Abstract
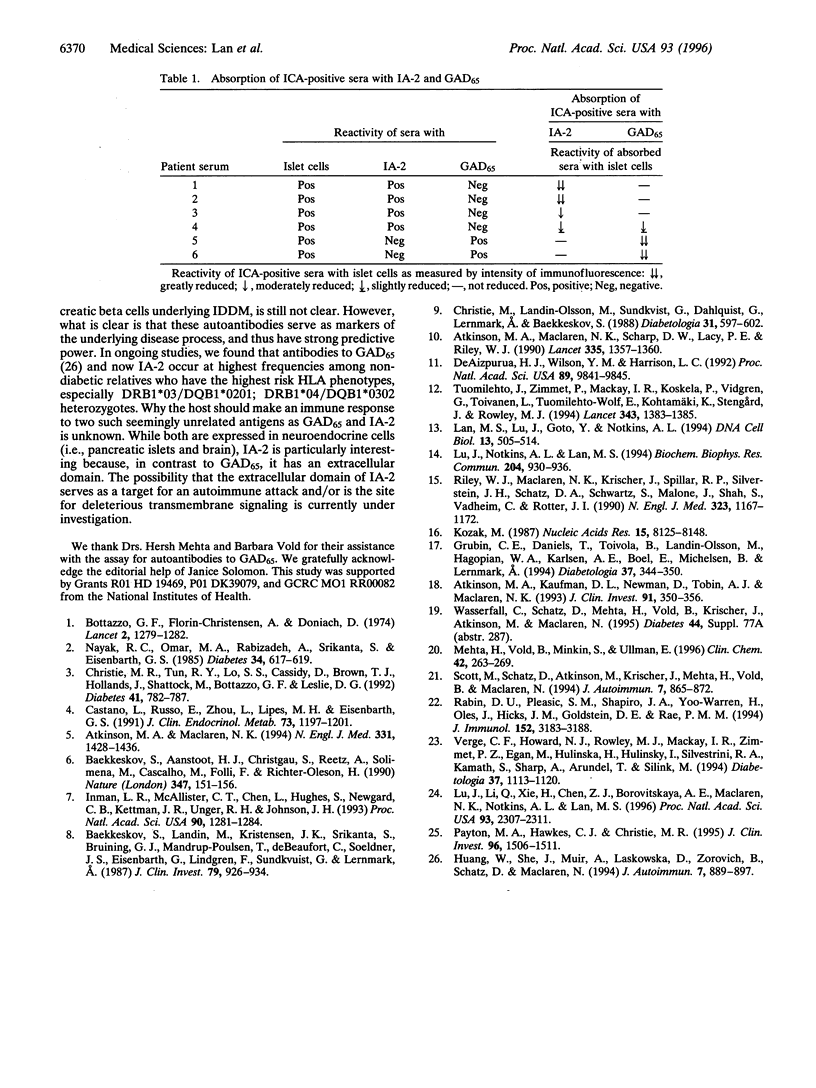
IA-2 is a 105,847 Da transmembrane protein that belongs to the protein tyrosine phosphatase family. Immunoperoxidase staining with antibody raised against IA-2 showed that this protein is expressed in human pancreatic islet cells. In this study, we expressed the full-length cDNA clone of IA-2 in a rabbit reticulocyte transcription/translation system and used the recombinant radiolabeled IA-2 protein to detect autoantibodies by immunoprecipitation. Coded sera (100) were tested: 50 from patients with newly diagnosed insulin-dependent diabetes mellitus (IDDM) and 50 from age-matched normal controls. Sixty-six percent of the sera from patients, but none of the sera from controls, reacted with IA-2. The same diabetic sera tested for autoantibodies to islet cells (ICA) by indirect immunofluorescence and glutamic acid decarboxylase (GAD65Ab) by depletion ELISA showed 68% and 52% positivity, respectively. Up to 86% of the IDDM patients had autoantibodies to IA-2 and/or GAD65. Moreover, greater than 90% (14 of 15) of the ICA-positive but GAD65Ab-negative sera had autoantibodies to IA-2. Absorption experiments showed that the immunofluorescence reactivity of ICA-positive sera was greatly reduced by prior incubation with recombinant IA-2 or GAD65 when the respective antibody was present. A little over one-half (9 of 16) of the IDDM sera that were negative for ICA were found to be positive for autoantibodies to IA-2 and/or GAD65, arguing that the immunofluorescence test for ICA is less sensitive than the recombinant tests for autoantibodies to IA-2 and GAD65. It is concluded that IA-2 is a major islet cell autoantigen in IDDM, and, together with GAD65, is responsible for much of the reactivity of ICA with pancreatic islets. Tests for the detection of autoantibodies to recombinant IA-2 and GAD65 may eventually replace ICA immunofluorescence for IDDM population screening.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Kaufman D. L., Newman D., Tobin A. J., Maclaren N. K. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J Clin Invest. 1993 Jan;91(1):350–356. doi: 10.1172/JCI116192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Scharp D. W., Lacy P. E., Riley W. J. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990 Jun 9;335(8702):1357–1360. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994 Nov 24;331(21):1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Landin M., Kristensen J. K., Srikanta S., Bruining G. J., Mandrup-Poulsen T., de Beaufort C., Soeldner J. S., Eisenbarth G., Lindgren F. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987 Mar;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Castaño L., Russo E., Zhou L., Lipes M. A., Eisenbarth G. S. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrinol Metab. 1991 Dec;73(6):1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Tun R. Y., Lo S. S., Cassidy D., Brown T. J., Hollands J., Shattock M., Bottazzo G. F., Leslie R. D. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992 Jul;41(7):782–787. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- Christie M., Landin-Olsson M., Sundkvist G., Dahlquist G., Lernmark A., Baekkeskov S. Antibodies to a Mr-64,000 islet cell protein in Swedish children with newly diagnosed type 1 (insulin-dependent) diabetes. Diabetologia. 1988 Aug;31(8):597–602. doi: 10.1007/BF00264766. [DOI] [PubMed] [Google Scholar]
- De Aizpurua H. J., Wilson Y. M., Harrison L. C. Glutamic acid decarboxylase autoantibodies in preclinical insulin-dependent diabetes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9841–9845. doi: 10.1073/pnas.89.20.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubin C. E., Daniels T., Toivola B., Landin-Olsson M., Hagopian W. A., Li L., Karlsen A. E., Boel E., Michelsen B., Lernmark A. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994 Apr;37(4):344–350. doi: 10.1007/BF00408469. [DOI] [PubMed] [Google Scholar]
