Abstract
Background
Chronic symptoms of orthostatic intolerance occur in postural tachycardia syndrome (POTS) and patients with orthostatic intolerance (OI) without tachycardia. We recently reported that deconditioning is almost universal in both patient groups. In this study, we focussed on the question of how much dysautonomia, besides orthostatic tachycardia, is there in POTS vs. OI, and how the two groups compare in regards to clinical, autonomic, laboratory, and exercise variables.
Methods
We retrospectively studied all patients referred for orthostatic intolerance at Mayo Clinic between January 2006 and June 2011, who underwent standardized autonomic and exercise testing.
Results
Eighty-four POTS and 100 OI fulfilled inclusion criteria, 89 % were females. The mean age was 25 and 32 years, respectively. Clinical presentation, autonomic parameters, laboratory findings, and degree of deconditioning were overall similar between the two groups, except for the excessive orthostatic heart rate (HR) rise and mild vasomotor findings observed in POTS but not in OI (slightly larger Valsalva ratio and incomplete blood pressure recovery during Valsalva). Both groups responded poorly to various medications. Severely deconditioned patients were similar to non-deconditioned patients, except for 24 h urine volume (1,555 vs. 2,417 ml), sweat loss on thermoregulatory sweat test (1.5 vs. 0.5 %), and few respiratory parameters during exercise, which are likely clinically insignificant.
Conclusion
Though similar in clinical presentation, POTS and OI are different entities with greater, albeit still mild, dysautonomia in POTS. The clinical and pathophysiological relevance of minimal dysautonomia in the absence of orthostatic tachycardia as seen in OI remain uncertain.
Keywords: Orthostatic intolerance, Postural tachycardia syndrome, Exercise capacity, Deconditioning
Background
We recently reported that deconditioning is an almost universal finding in patients with symptoms of orthostatic intolerance associated with orthostatic tachycardia (postural tachycardia syndrome, POTS) or without orthostatic tachycardia (orthostatic intolerance alone, OI) [1]. The focus of that report was on deconditioning. This part of the study is focussed specifically on the role of dysautonomia in POTS and OI. Chronic orthostatic intolerance (OI) occurs in neurogenic orthostatic hypotension and symptoms are due to cerebral hypoperfusion [2]. In contrast, cerebral hypoperfusion is only sometimes present in POTS and when present, is thought to relate to hypocapnia-induced cerebral vasoconstriction [2]. Psychologic factors such as catastrophic cognitions, somatic hypervigilance, anxiety sensitivity, and neuroticism have been reported to occur in higher frequency in patients with orthostatic intolerance and likely add to functional limitations and symptoms [3, 4]. Autonomic testing has focussed on identifying reliable autonomic indices that characterize the dysautonomic components of the syndrome. Orthostatic tachycardia has been the most reliable index and has been demonstrated not to be due to anxiety [5]. Since this index is absent at the time of testing in OI, we sought to evaluate, retrospectively, if the severity and distribution of other autonomic indices would distinguish the two conditions.
We examined if the clinical presentation, autonomic and exercise parameters are similar in the two conditions, and also evaluated the relationship of these parameters to the degree of deconditioning. We have reported previously that most POTS patients have functional disability and limited activities of daily living [4]. However, patients with OI have not been studied for such functional limitations. We previously reported that deconditioning is similar between POTS and OI [1]; however, other exercise parameters have not been compared between the two groups nor have they been related to the degree of deconditioning. Our large cohort of patients with POTS and OI, who underwent standardized exercise, autonomic, and laboratory evaluations at Mayo Clinic, provides a unique data set that allows us to undertake this analysis.
We hypothesized that POTS and OI are phenotypically similar entities with subtle differences in the degree and pattern of dysautonomia. We furthermore hypothesized that deconditioned patients have a clinical presentation similar to patients with no or mild degrees of deconditioning and that autonomic and laboratory parameters do not vary with the degree of deconditioning.
Schematic design of the study
Our study was approved by the Mayo Clinic Institutional Review Board. Electronic medical records of all patients, who presented with orthostatic symptoms at Mayo Clinic, Rochester, MN, between January 2006 and June 2011 and who underwent autonomic and exercise testing, were reviewed retrospectively. Patients who did not authorize the use of their medical records for research purpose, minors (<18 years), patients with medical conditions or on medications known to cause orthostatic tachycardia, and those with incomplete medical records were excluded from the study.
