Abstract
Transcranial Doppler (TCD) ultrasound provides rapid, noninvasive, real-time measures of cerebrovascular function. TCD can be used to measure flow velocity in the basal arteries of the brain to assess relative changes in flow, diagnose focal vascular stenosis, or to detect embolic signals within these arteries. TCD can also be used to assess the physiologic health of a particular vascular territory by measuring blood flow responses to changes in blood pressure (cerebral autoregulation), changes in end-tidal CO2 (cerebral vasoreactivity), or cognitive and motor activation (neurovascular coupling or functional hyperemia). TCD has established utility in the clinical diagnosis of a number of cerebrovascular disorders such as acute ischemic stroke, vasospasm, subarachnoid hemorrhage, sickle cell disease, as well as other conditions such as brain death. Clinical indication and research applications for this mode of imaging continue to expand. In this review, the authors summarize the basic principles and clinical utility of TCD and provide an overview of a few TCD research applications.
Keywords: transcranial Doppler, cerebral blood flow, ultrasound, cerebrovascular diseases
Transcranial Doppler (TCD) ultrasonography provides a relatively inexpensive, noninvasive real-time measurement of blood flow characteristics and cerebrovascular hemodynamics within the basal arteries of the brain. The physiologic data obtained from these measurements are complementary to structural data obtained from various modes of currently available vascular imaging. TCD is the most convenient way to monitor vascular changes in response to interventions during acute cerebrovascular events at the bedside. Given the convenience of this tool as a diagnostic modality, its clinical and research applications will continue to increase in the many disorders of the cerebral vessel.
Basic Principles
TCD ultrasonography is based on the principle of the Doppler effect. According to this principle, ultrasound waves emitted from the Doppler probe are transmitted through the skull and reflected by moving red blood cells within the intracerebral vessels. The difference in the frequency between the emitted and reflected waves, referred to as the “Doppler shift frequency,” is directly proportional to the speed of the moving red blood cells (blood flow velocity). Because blood flow within the vessel is laminar, the Doppler signal obtained actually represents a mixture of different Doppler frequency shifts forming a spectral display of the distribution of the velocities of individual red blood cells on the TCD monitor (Fig. 1).1,2 Spectral analysis can then be used to obtain measures of blood flow velocity, as well as a few other characteristics of flow within the insonated blood vessel. The specific parameters obtained from this spectral analysis include peak systolic velocity (Vs), end diastolic velocity (Vd), systolic upstroke or acceleration time, pulsatility index (PI), and time-averaged mean maximum velocity (Vmean).3 The Vmean is a continuous trace of peak velocities as a function of time and in most TCD instruments, it is calculated and displayed automatically.
Fig. 1.
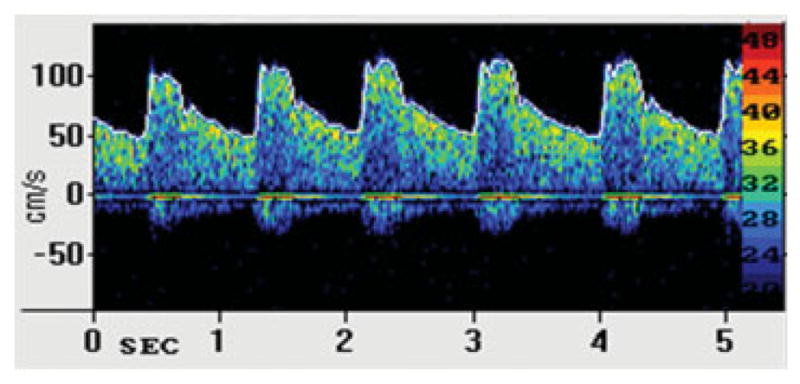
An example of spectral Doppler frequency display of the middle cerebral artery.
The formula that describes the relationship between flow velocity (reflector speed) and Doppler shift frequency is
The propagation speed of a wave is a constant that can be obtained for various mediums (speed in soft tissue is 1541 m/s). Theta (θ) is the angle of insonation or the angle of the emitted wave relative to the direction of vessel (blood flow). If the angle is zero, or the emitted wave is parallel to the direction of flow, the cosine of zero is 1, and we have achieved the most accurate measure of flow velocity. The larger the angle, the larger is the cosine of the angle; hence, the greater is the error in our velocity measure. Therefore, it is important to minimize this angle to less than 30 degrees to keep the error below 15%. In addition, the velocity of blood flow through a vessel is proportional to the fourth power of the vessel radius. However, the basic assumption for TCD measurements is that the diameter of the insonated vessel does not change during the course of a study and it remains constant in response to various physiologic variables such as blood pressure or changes in sub> has also been shown/sub>, which may easily occur as a result of treatment.4–6
Physiologic Determinants of Blood Flow Velocity and Indices
A number of physiologic variables can impact blood flow velocity as measured by TCD. The most robust of these variables are age, gender, hematocrit, viscosity, carbon dioxide, temperature, blood pressure, and mental or motor activity. Therefore, it is important to remember that during the course of a TCD study, any measured differences in blood flow velocity should be interpreted in the context of these variables. All studies should be conducted with the patients at rest—not speaking or moving their limbs.
