes that rendered the dried 18F.
The precursor, ethyl (p-tosyloxy) acetate (ETA), was purchased from ABX (Radeberg, Germany), dissolved in 0.3 mL acetonitrile and added to the dry 18F mixture. The reaction mixture was heated to 80°C and purged with a stream of nitrogen gas. The 18F-EFACE and acetonitrile solvent were evaporated from the reaction mixture under the stated conditions and were trapped by a second vessel which contained 0.3 mL of 0.1 M sodium hydroxide maintained at 15°C. Evaporation of acetonitrile and simultaneous hydrolysis of 18F-EFACE were completed within 5 min. The crude 18F-FACE product was then passed through a preconditioned ion exchange resin AG1-X8 (BioRad, Hercules, CA, USA). The resin with the trapped product was rinsed three times with sterile water for injection that was sent to waste. Saline solution for injection (0.9% sodium chloride USP) was then used to elute 18F-FACE. Hydrochloric acid (0.01 N) was added to bring the final pH of the product into the range of pH 6–8. The final product was analyzed using an HPLC model 1,100 (Agilent, Santa Clara, CA, USA) coupled to a UV–visible spectrum detector and a radioactivity detector Flowcount (Bioscan Inc., Washington, DC, USA), with a Supelcogel C-610H column (30 cm long; 7.8 mm internal diameter; Sigma-Aldrich, St. Louis, MO, USA) using 20 mM phosphoric acid aqueous solution as a mobile phase at a flow rate of 0.5 mL/min.
The final product was passed through a 0.22-µm sterile filter (Millipore) into a 30-mL sterile vial. A bubble point procedure was performed on the final filter to test filter integrity. Sterility was verified post hoc by incubation in soybean–casein digest medium for 14 days at 20–25°C. Pyrogenicity was determined by a Limulus amoebocyte lysate test using Endosafe®-PTS system (Charles River, Wilmington, MA, USA).
Determination of Specific Activity
Specific activity of 18F-FACE was calculated based on measurements of radioactivity concentration and molar concentration in the dosing injectate. The radioactivity concentration of 18F-FACE was determined using gamma spectrometer Packard 5500 gamma spectrometer (Perkin-Elmer, Waltham, MA, USA). The molar concentration of 18F-FACE was determined using ion chromatography system ICS-2000 (Dionex, Sunnyvale, CA, USA) with potassium hydroxide eluent generator and IonPac AS11-HC, 4× 250 mm column (Dionex) maintained at 30°C; the potassium hydroxide mobile phase gradient was as follows: 0–8 min 1 mM/L, 8–18 min 1–15 mM/L, 18–28 min 15–45 mM/L, and 28–30 min 45 mM/L. To measure the concentration and total amount of fluoroacetate in the dosing injectate, a standard curve was established (linear fit R2=0.9998) using authentic fluoroacetate standard (product number 31220; Sigma-Aldrich, St. Louis, MO, USA) at different concentrations ranging from 0.0241 to 24.1 µg/mL (0.0309 to 3.09 µM/L).
Experimental Animals
Six rhesus macaques (three female and three male animals) were included in this study (Table 1). The animals were housed separately in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, at The University of Texas MD Anderson Cancer Center. All experiments were performed following MD Anderson guidelines for conducting experiments in non-human primates under an Institutional Animal Care and Use Committee approved research protocol. Prior to imaging, the animals were fasted overnight but had free access to water. To facilitate the intubation of the trachea, the animals were pre-medicated with atropine sulfate 0.04 mg/kg intramuscularly (i.m.) and anesthetized with 2.2–4.4 mg/kg telazole i.m., or 10– 25 mg/kg ketamine i.m., followed by inhalation anesthesia with isoflurane 1.0–3.0% in oxygen and maintained by mechanical ventilation using an Ohmeda Excel 210 SE (Soma Technology, Bloomfield, CT, USA). After intubation and stabilization of vital signs under anesthesia, the animals were cannulated in both saphenous veins: One cannula was used for injection of 18F- FACE, and the other was used for repetitive blood sampling during imaging. Also, the urinary bladder was catheterized for collection of radioactive urine during PET/CT imaging. Body temperature was maintained at 37±0.8°C using an air-circulating heating device Model 505 (Arizant Healthcare, Praire, MN, USA). The electrocardiogram (ECG), blood pressure, pulse oxymetry, and respiration rates were monitored using a Solar 8000 system (Marquette Medical Systems, Milwaukee, WI, USA) throughout the imaging study.
Table 1.
Summary of animal characteristics and hematological profile before PET imaging with 18F-FACE
| Animal | Male | Female | All | Normal range |
|---|---|---|---|---|
| Sex, n | 3 | 3 | 6 | |
| Weight (kg) injected dose | 10.1 ±1.8 | 5.9±0.2 | 8.00±2.53 | N/A |
| (MBq) | 178.3±33.3 | 152.4±20.4 | 165.4±28.5 | N/A |
| (MBq/kg) | 18.1±5.2 | 25.9±4.1 | 21.8±5.9 | |
| Hb (g/dL) | 13.2±1.2 | 12.9±1.1 | 13.1± 1.0 | 12.1–14.5 |
| Hct (%) | 42.5±4.1 | 41.6±3.5 | 42.1±3.4 | 37.7–44.9 |
| WBC (×103/mL) | 7.0±1.0 | 6.7±1.2 | 6.8±1.0 | 6.5–14.7 |
| RBC (×106/mL) | 5.8±0.9 | 5.7±0.7 | 5.7±0.7 | 5.3–7.4 |
| Total bilirubin (mg/dL) | 0.3±0.1 | 0.3±0.0 | 0.3±0.1 | 0.1–0.4 |
| AST (IU/L) | 26.0±9.0 | 28.0±5.0 | 27.0±6.7 | 24.0–52.0 |
| ALT (IU/L) | 25.3±6.7 | 39.0±12.2 | 32.2±11.5 | 25.0–52.0 |
| ALP (IU/L) | 134.7±62.7 | 197.0±57.4 | 165.8±63.7 | 78–287 |
| Total protein (g/dL) | 7.0±0.8 | 7.1±0.6 | 7.1±0.7 | 7.2–8.2 |
| Albumin (g/dL) | 4.1±0.3 | 3.9±0.9 | 4.0±0.6 | 3.8–5.0 |
| Blood glucose (mg/dL) | 69.0±7.1 | 63.0±8.0 | 65.4±7.4 | 51.0–89.0 |
| Creatinine (mg/dL) | 1.1±0.1 | 1.3±0.4 | 1.2±0.3 | 0.7–1.2 |
| BUN (mg/dL) | 18.1±3.3 | 16.2±5.5 | 17.1±4.2 | 16.0–25.0 |
PET Imaging Protocol
PET images were acquired using an ECAT HR+ (Siemens Molecular Imaging, Knoxville, TN, USA). After a 10-min transmission scan, 165.4±28.5 MBq (21.8±5.9 MBq/kg) of 18F-FACE in a volume of 5 mL was administered intravenously over 2 min by a digital syringe pump (Harvard Apparatus, Holliston, MA, USA). After injection of the radiotracer, a total of 29 frames were acquired in the first 30 min with 2D mode acquisition (20 frames for 15 s each, 5 frames for 1 min each, and 4 frames for 5 min each) in the area from the lower thorax to the upper abdomen. This enabled the acquisition of time–activity curves for the lungs, heart, aorta (blood), liver, mediastinal structure, stomach, and upper tips of the kidneys during the first half-hour following administration. Thereafter, three consecutive whole-body static scans were performed with the following protocol: Transmission and emission scans were 3 and 5 min, respectively, for four to five bed positions with acquisition in a 2D mode. PET images were reconstructed using standard vendor-provided reconstruction algorithms, which incorporated ordered-subset expectation maximization and corrected for attenuation; the emission data were corrected for scatter, random events, and dead-time losses using the manufacturer’s software program. To ensure that the injected dosage was accurate in terms of measured activity using PET, a CRC-15R dose calibrator (Capintec, Pittsburgh, PA, USA) was cross-calibrated with the PET instrument.