Definition of different variables
Orthostatic intolerance was defined as the development of previously defined symptoms of cerebral hypoperfusion or sympathetic activation upon standing along with a heart rate (HR) increment <30 bpm on head-up tilt (HUT) [6], while those with symptomatic increase in heart rate on HUT ≥ 30 bpm were defined as POTS. Patients with predicted VO2 max of <85 % on exercise testing were considered deconditioned, <65 % severely deconditioned while those with ≥85 % were considered normal.
Exercise test parameters
Measured VO2 max was defined as the maximum capacity of an individual’s body to transport and use oxygen during incremental exercise, while predicted VO2 max was calculated as 60 − (age × 0.5) for males and 40 − (age × 0.4) for females. Predicted VO2 max % was calculated as measured VO2 max/predicted VO2 max multiplied by 100. HR recovery was calculated as peak HR − HR for 1 min during recovery period after the cessation of exercise. The following HR and BP variables were calculated: ΔHR_stage1: exercise stage1 HR − supine HR; ΔHR_peak: peak HR during − supine HR, ΔSBP_stage1: exercise stage 1 systolic blood pressure (SBP) − resting SBP, ΔDBP_stage1: exercise stage 1 diastolic blood pressure (DBP) − resting DBP; ΔSBP_peak: peak SBP during exercise − resting SBP, and ΔDBP_peak: peak DBP during exercise − resting DBP. Breathing reserve was defined as percentage of a subject’s maximum voluntary ventilation that is not utilized at peak exercise [7]. Peak tidal volume (TV) was defined as the patient’s highest value of TV sustained for at least 20 s and peak respiratory rate (RR) was defined as patient’s highest RR during exercise testing. Respiratory effort was defined based on the peak respiratory exchange ratio (RER): RER < 1.05, inadequate effort; RER 1.05–1.15, near maximum; RER > 1.15, maximum effort.
Valsalva test parameters
The change (Δ) in SBP and DBP during early phase II (IIE), late phase II (IIL) and phase IV (IV) of the Valsalva maneuver (VM) were assessed compared to baseline average BP.
Head-up tilt parameters
Supine HR and BP were calculated as the average HR and BP during 5 min of resting in supine position. HUT was done routinely for 10 min. ΔSBP_H, ΔDBP_H, ΔHR and Δ pulse pressure were calculated as follows: ΔSBP_H: average SBP during second half of HUT − resting SBP; ΔDBP_H: average DBP during second half of HUT − resting ΔDBP; ΔHR: average HR during second half of HUT − resting HR; and Δpulse pressure: HUT pulse pressure − resting pulse pressure.
Exercise test
All subjects underwent symptom-limited graded treadmill exercise testing to maximum or near maximum level using metabolic cart (Medical Graphic CPX; Medical Graphic Corporation; St. Paul, MN). Initial workload was 2 mph at 0 % grade (equivalent to 2.5 METs), and the load was increased every 2 min by 2 METs until symptom-limited termination. This was followed by 3 min of active recovery at a speed of 1.7 mph with 0 % grade, then three or more additional minutes of seated recovery. Expired gases were monitored from rest throughout exercise and 1 min of active recovery. Simultaneous ECG recordings were obtained. BP was measured by left arm sphygmomanometer at 1.5 min of every stage, at peak exercise, and at 30 s and 2.5 min of seated recovery.
Autonomic tests
The quantitative sudomotor axon reflex test (QSART), heart rate response to deep breathing (HRDB), beat-to-beat BP responses to the VM, Valsalva ratio (VR), and thermoregulatory sweat test (TST) were performed in a routine fashion as previously described [8, 9]. The composite autonomic severity score (CASS) is an age- and sex-adjusted semiquantitative score ranging from 0 to 10 (normal to maximum dysfunction) which combines scores for the three tested autonomic domains (i.e., sudomotor 0–3, cardiovagal 0–3, adrenergic 0–4) [10].
Data collection
Demographic, clinical symptoms, and other data were collected from electronic medical records, while exercise and autonomic parameters were collected from cardiophysiologic and autonomic databases, respectively. Laboratory test variables were obtained as per standard clinical laboratory protocols.
Statistical considerations
Continuous measures are reported as mean ± SD or median and range, while discrete variables are reported as counts and percentages. Comparisons between two groups were analyzed with Chi-square or Fisher exact test for categorical variables and with two-sample t test or Wilcoxon rank-sum test for continuous variables, as appropriate. Comparisons among three groups were performed using ANOVA for continuous variables and Chi-square test for categorical variables. ANOVA with p < 0.05 was followed by multiple comparisons between groups along with the Bonferroni correction for p value. Calculated p values were two-sided and p values <0.05 were considered statistically significant. All statistical analysis was performed using JMP 9 (SAS institute, Cary, NC).