Blood flow velocities in the basal arteries of the brain decline an average of 0.3 to 0.5% per year between 20 to 70 years of age.7–9 Women have been shown to have higher flow velocities than men between 20 to 60 years of age,7 and this difference may be explained by the lower hematocrit in premenopausal women.9 The magnitude of gender difference in this age group is ~10 to 15%.10 However, no detectable difference between the genders is observed after the age of 70 years.7
Hematocrit and viscosity are inversely related to cerebral blood flow velocity.11–14 This relationship is best exemplified in children with sickle cell anemia who have a significant drop in their mean flow velocities after a blood transfusion.15 Blood flow velocities increase by ~20% with a drop in hematocrit from 40% to 30%.10
Partial pressure of CO2 has also been shown to have a major influence on cerebral blood flow velocity10,16 (for a detailed review of this topic, please refer to the section Cerebral Vasoreactivity). Measured blood flow velocity can also be higher with higher systemic blood pressures despite an intact autoregulatory system.17 This relationship is particularly important in patients with subarachnoid hemorrhage (SAH) who are monitored for cerebral vasospasm as manifested by elevated cerebral blood flow velocity and who may simultaneously be undergoing induced hypertension to treat vasospasm. The analysis of velocity measurements are particularly challenging in these patients.
The effect of temperature on cerebral blood flow velocities is not well established. Although one study showed an inverse relationship between temperature and flow velocities,18 a more recent study of TCD flow velocities in postcardiac arrest patients treated with hypothermia does not support a relationship between temperature and flow velocities.19 More studies are needed to better understand the relationship between blood flow velocity and temperature.
Types of Transcranial Doppler Devices
Two types of TCD equipment are currently available: non-duplex (nonimaging) and duplex (imaging) devices. In non-duplex devices, the arteries are identified “blindly” based on the audible Doppler shift and the spectral display. Specific vessel identification is based on standard criteria, which includes the cranial window used, orientation of the probe, depth of sample volume, direction of blood flow, relationship to the terminal internal carotid artery, and response to various maneuvers such as the common carotid artery compression and eye opening and closing.20
The imaging B-mode transcranial color-coded duplex (TCCD) combines pulsed wave Doppler ultrasound with a cross-sectional view of the area of insonation, which allows identification of the arteries in relation to various anatomic locations. The color-coded Doppler also depicts the direction of the flow in relation to the probe (transducer) while recording blood flow velocities. In TCD, the angle of insonation is assumed < 30 degrees (as close to zero as possible) to minimize the Doppler shift measurement error. However, in TCCD, the angle of insonation can be measured and used to correct the flow velocity measurement. More recently, a more advanced technology, called the power motion-mode TCD (PMD/TCD), has also become available that provides multi-gate flow information simultaneously in the power M-mode display. It uses several overlapping sample volumes to simultaneously display flow signals. PMD/TCD appears to simplify handling of the TCD by facilitating the temporal window location and alignment of the incident signal to allow cerebral blood flow velocity recordings through multiple vessels.
Although these imaging TCD modalities significantly increase the reliability of TCD with better insonation angle correction,21 the clinical applications of the more recent imaging modalities are still under development and most of the currently utilized clinical applications have been best developed using the nonduplex mode of TCD. Therefore, the focus of this review will be on the examination and clinical applications of the nonduplex TCD. With time, the duplex TCD modalities will not only expand the clinical utility of TCD, but will also likely replace the nonduplex TCD in clinical practice.
The Transcranial Doppler Examination
The TCD examination is performed using a 2 MHz frequency ultrasound probe. The higher frequency probes used in extracranial Doppler studies are not applicable for intracranial measurements because higher frequency waves are not able to adequately penetrate through the skull.2,22 In addition to using a lower frequency probe, insonation of the cerebral arteries is only possible through thinner regions of the skull, termed acoustic windows. Therefore, familiarity with the anatomic location of cerebral arteries relative to the acoustic windows and blood flow velocities for the various arteries is critical for accurate blood flow measurements through the nonduplex mode.2,23,24
In general, four main acoustic windows have been described: (1) the transtemporal window,1 (2) the transorbital window,25 (3) the submandibular window, and (4) the suboccipital window (Fig. 2). Although each window has unique advantages for different arteries and indications, a complete TCD examination should include measurements from all four windows and the course of blood flow at various depths within each major branch of the circle of Willis should be assessed. (See references 10, 26, and 27 for detailed reviews on examination techniques and basal cerebral artery anatomy). Specific arteries of the circle of Willis are identified using the following criteria: (1) relative direction of the probe within a specific acoustic window, (2) direction of blood flow relative to the probe, (3) depth of insonation, and (4) in difficult cases when it is not possible to differentiate the anterior from the posterior circulation, the blood flow response to carotid compression or vibration may be used.