CT Study
A CT examination was performed using either a HiSpeed Advantage or a Light Speed 4-Slice helical CT scanner (GE Healthcare, Milwaukee, WI, USA) on the same day that the PET study was performed. The axial images were converted to Digital Imaging and Communications in Medicine Digital Imaging and Communications in Medicine (DICOM) image files for registration with the PET images.
Image Analysis
Regional dynamic and whole-body reconstructed PET and CT data were stored in the DICOM file format. Three-dimensional volumes of interest (VOIs) of individual source organs were constructed on the PET images to include all organ activity. These VOIs were used for PET image analysis. The following source organs were analyzed: the heart, liver, gallbladder, kidneys, urinary bladder, intestines, and whole body. Three-dimensional VOI definitions were used to visually inspect for movement artifacts between sequential scans in the same segment. Residual errors were manually corrected by redefining VOIs when necessary.
Time-Integrated Activity Coefficient and Absorbed Radiation Dose Calculations
Individual organ time–activity curves were estimated by fitting volume of interest data from the whole-body scans to a mono-exponential function using the Sigma Plot software program (version 11; Systat Software, Chicago, IL, USA) for five of the six animals. Only two whole-body scans were acquired for the sixth animal and so the rate of clearance was estimated by a linear fit to the logarithms of the two measured points. The whole-body time–activity curves were constructed by taking the activity in the entire animal (including the urinary bladder) in the first whole-body scan and decay-correcting it to the time of administration. This was assumed to represent the entirety of the administered activity. The second time point was constructed by taking the activity in the entire animal less that of the bladder at the time of the first whole-body scan. The remaining two time points, or in one case one time point, were constructed from the corresponding whole-body activity measurements less those of the urinary bladder. The time-integrated activity coefficient (formerly called the residence time [31]) was calculated by dividing the fractional uptake parameter of the exponential fit to the original data (not corrected for decay) by the decay constant of the fit.
The time-integrated activity coefficients were “humanized” using the mass of each of the identified source organs estimated from CT scans of the non-human primates and the masses of those organs tabulated by the OLINDA/EXM 1.1 (Vanderbilt University, Nashville, TN, USA) software for its 73.7-kg adult male model. The non-human primate and human organ masses were normalized by the respective whole-body masses, and each time-integrated activity coefficient was scaled by the ratio of the human to nonhuman normalized organ masses [32]. The human dosimetry of 18F-FACE was then estimated using these humanized time-integrated activity coefficients and the adult male model in the OLINDA/EXM 1.1 software program.
Blood Sampling and Analyses
Immediately before 18F-FACE injection in each animal, a 0.5-mL blood sample was drawn to measure the hematocrit and blood gases with an i-STAT portable analyzer (Abbott Point of Care, Princeton, NJ, USA). 18F-FACE was injected only if the hematological and blood chemical parameters in the blood sample were all within the normal ranges. Subsequently, 0.5-mL venous blood samples were obtained via the catheterized vein at 10, 20, 40, 60, and 90 s and 2, 3, 5, 8, 16, 32, 60, 90, 120, and 180 min after 18F-FACE injection. At the end of each PET/CT imaging session, an additional blood sample (0.5 mL) was obtained and analyzed using the i-STAT portable analyzer to determine potential acute toxic side effects from the diagnostic dosage of 18F-FACE.
The total radioactivity in the blood and plasma compartments was assayed for radioactivity concentration using a gamma counter (Cobra Quantum, Perkin-Elmer, Waltham, MA, USA). A portion of each plasma sample was ultrafiltered with a Biomax filter (Millipore, Billerica, MA, USA) before it was analyzed by HPLC The chromatographic conditions were the same as described in the section on the HPLC analytical method. The elution times of 18F-FACE and major potential radiolabeled metabolites in blood plasma (18F-fluorocitrate and 18F-fluoride) were determined using authentic cold standards under the same chromatographic conditions. Fractions of 18F-FACE and 18F-labeled metabolites were calculated for each sample based on the area under each peak. The total radioactivity in the plasma was expressed as %ID/mL and plotted against time post-injection of 18F-FACE. Also, the %ID/mL of intact 18F-FACE and 18F-metabolites were calculated, based on the results of the radio-HPLC analyses and were plotted against time post-injection of 18F-FACE to generate the corresponding time–activity curves.
Urine Analysis
Each animal’s urinary bladder was catheterized with a Foley catheter. The line was clamped until after the second whole-body image had been acquired. In five of the six animals, the line was then unclamped. At 120–150 min after administration of 18F-FACE, the urine was collected (mean volume of 38±21 mL). The total radioactivity of each sample was measured using a gamma counter. Samples were subjected to the same radio-HPLC analysis as described above for analysis of blood samples.
Results
18F-FACE Synthesis
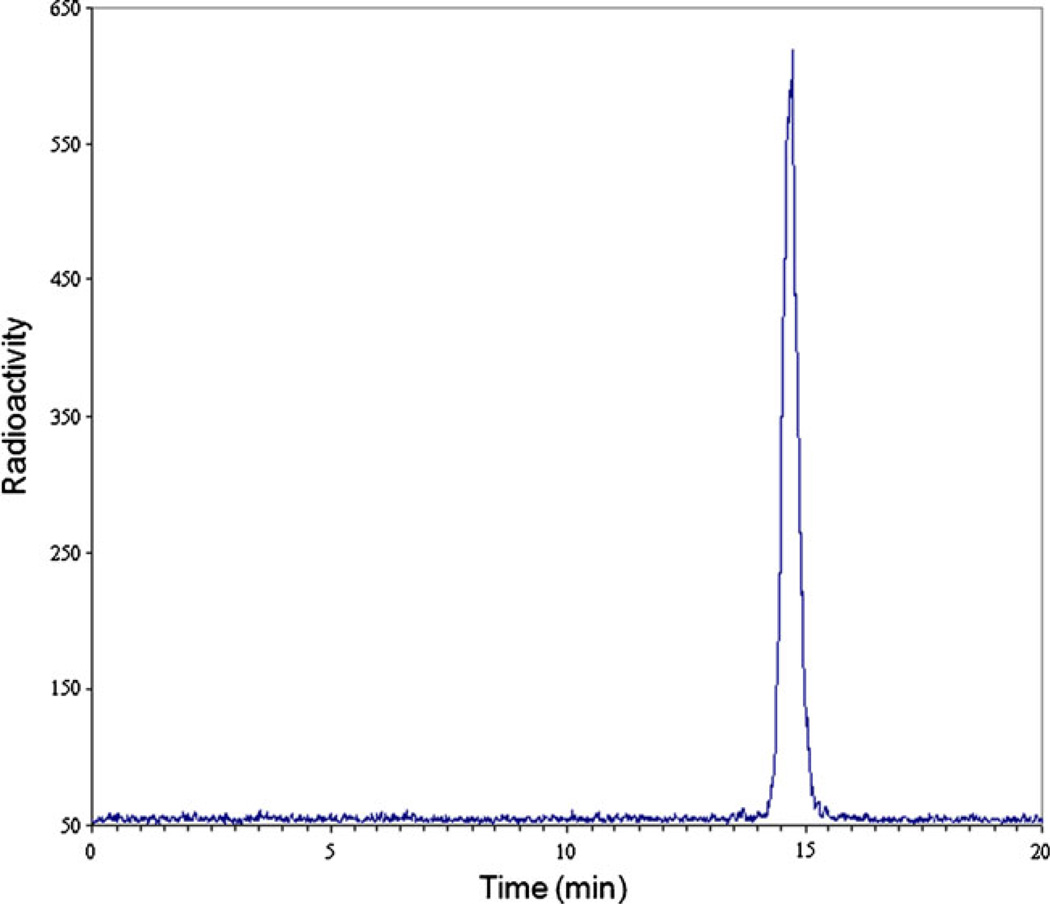
Using an automated synthesizer, we have obtained greater than 97% pure 18F-FACE at a radioactivity yield greater than 50%. Analysis of the final product using HPLC showed a major peak eluting at the same retention time, 14.7 min, as the sodium fluoroacetate standard. A trace amount of 18F-fluoride (<0.5%) eluting at 13.7 min in the product is also detected (Fig. 2). The product maintained its radiochemical purity for more than 8 h after the completion of the synthesis. Based on ion chromatography and radioactivity measurements, the specific activity of the synthesized 18FFACE was 1.162 Ci/µM (43 GBq/µ,M). The total pharmacologic “cold” equivalent of fluoroacetate administered to the animals in a single dose of 165–200 MBq 18F-FACE was 0.30–0.36 ng (or 1.815 pg/MBq).