Results
One-hundred and eighty-four patients fulfilled the study inclusion criteria. Eighty-four patients fulfilled the criteria for POTS, while the remaining 100 fulfilled those for OI. Median age at symptom onset was 27.5 (IQR 22–37) years, with the OI group being older (p < 0.0001) (Table 1). Patients were predominately female, 89 % (Table 1).
Table 1.
Baseline demographic and clinical presentation of POTS and OI
| POTS (84) | OI (100) | p value | |
|---|---|---|---|
| Age at onset of symptoms | 25 (IQR 0–32) | 32 (IQR 4–40) | <0.0001 |
| Females | 73 (87 %) | 91 (91 %) | 0.37 |
| Duration of illness (years) | 3 (IQR 1.1–6) | 5 (IQR 2–8) | 0.0094 |
| Lightheadedness | 82 (98 %) | 95 (93 %) | 0.18 |
| Weakness | 34 (41 %) | 49 (49 %) | 0.25 |
| Presyncope/syncope | 58 (69 %) | 68 (68 %) | 0.87 |
| Head pressure/headache | 55 (66 %) | 59 (59 %) | 0.37 |
| Palpitation | 68 (81 %) | 83 (83 %) | 0.72 |
| Tremulousness | 14 (17 %) | 14 (14 %) | 0.62 |
| Dyspnea | 31 (37 %) | 31 (31 %) | 0.40 |
| Chest pain | 27 (32 %) | 32 (32 %) | 0.98 |
| Decreased sweating | 3 (4 %) | 2 (2 %) | 0.51 |
| Increased sweating | 15 (18 %) | 15 (15 %) | 0.60 |
| Heat intolerance | 20 (24 %) | 21 (21 %) | 0.65 |
| Exercise intolerance | 84 (100 %) | 97 (97 %) | 0.25 |
| Post prandial intolerance | 2 (2 %) | 7 (7 %) | 0.18 |
| Bloating | 20 (24 %) | 20 (20 %) | 0.53 |
| Nausea | 48 (57 %) | 61 (61 %) | 0.60 |
| Vomiting | 15 (18 %) | 27 (27 %) | 0.14 |
| Abdominal pain | 19 (23 %) | 25 (25 %) | 0.71 |
| Constipation | 24 (29 %) | 41 (41 %) | 0.08 |
| Diarrhea | 25 (30 %) | 34 (34 %) | 0.54 |
| Visual abnormality (blurring, diplopia, etc.) | 14 (17 %) | 22 (22 %) | 0.36 |
| Bladder symptoms | 16 (19 %) | 16 (16 %) | 0.59 |
| Sleep disturbances | 45 (54 %) | 56 (56 %) | 0.42 |
| Fatigue | 62 (74 %) | 69 (69 %) | 0.47 |
| Migraine | 35 (42 %) | 47 (47 %) | 0.47 |
| Myofascial pain | 14 (17 %) | 16 (16 %) | 0.90 |
| Neuropathic pain | 3 (4 %) | 4 (4 %) | 1.00 |
| Orthostatic intolerance related to menstruation | 3 (4 %) | 4 (4 %) | 1.00 |
| Cognitive decline/mental clouding | 21 (25 %) | 19 (19 %) | 0.33 |
| Past history of orthostatic intolerance | 3 (4 %) | 7 (7 %) | 0.35 |
| Family history of orthostatic intolerance | 4 (5 %) | 1 (1 %) | 0.18 |
| Mitral valve regurgitation | 6 (7 %) | 11 (11 %) | 0.37 |
| Mitral valve prolapse | 1 (1 %) | 2 (2 %) | 1.00 |
| Panic attack | 6 (7 %) | 5 (5 %) | 0.54 |
| Bud Chiari syndrome | 2 (2 %) | 1 (1 %) | 0.59 |
| Motor examinationa | 84 (100 %) | 99 (99 %) | 1.00 |
| Sensory examinationa | 79 (94 %) | 95 (95 %) | 1.00 |
| Pupillary examinationa | 84 (100 %) | 96 (96 %) | 0.13 |
Bold values indicate statistical significance
Normal neuroexamination
Table 1 describes the details of clinical presentation of both groups. For POTS patients, the onset of illness was acute (<1 month) in 5 (6 %), subacute (1–3 months) in 2 (2 %), gradual (>3 months) in 72 (86 %), and unknown in 5 (6 %) patients. For patients with OI, the onset was acute in 2 (2 %) patients, subacute in 2 (2 %), gradual in 83 (83 %), and unknown in 13 (13 %) patients. The work capacity was reduced in 98 % of POTS and OI both, while 1 % of POTS and 2 % of OI had near normal function.