Fig. 2.
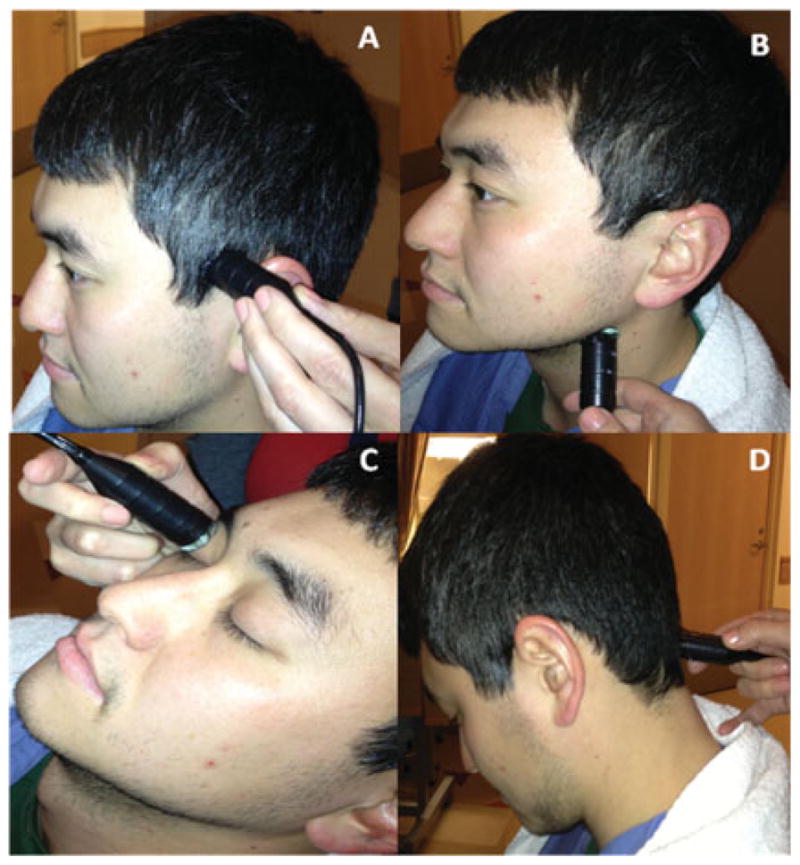
Four acoustic windows commonly used in transcranial Doppler examination: transtemporal window (A), submandibular window (B), transorbital window (C), suboccipital window (D).
The transtemporal window consists of an anterior, middle, and posterior window. However, in practice, there is usually only one useful window. Using this window, the intracranial carotid artery (ICA) bifurcation can be identified at depths of 55 to 65 mm with simultaneous flow toward and away from the probe as the ICA bifurcation terminates in the anterior (flow away from the probe) and middle (flow toward the probe) cerebral arteries (ACA and MCA).
The ICA terminus is a convenient anatomic landmark to locate the vessels of the anterior circulation. The MCA, viewed at depths of 35 to 55 mm, runs laterally and slightly anterior after its origin from the ICA. Flow in the MCA should be toward the probe until the MCA trifurcation where flow becomes bidirectional. The ACA, which can be viewed at depths of 60 to 70 mm, begins coursing medially and then anteriorly after the ICA bifurcation. The ACA flow should be away from the probe.1,28
The posterior cerebral artery (PCA) can also be insonated through the transtemporal window. In general, the PCA is found 1 to 2 cm posterior to the ICA bifurcation, but in the same plane as the circle of Willis. The PCA can be found posterior and deep to the ICA and MCA, at a depth of ~60 to 70 mm. Flow in the proximal PCA (P1 segment) is toward the probe and in the distal PCA (P2 segment) away from the probe. The PCA will always exhibit lower velocities than the MCA. It is important to note that in individuals where the PCA derives most of its flow from the ICA through a large posterior communicating artery (Pcom), the so-called fetal PCA configuration, the P1 segment is hypoplastic and may be very difficult to identify.1
The transorbital window can be used to examine the carotid siphon and the ophthalmic artery. The probe is placed over the closed eyelid and the beam power is kept under 10% of maximum power in order to minimize the risk of traumatic subluxation of the crystalline lens of the eye. In addition to the amount of energy, the total time of insonation also needs to be considered and kept to a minimum to avoid further soft tissue damage.29 The probe is directed toward the optic canal at a depth of 55 to 70 mm to insonate the carotid siphon. Flow direction can be used to identify the different segments of the siphon. In general, flow is toward the probe in the infraclinoid siphon, flow in the genu is bidirectional, and flow is away from the probe in the supraclinoid segment of the siphon. The ophthalmic artery can be found at depths of 40 to 50 mm. Flow in the ophthalmic artery should be toward the probe.