Fig. 2.
A representative radio-HPLC of 18F-FACE after purification: 18F-FACE (99.4%) elution at 14.7 min; 18F-F− (0.6%) elution at 13.7 min.
PET Imaging of Pharmacokinetics and Biodistribution of 18F-FACE
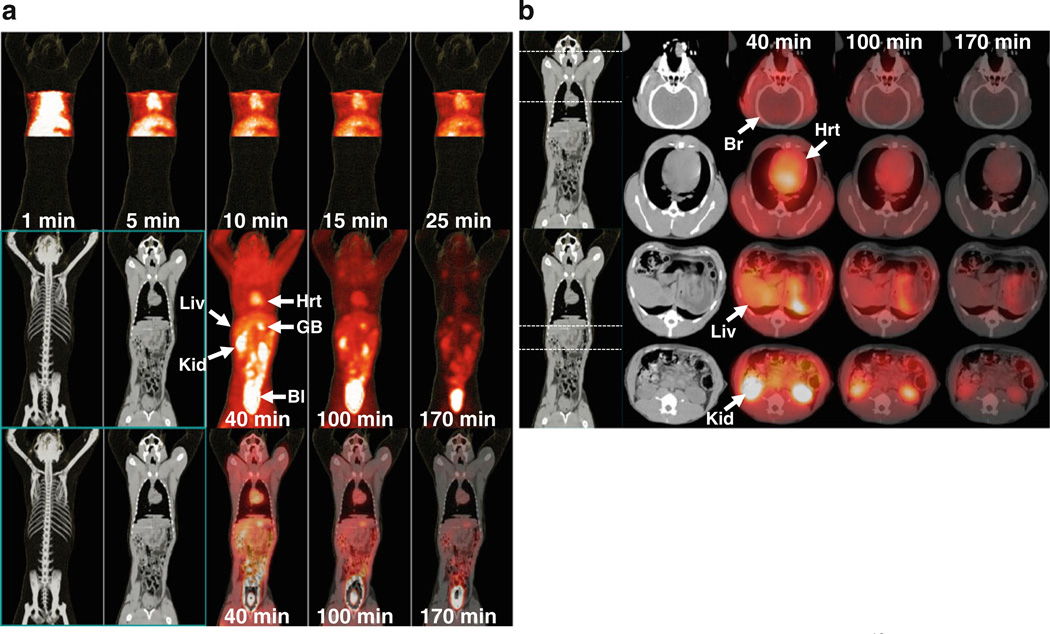
Dynamic PET imaging demonstrated that the hepatobiliary and renal systems were the principal pathways of clearance of 18F-FACE (Fig. 3). A relatively low accumulation of 18F-FACE-derived radioactivity was observed in the majority of organs not involved in biological clearance of 18F-FACE-derived radioactivity. Some accumulation of 18F-FACE was observed in the myocardium. No significant accumulation or retention of 18F-FACE was observed in the brain. There was some low level deposition of radioactivity in the skeletal structures, predominantly in the shoulder and hip joints, indicating a gradual defluorination occurring over 2 to 3 h post-injection of the radiotracer. Consistent with this observation, an increased level of free 18F-fluoride was detected in blood and urine samples collected at 2 to 3 h after the radiotracer administration.
Fig. 3.
PET and PET/CT fusion images obtained at different time points after intravenous injection of 18F-FACE. a Coronal maximum intensity projection (MIP) PET images of the thoracoabdominal section obtained at 1–25 min (top row); whole-body CT and MIP PET images obtained at 40, 100, and 170 min, average frame times (middle row); and corresponding fusion of coronal PET and CT images (bottom row). b Fusion of axial PET and CT images obtained at the level of the brain (top row), heart (second row), liver (third row), and kidneys (bottom row). Different organs are indicated by arrows and labeled as: Br brain, Hrt heart, Liv liver, GB gall bladder, Kid kidney, Bl urinary bladder.
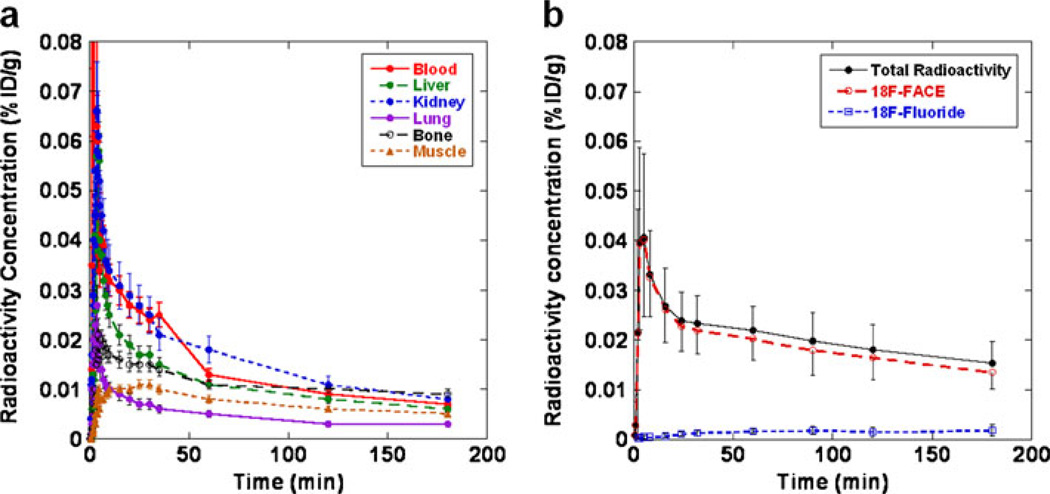
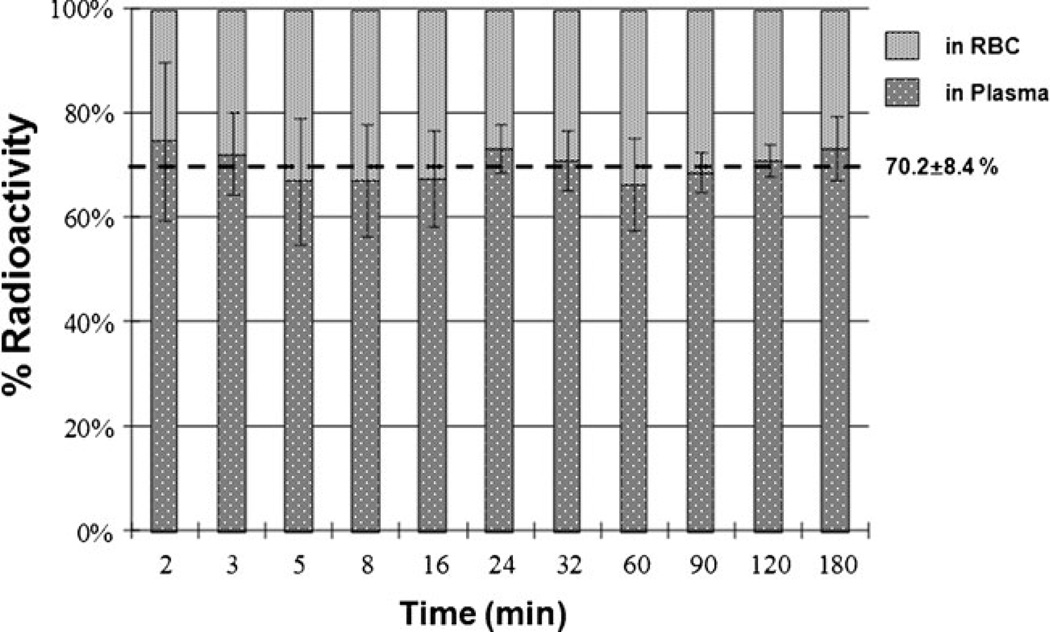
The time-dependent dynamics of 18F-FACE-derived radioactivity concentration in different organs and tissues were determined from PET images (Fig. 4a). Within 5 min, the level of radioactivity in the blood reached 0.040±0.02% ID/g and then cleared bi-exponentially with the fast phase between 3 and 18 min (half-life of 4 min) and then a slow phase until 180 min (half-life of 250 min). The fraction of 18F-fluoride in the blood gradually increased over time, but it was still less than 9% of the whole blood radioactivity at 180 min after 18F-FACE administration (Fig. 4b). The partition of radioactivity in the blood, 70% in the plasma and 30% in the red blood cells, was stable throughout the study (Fig. 5). Rapid accumulation of the radioactivity into the liver and kidney was observed, reaching concentrations of 0.040% ID/g (organ tissue) and 0.047% ID/g, respectively, at 5 min. There was a relatively low level of uptake in lung and muscle and other organs throughout the 180 min of the study; 27.6% of the total radioactivity in urine was determined to be 18F-fluoride, while 72.4% was 18F-FACE.