Clinical symptoms were similar between POTS and OI, but the duration of symptoms was longer among patients with OI compared to POTS (p = 0.0094) (Table 1). Seventy- four (40 %) of the 184 patients had additional disorders, including psychiatric diagnoses (depression, anxiety, posttraumatic stress disorder, attention deficit disorder, somatoform disorder), autoimmune disorders (diabetes mellitus, Hashimoto thyroiditis, celiac disease, Sjögren syndrome), asthma, myalgia, arthralgia, hypertension, and chronic fatigue syndrome. The frequencies of these conditions were similar between the two groups. Response to different medications was poor in both groups.
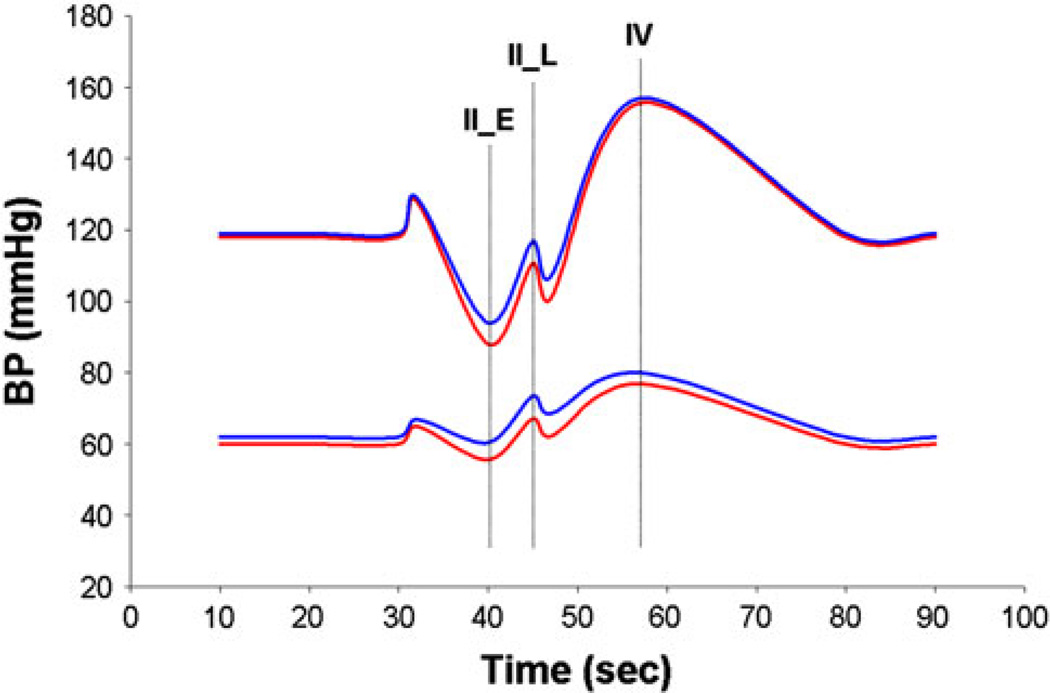
ΔHR_H was markedly higher in patient with POTS as expected. Measures of cardiovagal sensitivity (VR and HRDB) were higher among POTS compared to OI (p ≤ 0.002), possibly reflecting the slightly younger age of this group. BP responses to the Valsalva maneuver showed a trend toward a greater decrease during phase IIE, and there was a less complete BP recovery during phase IIL in POTS compared to OI (ΔIIL DBP, p = 0.04), consistent with a suggestion of mild vascular adrenergic impairment limited to the POTS group (Fig. 1). The rest of the autonomic parameters was similar between the two groups (Table 2A).
Fig. 1.
Blood pressure (systolic and diastolic) profile comparison between POTS (red) and OI (blue) during the Valsalva maneuver
Table 2.