The suboccipital window with the neck flexed, can be used to insonate the basilar and vertebral arteries. The basilar artery is typically found at depths of 60 to 70 mm and can sometimes be followed to depths up to 100 mm. Although the basilar artery is found with probe directed medially, vertebral arteries are best insonated with the probe slightly shifted laterally, at a depth of 80 to 115 mm. Flow at the top of the basilar and in the vertebral arteries is typically away from the probe.
The submandibular window is at the angle of jaw and can be used to locate the distal ICA in the neck at a depth of 40 to 60 mm. Flow at this point is usually away from the probe.
Clinical Applications
A detailed review of adult and pediatric TCD applications is beyond the scope of this article; this review will mostly focus on TCD in adults. For a detailed review of pediatric TCD applications, please refer to Verlhac.30
Subarachnoid Hemorrhage and Cerebral Vasospasm
Angiographic cerebral vasospasm (VSP) occurs in two-thirds of patients with aneurysmal SAH with half becoming symptomatic. There is a significant direct correlation between VSP severity after SAH and flow velocities in most cerebral arteries, although anatomic and technical factors weaken the association for the ICA and ACA.31 The sensitivity and specificity of TCD versus cerebral angiography for the detection of VSP after SAH in the proximal portions of each intracranial artery have been previously reported.32 TCD is much more sensitive for detecting proximal versus distal VSP. Proximal VSP in any intracranial artery results in segmental or diffuse elevations of the mean flow velocities without a parallel flow velocity increase in the feeding extracranial arteries such as the carotid or the vertebral arteries. The Lindegaard ratio (LR), defined as the ratio between the time mean average (Vmean) velocity of the MCA to ICA, is the most established of such ratios and helps differentiate hyperemia from VSP. Hyperemia would result in flow elevations in both the MCA and ICA and result in an LR < 3, whereas VSP would preferentially elevate the MCA flow over the ICA with LR > 6. LR between 3 and 6 is a sign of mild VSP and > 6 is an indication of severe VSP.33 There have been other proposed values for PCA/basilar and basilar/vertebral and MCA/ACA ratios, but these have not been well validated. Unlike proximal VSP, distal VSP cannot be insonated. Therefore, increased PI, indicating increased resistance distal to the site of insonation, is used as a surrogate measure of distal VSP.
In general, TCD flow velocity criteria appear to be most reliable for detecting angiographic MCA and basilar artery VSP. Some of the findings in MCA VSP include MCA Vmean ≥ 180 cm/s, a sudden rise in MCAVmean by > 65 cm/s or 20% increase within a day during posthemorrhage days 3 to 7, LR ≥ 6, and abrupt increase in PI > 1.5 in two or more arteries suggesting increases in ICP and/or VSP.34 TCD is most useful in monitoring the temporal course of angiographic VSP following SAH to help guide the timing of diagnostic and therapeutic angiographic interventions. Sporadic measurements, especially if started after the development of vasospasm, are less useful. In addition, given the impact of physiologic changes on blood flow velocity, any measured velocity or pulsatility changes should be interpreted in the context of the patient’s condition and in relation to variables that can impact flow velocity measurements such as technical issues, vessel anatomy, age, ICP, mean arterial pressure, hematocrit, arterial CO2 content, collateral flow pattern, and other therapeutic interventions.31,35
Intracranial Steno-Occlusive Disease
Intracranial atherosclerosis is a significant risk factor for ischemic strokes and transient ischemic attacks (TIAs), accounting for ~10% of such events.36,37 TCD can be used to detect stenosis and occlusion of the carotid siphon, proximal MCA, ACA, PCA, and basilar as well as intracranial vertebral arteries.31,34,38 Due to the greater tortuosity and anatomic variability of the vessels in the posterior circulation, the sensitivity, specificity, positive predictive value, and negative predictive value of TCD is generally higher in the anterior circulation. Diagnosis of stenosis > 50% using TCD is based on the following criteria: (1) acceleration of flow velocity through the stenotic segment; (2) decrease in velocity distal to the stenotic segment (poststenotic dilatation); (3) side-to-side differences in mean flow velocity, and (4) disturbances in flow (i.e., turbulence and murmurs).39 Diagnosis of an intracranial occlusion is based on the absence of flow at the normal position and depth for a specific vessel given that there is an adequate “acoustic window” and other vessels in the vicinity can be visualized. 40 In addition, one may also find that flow velocities are increased in other intracranial vessels due to activation of collateral vessels.