Fig. 4.
Time–activity curves of radioactivity concentration (% ID/g) (a) in selected organs and tissues determined from dynamic PET images and (b) of intact 18F-FACE and 18F-fluoride in blood plasma after intravenous administration of 18F-FACE in rhesus macaques. Data points: mean±SD.
Fig. 5.
Partition of radioactivity in blood between RBC and plasma after injection of 18F-FACE in rhesus macaques.
Evaluation of Acute Toxicity from 18F-FACE
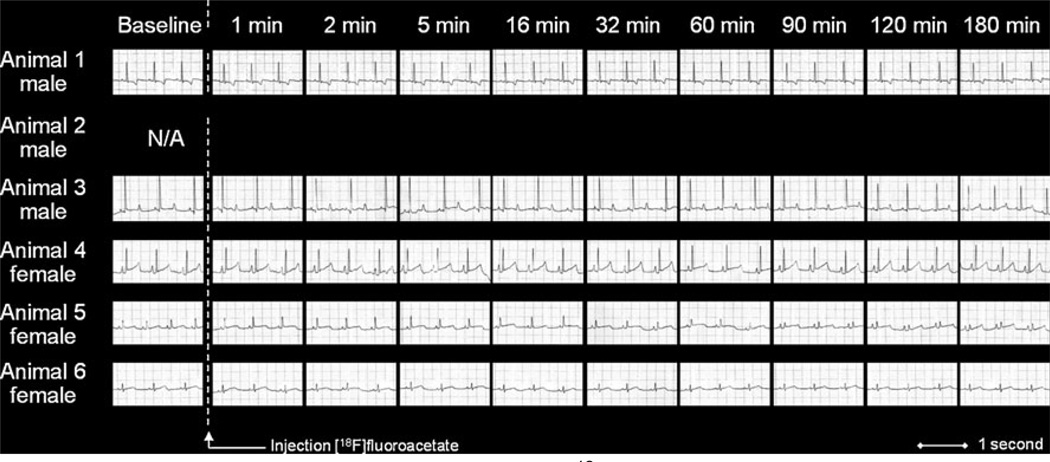
ECG traces after intravenously injection of [18F]-FACE and quantitative measures of different intervals are summarized in Fig. 6 and Table 2. None of the animals injected with 18F-FACE demonstrated any significant changes in cardiac rhythm or electrical activity patterns from the time of administration to 180 min post-injection. Hematologic parameters before and after the PET study with 18F-FACE are summarized in Table 3. There were no statistically significant changes in blood chemistry, liver enzymes, or renal function before, after 3 h, and over a period of 1– 3 months after the tracer administration, demonstrating the absence of any acute or chronic toxicity attributable to 18FFACE.
Fig. 6.
Representative ECG profiles of rhesus macaques during 18F-FACE PET imaging study. Quantitative measures of cardiac electrophysiological parameters are provided in Table 2
Table 2.
Quantitative measures of ECG parameters at different times before and during 18F-FACE PET imaging
| Time (min) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 5 | 16 | 32 | 60 | 90 | 120 | 180 | |
| Heart ratea | 92±12 | 88±8 | 88±8 | 88±7 | 87±6 | 89±7 | 89±7 | 87±5 | 88±3 | 93±8 |
| pb | 0.05±0.01 | 0.04±0.01 | 0.04±0.00 | 0.04±0.01 | 0.05±0.01 | 0.05±0.01 | 0.04±0.00 | 0.04±0.01 | 0.04±0.01 | 0.04±0.01 |
| PR | 0.11 ±0.01 | 0.11 ±0.01 | 0.11 ±0.01 | 0.11 ±0.02 | 0.10±0.01 | 0.11 ±0.01 | 0.11 ±0.01 | 0.11 ±0.01 | 0.11 ±0.01 | 0.10±0.01 |
| QRS | 0.04±0.01 | 0.05±0.01 | 0.04±0.00 | 0.04±0.01 | 0.04±0.00 | 0.04±0.00 | 0.04±0.01 | 0.04±0.00 | 0.05±0.01 | 0.04±0.00 |
| QT | 0.35±0.06 | 0.35±0.04 | 0.34±0.05 | 0.35±0.04 | 0.34±0.04 | 0.34±0.03 | 0.34±0.04 | 0.34±0.03 | 0.33±0.03 | 0.34±0.03 |
| ST | 0.80±0.74 | 0.80±0.45 | 1.00±0.50 | 0.95±0.51 | 0.80±0.41 | 0.60±0.38 | 0.70±0.54 | 0.70±0.54 | 0.60±0.38 | 0.85±0.63 |
Heart hare is in per minute
ECG measurements are in seconds
Table 3.
Comparison of hematological parameters before and after 18F-FACE PET imaging
| Parameter | Before imaging | After imaging | After 1–3 months |
|---|---|---|---|
| Hb (g/dL) | 13.1± 1.0 | 13.1± 1.2* | 13.4±0.4* |
| Hct (%) | 42.1±3.4 | 41.4±2.5* | 42.4±2.0* |
| WBC (×103/mL) | 6.8±1.0 | 6.8± 1.1* | 4.8±0.5* |
| RBC (×106/mL) | 5.7±0.7 | 5.7±0.6* | 5.7±0.1* |
| Plt (×106/mL) | 327.3±57.6 | 328.1±54.2* | 303.0±118.8* |
| Total bilirubin (mg/dL) | 0.3±0.1 | 0.3±0.1* | 0.2±0.1* |
| AST (IU/L) | 27.0±6.7 | 29.0±5.6* | 28.7±3.4* |
| ALT (IU/L) | 32.2±11.5 | 34.2±12.2* | 30.5±8.5* |
| ALP (IU/L) | 165.8±63.7 | 169.4±61.3* | 134.0±58.2* |
| Total protein (g/dL) | 7.1±0.7 | 7.0±0.5* | 6.1±0.9* |
| Albumin (g/dL) | 4.0±0.6 | 3.9±0.8* | 3.4±0.8* |
| BUN (mg/dL) | 17.1±4.2 | 17.9±4.6* | 17.9±4.9* |
p>0.1, no statistically significant differences
Radiation Dosimetry
To assess human radiation exposure due to diagnostic dosages of 18F-FACE, the radiation absorbed doses to organs were estimated using organ time-integrated activity coefficients from each individual animal that were “humanized” (Table 4). These doses are expressed in millisieverts per megabecquerel (Table 5) because the radiation-weighting factor of the ionizing radiation arising from F-18 is included in the organ dose calculations that were performed using the OLINDA/EXM 1.1 software. The effective dose for the adult male dosimetric model was estimated to be 0.0211 mSv/MBq. The higher absorbed doses were estimated for the liver (0.0307 mSv/MBq), the gallbladder wall (0.0288 mSv/MBq), the intestines (0.0366–0.0745 mSv/MBq), and the kidneys (0.0342 mSv/MBq), while much lower radiation absorbed doses were estimated for the brain (0.00762 mSv/MBq), breast (0.00774 mSv/MBq), thyroid (0.00888 mSv/MBq), and red bone marrow (0.0102 mSv/MBq).