Autonomic and laboratory (A) and exercise (B) parameters between POTS and OI
| POTS (n = 4) | OI (n = 00) | p value | |
|---|---|---|---|
| A | |||
| Baseline SBP | 118 ± 17 | 119 ± 16 | 0.46 |
| ΔSBP_H | −4.4 ± 15.2 | −2.1 ± 12.2 | 0.26 |
| Baseline DBP | 60 ± 14 | 62 ± 10 | 0.12 |
| ΔDBP_H | 8.6 ± 9.5 | 6.6 ± 7.1 | 0.11 |
| Baseline HR | 74 ± 12 | 74 ± 12 | 0.99 |
| ΔHR_H | 40.19 ± 8.6 | 19.9 ± 7.3 | <0.0001 |
| % Anhydrosis on TST | 1(IQR 0–4.3) | 1 (IQR 0–3) | 0.16 |
| ΔIIE SBP | −30.1 ± 19.3 | −25.0 ± 21.6 | 0.09 |
| ΔIIE DBP | −4.4 ± 10.4 | −1.6 ± 10.6 | 0.07 |
| ΔIIL SBP | −7.4 ± 22.5 | −2.2 ± 22.0 | 0.11 |
| ΔIIL DBP | 7.1 ± 14.2 | 11.4 ± 13.7 | 0.04 |
| ΔIV SBP | 37.6 ± 19.1 | 38.0 ± 20.6 | 0.91 |
| ΔIV DBP | 16.8 ± 9.7 | 18.0 ± 9.5 | 0.40 |
| Valsalva ratio | 2.32 ± 0.68 | 2.07 ± 0.48 | 0.0024 |
| HRDB | 24.25 ± 9.21 | 20.09 ± 8.81 | 0.0021 |
| Hemoglobin | 13.6 ± 1.2 | 13.6 ± 1.1 | 0.75 |
| 24 h Urine volume | 1,649.1 ± 836.6 | 1,667.9 ± 1025.5 | 0.90 |
| 24 h Urine sodium | 131.8 ± 6.9 | 119.6 ± 60.6 | 0.19 |
| Plasma norepinephrine supine | 252.9 ± 127.0 | 295.6 ± 241.9 | 0.18 |
| Plasma norepinephrine upright | 562.9 ± 349.8 | 573.1 ± 334.4 | 0.9 |
| B | |||
| Supine HR | 82.5 ± 13.6 | 85.3 ± 14.8 | 0.20 |
| Sitting HR | 87.6 ± 15.6 | 85.9 ± 14.1 | 0.44 |
| Standing HR | 95.6 ± 18 | 91.7 ± 16.4 | 0.11 |
| Stage 1 HR | 109 ± 16 | 106.2 ± 16.4 | 0.24 |
| Peak HR | 174.2 ± 17.9 | 166 ± 19.9 | 0.004 |
| ΔHR_stage1 | 26.5 ± 13.2 | 20.9 ± 10.6 | 0.002 |
| ΔHR_peak | 91.6 ± 21.6 | 80.7 ± 20.8 | 0.0006 |
| HR_cool down 1 min | 151.9 ± 19.8 | 144.2 ± 20.6 | 0.01 |
| HR Recovery (1 min) | 22.3 ± 9.2 | 21.9 ± 9.8 | 0.76 |
| Peak HR | 171.7 ± 9 | 164 ± 9.9 | <0.0001 |
| Resting SBP | 108.5 ± 12.4 | 110.1 ± 14.1 | 0.42 |
| Resting DBP | 75 ± 9.1 | 73.6 ± 13.8 | 0.42 |
| ΔSBP_stage1 | 7.2 ± 10.2 | 8.2 ± 12.7 | 0.55 |
| ΔDBP_stage1 | −3.1 ± 8.1 | −1.6 ± 11.8 | 0.33 |
| ΔSBP_peak | 38.6 ± 20.8 | 36.5 ± 20.6 | 0.50 |
| ΔDBP_peak | −10.8 ± 19.6 | −6.5 ± 19.8 | 0.15 |
| Peak RR | 34 ± 8.1 | 33 ± 0.8 | 0.39 |
| Peak TV | 1,714 ± 491.6 | 1,756.3 ± 517.8 | 0.57 |
| Peak O2 saturation | 97.4 ± 1.6 | 97.5 ± 2 | 0.55 |
| VO2 max % (predicted) | 64.1 ± 15.8 | 65.2 ± 14.6 | 0.61 |
| Breathing reserve | 58.9 ± 11.5 | 55.4 ± 10.6 | 0.03 |
Bold values indicate statistical significance
Peak HR, ΔHR_stage1, ΔHR_peak, HR_cool down 1 min, peak HR and breathing reserve were significantly higher among POTS compared to OI patients; however, predicted VO2 max % did not differ between the two groups (Table 2B).