Acute Ischemic Stroke
TCD is particularly useful in acute ischemic stroke where repeated TCD studies can be used to track the course of an arterial occlusion before and after thrombolysis.39 TCD can detect acute MCA occlusions with high (> 90%) sensitivity, specificity, and positive and negative predictive values.40–43 TCD can also detect occlusion in the ICA siphon, vertebral, and basilar arteries with reasonable (70 to 90%) sensitivity and positive predictive value and excellent specificity and negative predictive value (> 90%).44
Recent studies suggest that ultrasound may also have an independent effect in augmenting thrombolysis of the occluded vessel in patients presenting with acute thrombosis.45 Continuous TCD recording significantly increased tissue plasminogen activator-induced arterial recanalization in the Clotbust trial. In this trial, 83% of patients achieved either partial or complete recanalization with tPA and TCD monitoring compared with 50% recanalization with tPA treatment alone46 (for detailed review, please see reference47).
In addition, early TCD findings can be very useful for prognosis in patients presenting with acute ischemic stroke. In these patients, intracranial arterial occlusion detected by TCD is associated with poor 90-day outcome,48,49 whereas a normal TCD study is predictive of early recovery.50,51 Delayed (> 6 h) spontaneous recanalization as demonstrated by TCD, is also independently associated with greater risk of hemorrhagic transformation (odds ratio [OR]: 8.9, 95% confidence interval [CI]: 2.1–33.3).52 In a more recent study of 489 patients with recent TIA or minor stroke, mean flow velocity and the ratio of pulsatility to mean flow velocity were independent risk factors for not only stroke recurrence, but also the occurrence of other major vascular events (stroke, myocardial infarction, and vascular death).53
Collateral Flow
Knowledge of collateral flow patterns of the basal arteries of the brain has significant clinical implications in the management of patients with cerebrovascular atherothrombotic disease. A number of clinical studies have established that the degree of collateral flow is correlated with infarct volume and clinical outcome in patients with ischemic stroke.54,55 TCD can be used to provide real-time information regarding the direction and the velocity of blood flow in known intracranial collateral channels, which become active in acute and/or chronic steno-occlusive cerebrovascular diseases. Flow direction in these vessels will depend on the direction of collateralization and will identify the donor and recipient arterial systems.56,57 Some examples of collateral flow patterns include (1) reversal of flow in the ophthalmic artery, (2) reversal of flow in the ACA or MCA, and (3) presence of prominent Acom or Pcom flow.
Sickle Cell Disease
Children with sickle cell disease (SCD) have chronic hemolysis resulting in low hemoglobin content. Chronic anemia and hypoxia trigger angiogenesis and neovascularization. In addition, the interaction of the sickled red cells with the endothelium causes inflammation and intracranial stenosis. The compromised vascular system predisposes these children to both ischemic and hemorrhagic infarcts.58 An increase Vmean of ≥ 200 cm/s in the ICA or MCA detected by TCD has been shown to be associated with increased risk of ischemic stroke in these children.15 In the Stroke Prevention Trial in Sickle Cell Disease (STOP), children between 2 to 16 years old with no history of stroke and MCA velocity threshold of 200 cm/s were randomly allocated to standard care or to periodic blood transfusion therapy to lower the hemoglobin S concentration to < 30% of total hemoglobin. Blood transfusion based on mean flow velocity resulted in 92% stroke risk reduction.15
Following the TCD criteria in the STOP trial, a fivefold decrease in the rate of first stroke was observed in children with SCD.15 The STOP II trial assessed the safety of discontinuing long-term blood transfusion in children who had normal MCA flow velocities and who had received transfusions for 30 months or longer.59 The study was stopped early due to increased MCA flow velocities and new ischemic strokes in the group that discontinued transfusion. There were no strokes in the group that continued periodic transfusion. In another retrospective cohort of 475 children, the incidence of stroke declined 10-fold following TCD screening and prophylactic blood transfusion over an 8-year period.60
Because early TCD screening coupled with prophylactic transfusion seems to reduce overt stroke in children with SCD, TCD assessment should now be a routine component of preventive care for these children. TCD screening should be avoided during acute illnesses because factors such as hypoxia, fever, hypoglycemia, and worsening anemia may impact flow velocity measures.58 The impact of TCD based transfusion on subsequent stroke risk has not been studied in adults with SCD.