Table 4.
Humanized residence time (in hours) from rhesus macaques after intravenous administration of 18F-FACE
| Source organ | Male | Female | All | Male | Female | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean±SD | Mean±SD | Mean±SD | |
| Heart | 0.048 | 0.057 | 0.025 | 0.039 | 0.016 | 0.017 | 0.034±0.017 | 0.043±0.016 | 0.024±0.013 |
| Liver | 0.505 | 0.290 | 0.065 | 0.226 | 0.078 | 0.093 | 0.209±0.171 | 0.287±0.220 | 0.132±0.082 |
| Kidneys | 0.061 | 0.038 | 0.043 | 0.032 | 0.059 | 0.030 | 0.044±0.013 | 0.047±0.012 | 0.041±0.016 |
| Intestinesa | 0.235 | 0.426 | 0.540 | 0.261 | 0.256 | 0.196 | 0.319±0.134 | 0.400±0.154 | 0.228±0.036 |
| Gall bladder | 0.006 | 0.009 | – | 0.017 | 0.014 | – | 0.011±0.005 | 0.007±0.002 | 0.016±0.002 |
| Remainder of body | 1.706 | 1.668 | 1.960 | 1.138 | 1.804 | 2.228 | 1.751±0.363 | 1.778±0.159 | 1.724±0.549 |
Assigned in thirds to the small intestine, upper large intestine, and lower large intestine, respectively
Table 5.
Organ doses to the 70 kg adult human model in OLINDA/EXM 1.1 using humanized time-integrated activity coefficients from rhesus macaques after intravenous administration of 18F-FACE (mSv/MBq)
| Target organ | Male | Female | All | Male | Female | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean±SD | Mean±SD | Mean±SD | |
| Adrenals | 0.016 | 0.014 | 0.013 | 0.010 | 0.012 | 0.013 | 0.013±0.002 | 0.014±0.002 | 0.012±0.002 |
| Brain | 0.007 | 0.007 | 0.009 | 0.005 | 0.008 | 0.010 | 0.008±0.002 | 0.008±0.001 | 0.007±0.002 |
| Breasts | 0.008 | 0.008 | 0.008 | 0.005 | 0.008 | 0.009 | 0.008±0.001 | 0.008±0.001 | 0.007±0.002 |
| Gallbladder wall | 0.031 | 0.033 | 0.017 | 0.040 | 0.036 | 0.016 | 0.029±0.010 | 0.027±0.008 | 0.031±0.013 |
| LLI wall | 0.058 | 0.096 | 0.120 | 0.060 | 0.062 | 0.052 | 0.075±0.027 | 0.091±0.031 | 0.058±0.005 |
| Small intestine | 0.031 | 0.045 | 0.055 | 0.029 | 0.031 | 0.029 | 0.037±0.011 | 0.044±0.012 | 0.030±0.002 |
| Stomach wall | 0.013 | 0.013 | 0.014 | 0.009 | 0.012 | 0.013 | 0.012±0.002 | 0.013±0.001 | 0.011±0.002 |
| ULI wall | 0.045 | 0.071 | 0.088 | 0.045 | 0.047 | 0.040 | 0.056±0.019 | 0.068±0.021 | 0.044±0.003 |
| Heart wall | 0.035 | 0.038 | 0.020 | 0.027 | 0.015 | 0.017 | 0.025±0.010 | 0.031±0.010 | 0.019±0.006 |
| Kidneys | 0.046 | 0.032 | 0.034 | 0.026 | 0.042 | 0.026 | 0.034±0.008 | 0.037±0.008 | 0.031±0.010 |
| Liver | 0.067 | 0.041 | 0.014 | 0.032 | 0.015 | 0.017 | 0.031±0.021 | 0.040±0.026 | 0.021 ±0.009 |
| Lungs | 0.011 | 0.010 | 0.010 | 0.007 | 0.009 | 0.011 | 0.010±0.002 | 0.010±0.001 | 0.009±0.002 |
| Muscle | 0.010 | 0.010 | 0.011 | 0.007 | 0.010 | 0.011 | 0.010±0.002 | 0.010±0.001 | 0.009±0.002 |
| Ovaries | NA | NA | NA | 0.013 | 0.017 | 0.018 | 0.016±0.002 | N/A | 0.016±0.002 |
| Pancreas | 0.016 | 0.014 | 0.014 | 0.010 | 0.013 | 0.014 | 0.013±0.002 | 0.015±0.001 | 0.012±0.002 |
| Red marrow | 0.011 | 0.011 | 0.012 | 0.007 | 0.010 | 0.011 | 0.010±0.002 | 0.011±0.001 | 0.009±0.002 |
| Osteogenic cells | 0.014 | 0.014 | 0.016 | 0.010 | 0.014 | 0.017 | 0.014±0.003 | 0.015±0.001 | 0.014±0.004 |
| Skin | 0.007 | 0.007 | 0.008 | 0.005 | 0.007 | 0.009 | 0.007±0.001 | 0.008±0.001 | 0.007±0.002 |
| Spleen | 0.012 | 0.011 | 0.012 | 0.008 | 0.011 | 0.013 | 0.011±0.002 | 0.012±0.001 | 0.010±0.003 |
| Testes | 0.009 | 0.009 | 0.010 | NA | NA | NA | 0.009±0.001 | 0.009±0.001 | NA |
| Thymus | 0.010 | 0.010 | 0.010 | 0.007 | 0.009 | 0.011 | 0.010±0.002 | 0.010±0.001 | 0.009±0.002 |
| Thyroid | 0.009 | 0.009 | 0.010 | 0.006 | 0.009 | 0.011 | 0.009±0.002 | 0.009±0.001 | 0.009±0.003 |
| Urinary bladder wall | 0.042 | 0.015 | 0.017 | 0.014 | 0.017 | 0.021 | 0.021±0.010 | 0.025±0.015 | 0.017±0.004 |
| Uterus | NA | NA | NA | 0.011 | 0.014 | 0.016 | 0.014±0.003 | NA | 0.014±0.003 |
| Total body | 0.012 | 0.012 | 0.012 | 0.008 | 0.010 | 0.012 | 0.011±0.002 | 0.012±0.001 | 0.010±0.002 |
| EDE | 0.024 | 0.027 | 0.030 | 0.019 | 0.021 | 0.019 | 0.023±0.004 | 0.027±0.003 | 0.020±0.001 |
| ED | 0.022 | 0.025 | 0.028 | 0.016 | 0.018 | 0.018 | 0.021±0.004 | 0.025±0.003 | 0.018±0.001 |
EDE effective dose equivalent, ED effective dose, LLI lower large intestine, ULI upper large intestine, NA not applicable
Discussion
This study was conducted to facilitate the clinical phase I study with 18F-FACE, as an alternative to 11C-ACE for imaging 18F-FDG-nonavid tumors. Previously, 11C-ACE PET was reported to be up to 100% effective for detection of primary tumors in patients with untreated prostate cancer, in contrast to other conventional imaging methods such as 18F-FDG PET, and to have a 59% sensitivity for detection of recurrent prostate cancer in patients with prostate-specific antigen relapse [19, 20, 33]. The use of 18F-FACE PET/CT has been reported in only one prostate cancer patient [25]. In the latter study, a dosage of 280 MBq 18F-FACE was injected intravenously, and PET imaging was started 70 min after dosing. In that study, 18F-FACE PET/CT images demonstrated moderate to intense uptake in several, but not all, of the metastatic lesions.