Demographics and clinical presentation were similar between normal and deconditioned groups except for bloating and sleep disturbances, both of which were less frequent among deconditioned patients (p = 0.04 for both). Demographics and clinical presentation were also similar between mildly and severely deconditioned groups except for a history of vomiting (severely deconditioned 30 % vs. mildly deconditioned 15 %, p = 0.03).
Baseline DBP and TST anhydrosis were slightly different across the three groups (normal, mildly, and severely deconditioned), both slightly higher among severely deconditioned patients (Table 3). VR, ΔIV SBP, and 24 h urine volume also varied across the three groups (Table 3). VR was mildly but significantly higher for the mildly deconditioned compared to the severely deconditioned group (p = 0.03 after Bonferroni correction). Urine volume did not differ between mildly and severely deconditioned groups (p = 0.16) but was lower among both groups compared to normal (normal vs. mild, p = 0.02, normal vs. severe p = 0.016 after Bonferroni correction). Using multiple comparisons, ΔIV SBP was not significant between the different groups after Bonferroni correction. Looking only at POTS patients, only 24 h urine volume and upright plasma NE were different between the degree of deconditioning, lower among severely deconditioned patients compared to normal (p ≤ 0.03). Peak RER (indicator of respiratory efforts) was almost identical among normal (1.19 ± 0.1), mildly deconditioned (1.22 ± 0.1) and severely deconditioned patients (1.18 ± 0.13) (p = 0.05).
Table 3.
Autonomic characteristics of normal and deconditioned patients
| Normal (13) | Mildly deconditioned (78) | Severely deconditioned (93) | p value | |
|---|---|---|---|---|
| Baseline SBP | 117.3 ± 12.8 | 115 ± 16.2 | 120.8 ± 16.7 | 0.13 |
| Baseline DBP | 56.8 ± 6.2 | 58.9 ± 11.8 | 63.3 ± 12.2 | 0.02 |
| Baseline HR | 69.2 ± 10.8 | 73.2 ± 12.1 | 75.3 ± 12.2 | 0.18 |
| ΔSBP_H | −5.7 ± 11.5 | −4.1 ± 12.8 | −2.0 ± 14.6 | 0.48 |
| ΔDBP_H | 8.8 ± 4.9 | 8.0 ± 7.8 | 6.9 ± 9.1 | 0.58 |
| ΔHR_H | 30.1 ± 14.4 | 30.9 ± 11.7 | 27.5 ± 13.4 | 0.22 |
| ΔPulse pressure | −14.5 ± 8.4 | −12.1 ± 9.4 | −8.9 ± 13 | 0.08 |
| Valsalva ratio | 2.1 ± 0.5 | 2.3 ± 0.7 | 2.1 ± 0.5 | 0.04 |
| HRDB | 17.5 ± 7.5 | 23.4 ± 9.0 | 21.4 ± 9.4 | 0.07 |
| TST % | 0.5 (IQR 0–6.25) | 0 (IQR 0–2) | 1.5 (IQR 0–6) | 0.017 |
| Valsalva baseline HR | 78.5 ± 11.9 | 79.1 ± 12.2 | 79.3 ± 12.1 | 1.0 |
| Valsalva baseline SBP | 117 ± 21.9 | 120.8 ± 16.5 | 125.3 ± 17.7 | 0.12 |
| Valsalva baseline DBP | 59.6 ± 9.6 | 64.5 ± 11.7 | 66.6 ± 11.4 | 0.09 |
| ΔIIE SBP | −31.8 ± 29.1 | −27.6 ± 18.8 | −26.4 ± 21.1 | 0.68 |
| ΔIIE DBP | −2.4 ± 14.2 | −2.7 ± 10.3 | −3.2 ± 10.4 | 0.94 |
| ΔIIL SBP | −10.5 ± 24.9 | −3.8 ± 21.7 | −4.3 ± 22.6 | 0.61 |
| ΔIIL DBP | 8.7 ± 14.4 | 9.8 ± 13.6 | 9.2 ± 14.4 | 0.94 |
| ΔIV SBP | 42.8 ± 20.0 | 41.4 ± 21.0 | 34.2 ± 18.4 | 0.04 |
| ΔIV DBP | 15.8 ± 8.4 | 17.6 ± 9.4 | 17.6 ± 10.0 | 0.81 |
| 24 h urine volume | 2,416.5 ± 1,045.2 | 1,661.4 ± 842.2 | 1,554.7 ± 971.8 | 0.012 |
| 24 h urine sodium | 130.3 ± 52.4 | 130.9 ± 64.2 | 119.5 ± 57.2 | 0.48 |
| Plasma NE supine | 220.2 ± 91.1 | 261.7 ± 163.9 | 299.8 ± 238.3 | 0.31 |
| Plasma NE upright | 610.1 ± 202.8 | 562.3 ± 274.3 | 567.8 ± 409 | 0.90 |
| Hemoglobin | 14.0 ± 1.1 | 13.6 ± 1.1 | 13.5 ± 1.2 | 0.44 |
Bold values indicate statistical significance
Discussion
Even though many autonomic researchers have focused on POTS, OI has remained a nondescript entity. Some have regarded these patients as having a functional or psychiatric disorder. Others have considered OI to be a POTS variant (or in the POTS spectrum). Our findings support the hypothesis that POTS as a group is associated with greater dysautonomia (with a greater orthostatic heart rate increment, by definition) than OI, which is further supported by mild differences in the blood pressure responses during phase II between the two groups. Otherwise, autonomic deficits are similar, but not illuminating here, since they are minimal (at least in the majority of patients). As the wide spectrum of clinical complaints is generally disproportionate to the degree of abnormality found on testing, it cannot be completely explained by the autonomic abnormalities, suggesting that the two disorders may share a psychologic substrate or overlay. However, the abnormal autonomic responses are undeniably present, albeit mild. Their etiology remains still incompletely understood and is likely heterogeneous.