- Huang W., She J. X., Muir A., Laskowska D., Zorovich B., Schatz D., Maclaren N. K. High risk HLA-DR/DQ genotypes for IDD confer susceptibility to autoantibodies but DQB1*0602 does not prevent them. J Autoimmun. 1994 Dec;7(6):889–897. doi: 10.1006/jaut.1994.1073. [DOI] [PubMed] [Google Scholar]
- Inman L. R., McAllister C. T., Chen L., Hughes S., Newgard C. B., Kettman J. R., Unger R. H., Johnson J. H. Autoantibodies to the GLUT-2 glucose transporter of beta cells in insulin-dependent diabetes mellitus of recent onset. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1281–1284. doi: 10.1073/pnas.90.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M. S., Lu J., Goto Y., Notkins A. L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994 May;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lu J., Li Q., Xie H., Chen Z. J., Borovitskaya A. E., Maclaren N. K., Notkins A. L., Lan M. S. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2307–2311. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Notkins A. L., Lan M. S. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994 Oct 28;204(2):930–936. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- Mehta H. B., Vold B. S., Minkin S., Ullman E. F. DELISA: sensitive nonisotopic assay for GAD65 autoantibodies, a key risk-assessment marker for insulin-dependent diabetes mellitus. Clin Chem. 1996 Feb;42(2):263–269. [PubMed] [Google Scholar]
- Nayak R. C., Omar M. A., Rabizadeh A., Srikanta S., Eisenbarth G. S. "Cytoplasmic" islet cell antibodies. Evidence that the target antigen is a sialoglycoconjugate. Diabetes. 1985 Jun;34(6):617–619. doi: 10.2337/diab.34.6.617. [DOI] [PubMed] [Google Scholar]
- Payton M. A., Hawkes C. J., Christie M. R. Relationship of the 37,000- and 40,000-M(r) tryptic fragments of islet antigens in insulin-dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA512). J Clin Invest. 1995 Sep;96(3):1506–1511. doi: 10.1172/JCI118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Shapiro J. A., Yoo-Warren H., Oles J., Hicks J. M., Goldstein D. E., Rae P. M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994 Mar 15;152(6):3183–3188. [PubMed] [Google Scholar]
- Riley W. J., Maclaren N. K., Krischer J., Spillar R. P., Silverstein J. H., Schatz D. A., Schwartz S., Malone J., Shah S., Vadheim C. A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med. 1990 Oct 25;323(17):1167–1172. doi: 10.1056/NEJM199010253231704. [DOI] [PubMed] [Google Scholar]
- Schott M., Schatz D., Atkinson M., Krischer J., Mehta H., Vold B., Maclaren N. GAD65 autoantibodies increase the predictability but not the sensitivity of islet cell and insulin autoantibodies for developing insulin dependent diabetes mellitus. J Autoimmun. 1994 Dec;7(6):865–872. doi: 10.1006/jaut.1994.1070. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J., Zimmet P., Mackay I. R., Koskela P., Vidgren G., Toivanen L., Tuomilehto-Wolf E., Kohtamäki K., Stengård J., Rowley M. J. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994 Jun 4;343(8910):1383–1385. doi: 10.1016/s0140-6736(94)92521-6. [DOI] [PubMed] [Google Scholar]
- Verge C. F., Howard N. J., Rowley M. J., Mackay I. R., Zimmet P. Z., Egan M., Hulinska H., Hulinsky I., Silvestrini R. A., Kamath S. Anti-glutamate decarboxylase and other antibodies at the onset of childhood IDDM: a population-based study. Diabetologia. 1994 Nov;37(11):1113–1120. doi: 10.1007/BF00418375. [DOI] [PubMed] [Google Scholar]