In this current study, the pattern of biodistribution and clearance of 18F-FACE in rhesus macaques was similar to that reported before in a baboon [26] and in cynomologous monkeys [27]. The present work reports for the first time radiation dosimetry estimates made in non-human primates and scaled to human patients. Based on the results of the current studies in rhesus macaques, the estimated effective dose to human patients after administration of 185–370 MBq of 18F-FACE is 3.90–7.81 mSv. This is consistent with the previously reported range of 5.7–7.0 mSv for 300–370 MBq [34] and 3.8–10.7 mSv for 185–370 MBq [35] of 18F-FDG. The critical organs for 18F-FACE were the intestines (0.0366–0.0745 mSv/MBq), which is due to combined radiation exposure from the surrounding organs such as the liver, kidney, and urinary bladder. For comparison, the reported radiation absorbed doses after administration of 11C-ACE in humans were the highest in the pancreas (0.017 mGy/MBq), intestines (0.01–0.011 mGy/MBq), and kidneys (0.0093 mGy/MBq), followed by the liver and the urinary bladder with 0.006 and 0.0028 mGy/MBq, respectively. The effective dose was estimated to be 0.0049 mSv/MBq [36]. In contrast to the well-established hepatobiliary clearance of 11C-ACE from the circulation, 18F-FACE cleared predominantly through the kidneys into the urinary bladder, which corroborates previous reports [26, 27]. These results suggest that the diagnostic dosage of 370 MBq of 18F-FACE should be acceptable for administration in human patients and that its effects are well below the limit of 50 mSv per organ per year, which is set forth in the FDA regulations (21 CFR 361.1) [32, 37, 38].
This study demonstrated that the uptake of 18F-FACE-derived radioactivity in skeletal structures in monkeys was limited and that defluorination (measured in blood samples) was no more than 9% of the total at the end of the 3-h study and 18F-fluorocitrate anabolite was not detectable. Our results are in agreement with those reported previously in non-human primates, where only a minimal level of bone uptake of 18F-FACE-derived radioactivity was observed [26, 27]. In contrast, defluorination of 18F-FACE in rodents has been significant [39], and large amounts of 18F-fluoride liberated from 18F-FACE accumulate in rodent skeletal structures [26]. The high expression of glutathione S-transferase in rats and mice is responsible for the observed defluorination of fluoroacetate [40], which makes it a suboptimal radiotracer for PET imaging studies in rodents. Nevertheless, recent studies by Marik et al. [41] demonstrated the potential utility of 18F-FACE for imaging of glial activation in a murine model of intracerebral glioma, stroke, and cerebral ischemia–hypoxia. In contrast, Lindhe et al. [27] drew the paradoxical conclusion that 18F-FACE could not be regarded as a functional analog of 11C-ACE and that it is of little use for studies of organ blood flow, intermediary metabolism, or lipid synthesis. Therefore, the mechanism of 18F-FACE uptake and accumulation in tumor cells requires additional discussion.
The biochemical perspectives of PET imaging with 11C-ACE have been discussed recently in the literature [24]. The mechanism of 18F-FACE uptake and accumulation in cells is similar to that of 11C-ACE. Fluoroacetate is transported through cell membranes by a monocarboxylate transporter or by diffusion and is subsequently converted to fluoroacetyl-CoA by the enzyme acetyl-CoA synthases 1 and 2 (ACS1, ACS2) [42, 43]. At least three main metabolic pathways can utilize the 18F-fluoroacetyl-CoA. One is the TCA cycle [44, 45]. However, unlike acetate, fluoroacetate stops the TCA cycle from its completion after the formation of fluorocitrate. Fluorocitrate acts as an irreversible inhibitor of aconitase [46], which is the catalytic enzyme for the conversion of citrate to cis-citrate, a necessary intermediate for the successful completion of the TCA cycle. Hence, 18F-fluorocitrate is accumulated in cells until it degrades radiochemically. While citrate can be utilized by the ATP citrate lyase, which is one of the key lipogeneic enzymes that is over-expressed in many cancer cells [47], fluorocitrate cannot be utilized by ATP citrate lyase because it is irreversibly bound to aconitase. Another metabolic pathway of acetyl-CoA utilization is fatty acid synthesis catalyzed by the enzyme FAS, which is upregulated in many types of cancer cells [47]. However, fluoroacetyl-CoA is not an efficient substrate for FAS, and therefore, the fluoroacetyl moiety is not incorporated into the de novo synthesized fatty acids. Nevertheless, the expression and activity of acetyl-coA synthase is proportionally upregulated in tumor cells either with high glycolytic activity (TCA cycle) or high FAS activity, such as in prostate carcinoma cells, which mediates formation and accumulation of 18F-fluoroacetyl-CoA and may explain increased uptake and accumulation of 18F-FACE.
The third metabolic pathway that may utilize acetyl-coA is ketone body synthesis through formation of acetoacetate mediated by the cooperative action of acetyl-CoA transferase and acetoacetyl-CoA thiolase followed by succinyl coenzyme A/acetoacetate coenzyme transferase, an initiator of ketone body utilization in tumor cells [48, 49]. Therefore, it is conceivable that fluoroacetate in tracer dosages may be converted in part to fluoroacetoacetate, which would reflect de novo ketone synthetic activity in cancer cells, which utilize ketone bodies for energy production. Thus, there is plenty of evidence that 18F-FACE can be used for metabolic studies and imaging tumors with upregulated glycolysis, fatty acid synthesis, and ketone metabolism.
Although 18F-FACE may be a promising PET radiotracer, there have been concerns raised about potential toxicity of this compound in tracer dosages, which may have hindered its clinical translation. The data obtained from our studies and those of the others put these concerns in perspective. Fluoroacetate is a naturally occurring substance that can be found in the leaves of Dichapetalum cymosum, a poisonous South African plant, and in 40 other plant species including tea leaves. For the past half-century, chemically synthesized sodium fluoroacetate has been used as a vertebrate poison to help control opossum overpopulation in New Zealand. In the USA, sodium fluoroacetate is used in collars worn by livestock to protect them from aggressive predators. The pharmacology and toxicology of sodium fluoroacetate have been studied extensively in many animal species including rodents, dogs [50, 51], various livestock, and monkeys and have been recently reviewed [52]. The LD50 for dog is 60 µg/kg if administered intravenously and 66 µg/kg if administered orally. The LD50 for monkey is 5 and 300 mg/kg through intravenous injection and oral consumption, respectively. The estimated lethal dose for human is approximately 2–10 mg/kg [53–55]. Tea leaves are found to contain fluoroacetate at 0.19 µg/g of leaves [56]. If 3–6 g of tea leaves is commonly used to make a cup of tea, they could release fluoroacetate in the range of 0.57 to 1.14 µg. The anticipated initial dosage of 260 MBq of 18F-FACE for PET imaging in a phase I study equates to a pharmacologic mass dose of 0.47 ng, determined based on our measurements of specific activity of 18F-FACE (43 GBq/µ,M). For comparison, a cup of tea may contain approximately 1,000– 2,500 times more fluoroacetate than that in one diagnostic administration of 18F-FACE. It is well established that fluoroacetate toxicity is due to biosynthesis of fluorocitrate, which blocks the TCA cycle and causes cell death [57]. The fluorocitrate inhibition of aconitase and citrate transporters has been studied extensively [46, 58–61]. Within 1 h, the level of citrate in many organs increases more than tenfold. Citrate buildup further inhibits glucose metabolism by inhibiting the enzyme phosphofructokinase, which causes toxicity. However, at tracer doses administered to humans (i.e., <1 ng total dose), 18F-FACE cannot elicit any pharmacological or toxic effects. Furthermore, unlike “cold” (non-radiofluorinated) fluoroacetate, which contains a stable isotope of fluorine, in 18F-FACE the 18F radionuclide decays to 18O, resulting in a molecule that is no longer fluoroacetate and which dissociates from aconitase and citrate transporters, thus restoring the normal activity of TCA cycle.
Finally, in the current study, no significant changes to the ECG were observed during imaging studies, which indicates that there was no acute myocardial toxicity from the injection of 18F-FACE in rhesus macaques. Follow-up examinations immediately after PET imaging with 18F-FACE as well as 1– 3 month later revealed no significant changes in CBC, liver enzymes, or renal function when compared to data obtained prior to this radiotracer administration. Therefore, based on volumes of well-established toxicology of fluoroacetate in various species together with results of the current study in rhesus macaques, we are confident that it is pharmacologically safe to employ 18F-FACE as a PET tracer for human use at the proposed diagnostic doses.