A prospective study similar to our previous one on POTS would be required to assess and compare this notion satisfactorily [4].
The exercise abnormalities in both conditions indicate a high prevalence of deconditioning in POTS and OI alike. We found that deconditioned patients had a clinical presentation and symptom aggravating factors similar to those with normal predicted VO2 max %. Subtle statistical differences between different degrees of deconditioning observed in certain autonomic and laboratory variables are unlikely to be of clinical significance. Some patients with POTS show evidence of a limited autonomic neuropathy [9], and our sudomotor findings would indicate that such findings are most prominent among severely deconditioned patients. Beside cardiovascular compromise, deconditioned patients also had lower peak TV, peak RR and higher breathing reserve. Peak RR was not different between normal and mildly deconditioned patients; however, it was lower in the severely deconditioned group. These findings suggest mechanical respiratory impairment in the deconditioned groups, especially the severely deconditioned patients given that peak RER (measure of respiratory effort) was similar between deconditioned and normal groups. Significant correlation between peak TV and vital capacity (r = 0.827, p < 0.000l) described by Gowda et al. [11] supports our findings of mechanical respiratory impairment in deconditioned patients but requires further evidence to make definitive conclusions.
Symptom onset was mostly gradual in both groups with few patients having subacute and acute onset. About one-third of the patients had an antecedent trigger with viral illness being the most common in both groups; yet, this finding was less frequent than reported previously [9, 12]. However, a direct association between different risk factors and onset of orthostatic intolerance is difficult because most patients are not able to precisely identify the date and nature of those potential factors. Prolonged immobilization during a recovery phase after a major illness or surgery can lead to deconditioning, thus exaggerating the symptoms. Other triggers could also elicit orthostatic symptoms and affect autonomic nervous system function, and this aspect clearly requires further study.
In OI patients, the duration of illness was longer and OI patients were generally older than POTS patients. That may explain lower vagal sensitivity in OI patients as suggested by VR and HRBD, but the reason for the age difference remains elusive.
What interim conclusions can we draw and what management recommendations can we make? First, we should continue to use autonomic function testing to identify patients with orthostatic tachycardia and identify those with an autonomic neuropathy or hyperadrenergic state, since they need specific treatment. Second, both POTS and OI have underlying deconditioning, which requires treatment. Even though promising short-term response to different medications has been reported previously [13, 14], we found evidence of poor long-term responses to all medications in both groups. Furthermore, many side effects related to different medications have been described [15]. Hence, POTS and OI should be managed with a combination of exercise and other measures including suitable medications, as well as behavioral therapy in selected patients that present with a somatic hypervigilance component.