Microemboli Detection
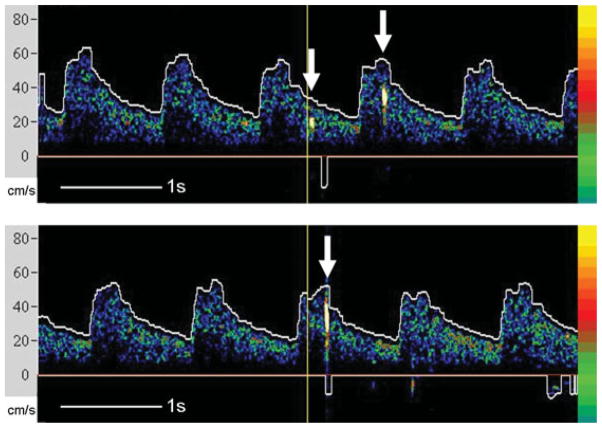
TCD is the only medical device that can detect circulating cerebral microemboli, both solid and gaseous, in real-time. TCD microemboli detection is based on backscatter of the ultrasound waves from the emboli resulting in high-intensity transient signals (HITS) or embolic signals in the Doppler spectrum as they travel through the insonated vessel (Fig. 3).61 The backscatter of the ultrasound from gaseous emboli are higher than that of solid emboli of a similar size, which in turn is higher than the backscatter observed from red blood cells within normal flow. Embolic signals using TCD ultrasound have been detected in patients with carotid stenosis, myocardial infarction, atrial fibrillation, and mechanical cardiac valves.
Fig. 3.
Embolic signals on transcranial Doppler recordings of the left middle cerebral artery. Arrows indicate the high-intensity transient signals (HITS) seen with emboli.
Ipsilateral MCA TCD recordings for 111 patients with > 60% carotid artery stenosis (69 symptomatic and 42 asymptomatic) were examined for embolic signals and the patients were followed from their first TCD recording until the occurrence of stroke, ischemic events, carotid endarterectomy, angioplasty, or completion of the study. Although the presence of embolic signals was predictive of ischemic events during follow-up in both symptomatic and asymptomatic patients, for the asymptomatic patients embolic signals predicted a further ischemic event with an adjusted OR of 8.10 (95% CI: 1.58–41.57; p = 0.01).62 The study was extended to include 200 patients with > 50% ICA stenosis. In this group, embolic signals in asymptomatic patients predicted short-term ipsilateral stroke and TIA risk with an OR of 4.67 (95% CI =1.99–11.01; p < 0.0001).63
The role of TCD in antithrombotic therapy was subsequently investigated in the CARESS (Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis) trial, which tested the effect of antithrombotic medications on patients with symptomatic carotid stenosis ≥ 50%. Patients with embolic signals were randomized to combination antithrombotic therapy with clopidogrel and aspirin or to aspirin therapy alone. TCD recording in the ipsilateral MCA on day 7 of the treatment showed that the combination therapy was more effective than aspirin alone in reducing embolic signals.64
The ACES (Asymptomatic Carotid Emboli Study) trial examined the presence of silent embolic signals in patients with ≥ 70% asymptomatic carotid artery stenosis and their relation with subsequent stroke risk. Embolic signals were present in 77 of the 467 patients screened and the hazard ratio (HR) for the risk of ipsilateral stroke and TIA from baseline to 2 years in patients with embolic signals compared with those without was 2.54 (1.20–5.36; p = 0.015); the HR for ipsilateral stroke alone was 5.57 (1.61–19.32; p = 0.007).65 Although carotid endarterectomy is proven to reduce the risk of ipsilateral stroke by 75% in patients with 50 to 70% symptomatic carotid stenosis,66 the management of patients with asymptomatic carotid stenosis is not clear. The results of the ACES trial suggest that TCD microemboli detection may be a useful approach to not only identify patients with asymptomatic carotid stenosis who are at high risk of stroke and TIA and who may benefit from endarterectomy, but to also identify those low-risk patients in whom surgical intervention may not be beneficial.
Cerebral Circulatory Arrest
A decrease in cerebral perfusion pressure associated with increases in ICP and PI result in compression of the intracranial arteries and cessation of flow to the brain, leading to cerebral circulatory arrest (CCA).34 The pattern of cerebral blood flow leading to CCA and brain death can be visualized by TCD and monitored continuously at bedside (Fig. 4). When the ICP increases to match the diastolic perfusion pressure, diastolic cerebral blood flow approaches zero. With continued rise in ICP, diastolic blood flow reappears, but it is in the opposite direction (reversed flow), visualized as retrograde flow in the TCD. Systolic waveforms also become spiked. The retrograde or oscillatory diastolic flow along with systolic spikes, result in no net forward cerebral blood flow and are characteristic of CCA. TCD has very high sensitivity (96.5%) and specificity (100%) in the diagnosis of cerebral circulatory arrest, but the possibility of temporary arrest should be excluded by having the systolic blood pressure > 70 mm Hg during the TCD assessment.39
Fig. 4.