Conclusions
The pharmacokinetics, metabolism, biodistribution, radiation dosimetry characteristics, and lack of acute and delayed toxicity of 18F-FACE determined in non-human primates indicate that diagnostic doses of this radiotracer are similar to those of other agents used for PET imaging in human patients. The highest radiation dose is delivered to the intestines. This dose estimation, as well as the radiation doses delivered to other radiosensitive organs, must be considered in evaluating the dosimetry of multiple administration of 18F-FACE to human patients.
Acknowledgments
This work was supported by the NIH-NCI CA-016672 (MD Anderson Cancer Center Support Grant) and new project development funds of the Department of Experimental Diagnostic Imaging, MDACC. We thank Nancy Swanston, CNMT, for help with the PET studies, Karen Yoas for help in coordinating this study, and the anonymous reviewers for their insights and advice.
Footnotes
Conflict of Interest Disclosure. The authors declare that they have no conflict of interest.
References
- 1.Hustinx R, Benard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med. 2002;32:35–46. doi: 10.1053/snuc.2002.29272. [DOI] [PubMed] [Google Scholar]
- 2.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 3.Ide M. Cancer screening with FDG-PET. Q J Nucl Med Mol Imaging. 2006;50:23–27. [PubMed] [Google Scholar]
- 4.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Busk M, Horsman MR, Jakobsen S, Bussink J, van der Kogel A, Overgaard J. Cellular uptake of PET tracers of glucose metabolism and hypoxia and their linkage. Eur J Nucl Med Mol Imaging. 2008;35:2294–2303. doi: 10.1007/s00259-008-0888-9. [DOI] [PubMed] [Google Scholar]
- 7.Busk M, Horsman MR, Kristjansen PE, van der Kogel AJ, Bussink J, Overgaard J. Aerobic glycolysis in cancers: implications for the usability of oxygen-responsive genes and fluorodeoxyglucose-PET as markers of tissue hypoxia. Int J Cancer. 2008;122:2726–2734. doi: 10.1002/ijc.23449. [DOI] [PubMed] [Google Scholar]
- 8.Bouchelouche K, Oehr P. Recent developments in urologic oncology: positron emission tomography molecular imaging. Curr Opin Oncol. 2008;20:321–326. doi: 10.1097/CCO.0b013e3282f8b02b. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Zhuang H, Alavi A. PET in the management of urologic malignancies. Radiol Clin North Am. 2004;42:1141–1153. ix. doi: 10.1016/j.rcl.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Functional imaging of renal cell carcinoma. Nat Rev Urol. 2010;7:258–266. doi: 10.1038/nrurol.2010.40. [DOI] [PubMed] [Google Scholar]
- 11.Wolfort RM, Papillion PW, Turnage RH, Lillien DL, Ramaswamy MR, Zibari GB. Role of FDG-PET in the evaluation and staging of hepatocellular carcinoma with comparison of tumor size, AFP level, and histologic grade. Int Surg. 2011;95:67–75. [PubMed] [Google Scholar]
- 12.Ott K, Herrmann K, Krause BJ, Lordick F. The value of PET imaging in patients with localized gastroesophageal cancer. Gastrointest Cancer Res. 2008;2:287–294. [PMC free article] [PubMed] [Google Scholar]
- 13.Lau EW, Drummond KJ, Ware RE, Drummond E, Hogg A, Ryan G, Grigg A, Callahan J, Hicks RJ. Comparative PET study using F-18 FET and F-18 FDG for the evaluation of patients with suspected brain tumour. J Clin Neurosci. 2011;17:43–49. doi: 10.1016/j.jocn.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Nanni C, Fantini L, Nicolini S, Fanti S. Non FDG PET. Clin Radiol. 2010;65:536–548. doi: 10.1016/j.crad.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Pike VW, Eakins MN, Allan RM, Selwyn AP. Preparation of [1–11C]acetate—an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33:505–512. doi: 10.1016/0020-708x(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 16.Schelbert HR. PET contributions to understanding normal and abnormal cardiac perfusion and metabolism. Ann Biomed Eng. 2000;28:922–929. doi: 10.1114/1.1310216. [DOI] [PubMed] [Google Scholar]
- 17.Timmer SA, Germans T, Gotte MJ, Russel IK, Dijkmans PA, Lubberink M, ten Berg JM, ten Cate FJ, Lammertsma AA, Knaapen P, van Rossum AC. Determinants of myocardial energetics and efficiency in symptomatic hypertrophic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2010;37:779–788. doi: 10.1007/s00259-009-1350-3. [DOI] [PubMed] [Google Scholar]
- 18.Timmer SA, Lubberink M, Germans T, Gotte MJ, ten Berg JM, ten Cate FJ, van Rossum AC, Lammertsma AA, Knaapen P. Potential of [11C] acetate for measuring myocardial blood flow: studies in normal subjects and patients with hypertrophic cardiomyopathy. J Nucl Cardiol. 2010;17:264–275. doi: 10.1007/s12350-009-9181-y. [DOI] [PubMed] [Google Scholar]
- 19.Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, Sadato N, Yamamoto K, Okada K. 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181–186. [PubMed] [Google Scholar]
- 20.Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, Kibel AS, Andriole GL, Picus J, Welch MJ. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549–555. [PubMed] [Google Scholar]
- 21.Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1380–1384. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 22.Fricke E, Machtens S, Hofmann M, van den Hoff J, Bergh S, Brunkhorst T, Meyer GJ, Karstens JH, Knapp WH, Boerner AR. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 23.Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996–1000. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Soloviev D, Fini A, Chierichetti F, Al-Nahhas A, Rubello D. PET imaging with 11C-acetate in prostate cancer: a biochemical, radiochemical and clinical perspective. Eur J Nucl Med Mol Imaging. 2008;35:942–949. doi: 10.1007/s00259-007-0662-4. [DOI] [PubMed] [Google Scholar]
- 25.Matthies A, Ezziddin S, Ulrich EM, Palmedo H, Biersack HJ, Bender H, Guhlke S. Imaging of prostate cancer metastases with 18F-fluoroacetate using PET/CT. Eur J Nucl Med Mol Imaging. 2004;31:797. doi: 10.1007/s00259-003-1437-1. [DOI] [PubMed] [Google Scholar]
- 26.Ponde DE, Dence CS, Oyama N, Kim J, Tai YC, Laforest R, Siegel BA, Welch MJ. 18F–fluoroacetate: a potential acetate analog for prostate tumor imaging—in vivo evaluation of 18F–fluoroacetate versus 11C–acetate. J Nucl Med. 2007;48:420–428. [PubMed] [Google Scholar]
- 27.Lindhe O, Sun A, Ulin J, Rahman O, Langstrom B, Sorensen J. [18F] Fluoroacetate is not a functional analogue of [11C]acetate in normal physiology. Eur J Nucl Med Mol Imaging. 2009;36:1453–1459. doi: 10.1007/s00259-009-1128-7. [DOI] [PubMed] [Google Scholar]
- 28.Sykes TR, Ruth TJ, Adam MJ. Synthesis and murine tissue uptake of sodium [18F]fluoroacetate. Int J Radiat Appl Instrum B. 1986;13:497–500. doi: 10.1016/0883-2897(86)90126-1. [DOI] [PubMed] [Google Scholar]
- 29.Jeong JMLD, Chung J-K, Lee MC, Koh C-S, Kang SS. Synthesis of no-carrier-added [18F]fluoroacetate. J Labelled Compd Radiopharm. 1997;39:395–399. [Google Scholar]
- 30.Sun LQ, Mori T, Dence CS, Ponde DE, Welch MJ, Furukawa T, Yonekura Y, Fujibayashi Y. New approach to fully automated synthesis of sodium [18 F]fluoroacetate—a simple and fast method using a commercial synthesizer. Nucl Med Biol. 2006;33:153–158. doi: 10.1016/j.nucmedbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry-standardization of nomenclature. J Nucl Med. 2009;50:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- 32.Macey DJWL, Breitz HB, Liu A, Johnson TK, Zanzonico PB. A primer for radioimmunotherapy and radionuclide therapy. AAPM report no 7. 2001 http://www.aapm.org/pubs/reports/rpt_71.pdf.