Our study has certain limitations which include its retrospective nature, the fact that the magnitude of HR increment during tilt may fluctuate during the day, being maximal usually in the morning. Thus, some OI patients may have fulfilled POTS criteria if tested at different time. Other limitations include the bias of a tertiary referral center, a small number of patients with normal predicted VO2 max %, and inadequate effort during exercise testing in some patients. However, patients referred for exercise testing comprised >80 % of the patients with orthostatic intolerance seen at Mayo Clinic during the timeframe of the study, and therefore, we are confident they are representative of this population seen at Mayo Clinic, Rochester, Minnesota. A key unanswered question is whether a postulated psychologic contribution to the clinical syndrome is different in OI and POTS. A prospective evaluation similar to what we have undertaken in POTS previously is needed to address this important question [3, 4].
Acknowledgments
This work was supported in part by National Institutes of Health (NS 44233 Pathogenesis and Diagnosis of Multiple System Atrophy, U54 NS065736 Autonomic Rare Disease Clinical Consortium), Mayo CTSA (UL1 TR000135), and Mayo Funds. The Autonomic Diseases Consortium is a part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54 NS065736 from the National Institute of Neurological Diseases and Stroke (NINDS) and the NIH Office of Rare Diseases Research (ORDR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
- POTS
Postural tachycardia syndrome
- OI
Orthostatic intolerance without excessive tachycardia
- HR
Heart rate
- BP
Blood pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- HUT
Head-up tilt
- ΔHR
Change/difference in heart rate
- ΔSBP
Change/difference in systolic blood pressure
- ΔDBP
Change/difference in diastolic blood pressure
- MET
Metabolic equivalent of task
- TV
Tidal volume
- RER
Respiratory exchange ratio
- RR
Respiratory rate
- IIE
Early phase II of Valsalva maneuver
- IIL
Late phase II of Valsalva maneuver
- IV
Phase IV of Valsalva maneuver
- ΔHR_H, ΔSBP/DBP_H
Change in heart rate and blood pressures during head-up tilt
- QSART
Quantitative sudomotor axon reflex test
- HRDB
Heart rate response to deep breathing
- VM
Valsalva maneuver
- VR
Valsalva ratio
- TST
Thermoregulatory sweat test
- CASS
Composite autonomic severity score
Footnotes
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ, Benarroch EE, Low PA, Sandroni P. Deconditioning in patients with orthostatic intolerance. Neurology. 2012;79(14):1435–1439. doi: 10.1212/WNL.0b013e31826d5f95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29(9):1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 3.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77(6):531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson LM, Sandroni P, Haythornthwaite JA, Rummans TA, Low PA. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross-sectional findings. Health Psychol. 2003;22(6):643–648. doi: 10.1037/0278-6133.22.6.643. [DOI] [PubMed] [Google Scholar]
- 5.Masuki S, Eisenach JH, Johnson CP, Dietz NM, Benrud-Larson LM, Schrage WG, Curry TB, Sandroni P, Low PA, Joyner MJ. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol. 2007;102:896–903. doi: 10.1152/japplphysiol.00927.2006. [DOI] [PubMed] [Google Scholar]
- 6.Low PA, Sandroni P, Joyner MJ, Shen WK. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20(3):352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman DE, Myers J, Lavie CJ, Guazzi M, Celli B, Arena R. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122(6):68–86. doi: 10.3810/pgm.2010.11.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10(1):14–27. doi: 10.1097/00004691-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Thieben M, Sandroni P, Sletten D, Benrud-Larson L, Fealey R, Vernino S, Lennon V, Shen W, Low PA. Postural orthostatic tachycardia syndrome—Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 10.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 11.Gowda K, Zintel T, McParland C, Orchard R, Gallagher CG. Diagnostic value of maximal exercise tidal volume. Chest. 1990;98(6):1351–1354. doi: 10.1378/chest.98.6.1351. [DOI] [PubMed] [Google Scholar]
- 12.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43(1):132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 13.Lai CC, Fischer PR, Brands CK, Fisher JL, Porter CB, Driscoll SW, Graner KK. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol. 2009;2(2):234–238. doi: 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74(11):1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JN, Mack KJ, Kuntz NL, Brands CK, Porter CJ, Fischer PR. Postural orthostatic tachycardia syndrome: a clinical review. Pediatr Neurol. 2010;42(2):77–85. doi: 10.1016/j.pediatrneurol.2009.07.002. [DOI] [PubMed] [Google Scholar]