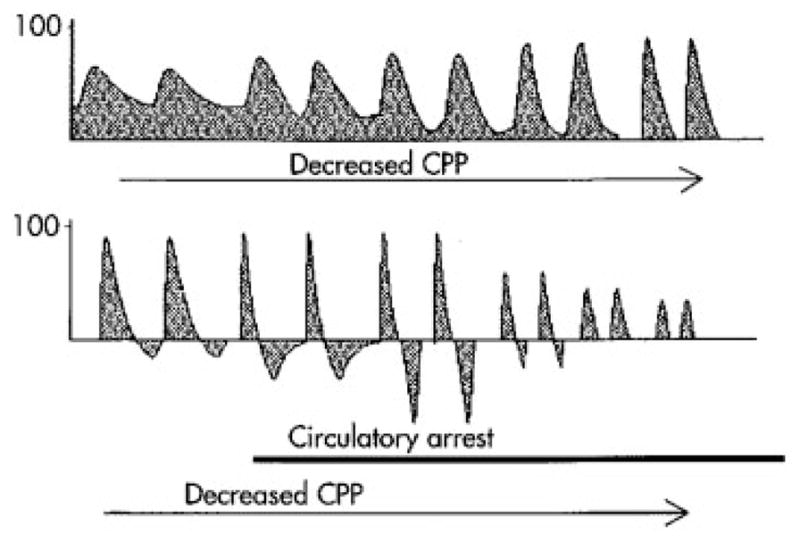
Cerebral circulatory arrest. Progressive changes in the waveform morphology of the middle cerebral artery. (From Hassler W, Steinmetz H, Pirschel J. Transcranial Doppler study of intracranial circulatory arrest. J Neurosurg 1989; 71(2):195–201).
Research Applications and Future Implications
Cerebral Autoregulation
Cerebral autoregulation is the intrinsic property of the blood vessels to maintain cerebral blood flow relatively constant by rapidly adjusting cerebrovascular resistance and compensating for fluctuations in cerebral perfusion pressure.67 Changes in cerebrovascular resistance usually take place at the level of the arterioles, although larger vessels may also contribute. Cerebral autoregulation maintains cerebral blood flow constant between mean arterial pressures of 50 to 170 mm Hg. Beyond the autoregulatory range cerebral blood flow is pressure passive—at low blood pressures the brain is at risk of ischemic injury, whereas at high blood pressures cerebral edema and breakdown of the blood–brain barrier may occur.
Two types of autoregulation exist; dynamic autoregulation, which responds to immediate changes (within seconds) and static autoregulation, which responds to long-term(from minutes to hours) changes in blood pressures. Earlier investigations of cerebral blood flow regulation relied on steady-state blood pressures for valid measures of cerebral autoregulation. This method was time consuming and required invasive procedures such as the Kety-Schmidt technique using Xenon Xe 133 as a tracer. Moreover, the traditional steady-state techniques lacked the temporal resolution to identify dynamic vascular changes that occur within seconds. TCD ultrasound is a powerful noninvasive tool with high temporal resolution for assessment of dynamic cerebral blood flow responses to various stimuli including changes in arterial pressure. TCD provides continuous beat-to-beat measurements of cerebral blood flow velocity in the basal cerebral arteries and has become the most commonly utilized tool to study cerebral blood flow regulation in humans. Assessment of dynamic cerebral autoregulation is based on transient changes in cerebral blood flow in response to sudden changes in arterial pressure. The sudden changes in arterial pressure can be induced by a variety of techniques such as deflation of bilateral thigh cuffs, postural alteration, Valsalva maneuver, lower-body negative pressure, isometric hand-grip exercise, and pharmacologic interventions.68–70 Several analyses using the time and the frequency domains are utilized to examine dynamic cerebral autoregulation with the TCD.69,70 Abnormalities in cerebral autoregulation are thought to occur in a number of clinical disorders such as stroke, subarachnoid hemorrhage, postpartum angiopathy, eclampsia, syncope, and traumatic brain injury.