- 33.Morris MJ, Scher HI. 11C-acetate PET imaging in prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:181–184. doi: 10.1007/s00259-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 34.Brix G, Lechel U, Glatting G, Ziegler SI, Munzing W, Muller SP, Beyer T. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med. 2005;46:608–613. [PubMed] [Google Scholar]
- 35.Deloar HM, Fujiwara T, Shidahara M, Nakamura T, Watabe H, Narita Y, Itoh M, Miyake M, Watanuki S. Estimation of absorbed dose for 2-[F-18]fluoro-2-deoxy-D-glucose using whole-body positron emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25:565–574. doi: 10.1007/s002590050257. [DOI] [PubMed] [Google Scholar]
- 36.Seltzer MA, Jahan SA, Sparks R, Stout DB, Satyamurthy N, Dahlbom M, Phelps ME, Barrio JR. Radiation dose estimates in humans for 11C-acetate whole-body PET. J Nucl Med. 2004;45:1233–1236. [PubMed] [Google Scholar]
- 37.Anonymous. Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53) Ann ICRP. 1998;28:1–126. doi: 10.1016/s0146-6453(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 38.Kase KR. Radiation protection principles of NCRP. Health Phys. 2004;87:251–257. doi: 10.1097/00004032-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Liu RS, Chou TK, Chang CH, Wu CY, Chang CW, Chang TJ, Wang SJ, Lin WJ, Wang HE. Biodistribution, pharmacokinetics and PET imaging of [(18)F]FMISO, [18F]FDG and [18F]FAc in a sarcoma-and inflammation-bearing mouse model. Nucl Med Biol. 2009;36:305–312. doi: 10.1016/j.nucmedbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Tecle B, Casida JE. Enzymatic defluorination and metabolism of fluoroacetate, fluoroacetamide, fluoroethanol, and (−)-erythro-fluorocitrate in rats and mice examined by 19F and 13C NMR. Chem Res Toxicol. 1989;2:429–435. doi: 10.1021/tx00012a012. [DOI] [PubMed] [Google Scholar]
- 41.Marik J, Ogasawara A, Martin-McNulty B, Ross J, Flores JE, Gill HS, Tinianow JN, Vanderbilt AN, Nishimura M, Peale F, Pastuskovas C, Greve JM, van Bruggen N, Williams SP. PET of glial metabolism using 2-18F-fluoroacetate. J Nucl Med. 2009;50:982–990. doi: 10.2967/jnumed.108.057356. [DOI] [PubMed] [Google Scholar]
- 42.Muir D, Berl S, Clarke DD. Acetate and fluoroacetate as possible markers for glial metabolism in vivo . Brain Res. 1986;380:336–340. doi: 10.1016/0006-8993(86)90231-3. [DOI] [PubMed] [Google Scholar]
- 43.Clarke DD. Fluoroacetate and fluorocitrate: mechanism of action. Neurochem Res. 1991;16:1055–1058. doi: 10.1007/BF00965850. [DOI] [PubMed] [Google Scholar]
- 44.Peters R, Wakelin RW. Biochemistry of fluoroacetate poisoning; the isolation and some properties of the fluorotricarboxylic acid inhibitor of citrate metabolism. Proc R Soc Lond B Biol Sci. 1953;140:497–507. doi: 10.1098/rspb.1953.0004. [DOI] [PubMed] [Google Scholar]
- 45.Proudfoot AT, Bradberry SM, Vale JA. Sodium fluoroacetate poisoning. Toxicol Rev. 2006;25:213–219. doi: 10.2165/00139709-200625040-00002. [DOI] [PubMed] [Google Scholar]
- 46.Lauble H, Kennedy MC, Emptage MH, Beinert H, Stout CD. The reaction of fluorocitrate with aconitase and the crystal structure of the enzyme-inhibitor complex. Proc Natl Acad Sci USA. 1996;93:13699–13703. doi: 10.1073/pnas.93.24.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 48.Lopes-Cardozo M, Mulder I, van Vugt F, Hermans PG, van den Bergh SG, Klazinga W, de Vries-Akkerman E. Aspects of ketogenesis: control and mechanism of ketone-body formation in isolated rat-liver mitochondria. Mol Cell Biochem. 1975;9:155–173. doi: 10.1007/BF01751311. [DOI] [PubMed] [Google Scholar]
- 49.Fenselau A, Wallis K, Morris HP. Subcellular localization of acetoacetate coenzyme A transferase in rat hepatomas. Cancer Res. 1976;36:4429–4433. [PubMed] [Google Scholar]
- 50.Yamashita K, Yada H, Ariyoshi T. Neurotoxic effects of alpha-fluoro-beta-alanine (FBAL) and fluoroacetic acid (FA) on dogs. J Toxicol Sci. 2004;29:155–166. doi: 10.2131/jts.29.155. [DOI] [PubMed] [Google Scholar]
- 51.Goh CS, Hodgson DR, Fearnside SM, Heller J, Malikides N. Sodium monofluoroacetate (compound 1080) poisoning in dogs. Aust Vet J. 2005;83:474–479. doi: 10.1111/j.1751-0813.2005.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 52.Goncharov NV, Jenkins RO, Radilov AS. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J Appl Toxicol. 2006;26:148–161. doi: 10.1002/jat.1118. [DOI] [PubMed] [Google Scholar]
- 53.Gajdusek DC, Luther G. Fluoroacetate poisoning a review and report of a case. Am J Dis Child. 1950;79:310–320. doi: 10.1001/archpedi.1950.04040010321007. [DOI] [PubMed] [Google Scholar]
- 54.Harrisson JW, Ambrus JL, Ambrus CM. Fluoroacetate 1080 poisoning. Ind Med Surg. 1952;21:440–442. [PubMed] [Google Scholar]
- 55.Harrisson JW, Ambrus JL, Ambrus CM, Rees EW, Peters RH, Jr, Reese LC. Acute poisoning with sodium fluoroacetate (compound 1080) J Am Med Assoc. 1952;149:1520–1522. doi: 10.1001/jama.1952.02930340004002. [DOI] [PubMed] [Google Scholar]
- 56.Vartiainen T, Gynther J. Fluoroacetic acid in guar gum. Food Chem Toxicol. 1984;22:307–308. doi: 10.1016/0278-6915(84)90011-5. [DOI] [PubMed] [Google Scholar]
- 57.Savarie P. Toxic characteristics of fluorocitrate, the toxic metabolite of compound 1080. 11th Vertebrate Pest Conference University of Nebraska, Lincoln, University of Nebraska; Lincoln. 1984. pp. 132–137. [Google Scholar]
- 58.Carrell HL, Glusker JP, Villafranca JJ, Mildvan AS, Dummel RJ, Kun E. Fluorocitrate inhibition of aconitase: relative configuration of inhibitory isomer by x-ray crystallography. Science. 1970;170:1412–1414. doi: 10.1126/science.170.3965.1412. [DOI] [PubMed] [Google Scholar]
- 59.Villafranca JJ, Platus E. Fluorocitrate inhibition of aconitase. Reversibility of the inactivation. Biochem Biophys Res Commun. 1973;55:1197–1207. doi: 10.1016/s0006-291x(73)80021-x. [DOI] [PubMed] [Google Scholar]
- 60.Brand MD, Evans SM, Mendes-Mourao J, Chappell JB. Fluorocitrate inhibition of aconitate hydratase and the tricarboxylate carrier of rat liver mitochondria. Biochem J. 1973;134:217–224. doi: 10.1042/bj1340217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eanes RZ, Kun E. Inhibition of liver aconitase isozymes by (−)-erythro-fluorocitrate. Mol Pharmacol. 1974;10:130–139. [PubMed] [Google Scholar]