Cerebral Vasoreactivity
The cerebrovascular bed is extremely sensitive to changes in arterial PaCO2. Increased CO2 causes arteriolar vasodilatation resulting in increased velocity in the upstream larger cerebral arteries (insonated vessels). On the other hand, decreased CO2 results in decreased cerebral blood flow velocity due to arteriolar vasoconstriction. Changes in ventilation (hyper- and hypoventilation or breathholding) and use of drugs such as acetazolamide can also induce CO2-mediated changes on cerebral blood flow velocity.71,72
Cerebral vasoreactivity describes the ratio of the percentage changes in cerebral blood flow velocity to changes in PaCO2. It is important to note that cerebral vasoreactivity, vasomotor reactivity, or CO2 vasoreactivity are not the same as cerebral autoregulation; therefore, the terms should not be used interchangeably. In fact, cerebral vasoreactivity and cerebral autoregulation may be decoupled in many conditions. 73 Diseases such as sleep apnea, congestive heart failure, carotid artery stenosis, and cerebral ischemia are associated with impaired vasoreactivity.70
Neurovascular Coupling (Functional Hyperemia)
The relationship between neuronal activation and cerebral blood flow—termed neurovascular coupling—ensures delivery of adequate oxygen and glucose to the activated neurons. In other words, an increase in metabolic demand from neuronal activity will lead to an increase in cerebral blood flow, and this link between regional neuronal activity and regional cerebral blood flow is referred to as neurovascular coupling or functional hyperemia. Functional TCD can be used to measure neurovascular coupling by recording cerebral blood flow velocity during cognitive tasks74 and motor tasks.75 Neurovascular coupling is disrupted in diseases such as hypertension and ischemic stroke.76
Future Implications
Traumatic Brain Injury
Traumatic brain injury (TBI) is one of the leading causes of death and disability. Cerebral vascular injury and hemodynamic compromise is a significant contributor to poor outcome in patients with TBI. Our current understanding of the mechanisms underlying cerebrovascular damage in TBI is limited. Disturbances in cerebral hemodynamics with an early phase of cerebral hypoperfusion followed by hyperemia and secondary elevations in ICP may be one possible mechanism. 77 Chan et al used TCD to detect hypoperfusion in TBI patients and showed that an initial Vmean of < 28 cm/s was associated with 80% likelihood of early death.78 In a prospective study of 36 children with moderate to severe TBI, diastolic flow velocity < 25 cm/s and PI >1.31 (indicative of high cerebrovascular resistance) at the time of admission was also associated with poor prognosis.79
The usefulness of early TCD-directed therapy was examined in a study of adults with severe TBI.80 TCD measurements were available within 18 ± 11 minutes after admission and were considered abnormal when two out of three measures were outside the following range: Vmean < 30 cm/s, Vd < 20 cm/s, PI > 1.4. Patients with abnormal TCD received treatment to increase cerebral perfusion pressure and/or decrease cerebral edema. Cerebral invasive monitoring was available at ~4 hours after admission and showed similar cerebral perfusion pressure and jugular venous oxygen saturation between the two groups despite increased intracranial pressure in patients with abnormal TCD. These findings show that early TCD monitoring can help prevent cerebral hypoperfusion, which may help reduce the extent of secondary ischemic injuries in TBI patients with elevated ICP. TCD measures predictive of clinical outcome that can be used as therapeutic targets in clinical trials of TBI will have a significant impact on how we manage TBI in the future.
Intraoperative TCD Monitoring
Monitoring cerebral blood flow velocity noninvasively during surgery can also provide real-time information about changes in velocity or occurrence of microemboli, which could be immediately corrected to prevent intraoperative cerebral ischemic injury. A number of studies have previously investigated cerebrovascular hemodynamics during coronary artery bypass graft81 and carotid endarterectomy.82,83 However, more studies are needed to validate these findings and to show that altering intraoperative care in response to TCD measures has a positive impact on patient outcomes.
TCD in Dementia
TCD may also have significant utility in dementia research (for a detailed review of these studies, please see reference84). One of the more recent studies has shown that patients with Alzheimer’s disease and vascular dementia had lower cerebral blood flow velocity and higher PI when compared with the healthy age-matched controls, supporting the association between dementia and hemodynamic disturbances.85 There is another recent study linking microemboli to accelerated cognitive decline in patients with dementia.86 The application of TCD to cognitive research is rapidly expanding.
Limitations of the TCD
Two major limitations of TCD impede its more widespread use. It is highly operator dependent, with the handheld technique requiring detailed three-dimensional knowledge of cerebrovascular anatomy and its variations. The use of TCD is also hampered by the 10 to 15% rate of inadequate acoustic windows prevalent in Blacks, Asians, and elderly women. This may be related to thickness and porosity of the bone around the acoustic windows and attenuation of the ultrasound energy transmission. TCD measurements are also limited to the large basal arteries and can only provide an index of global rather than local cerebral blood flow velocity.
Conclusions
TCD is an inexpensive, but essential tool that can be used along with a battery of other tests in clinical diagnosis of a number of cerebrovascular disorders such as acute ischemic stroke, vasospasm, traumatic brain injury, and cerebral microembolization. TCD is also useful in detecting collateral flow and managing cerebrovascular atherosclerotic diseases. Children with SCD who are at risk of stroke can be screened with TCD and managed with blood transfusion. TCD is also useful in confirming brain death. TCD is also extensively used in research settings to study cerebral autoregulation, vasoreactivity to CO2, and neurovascular coupling in healthy and diseased population. A better understanding of these physiologic processes may lead to novel therapeutic targets in acute ischemic stroke, vasospasm, TBI, and dementia where our clinical interventions are most limited.
References
- 1.Aaslid R. Transcranial Doppler Sonography. New York: Springer-Verlag; 1986. [Google Scholar]
- 2.DeWitt LD, Wechsler LR. Transcranial Doppler. Stroke. 1988;19(7):915–921. doi: 10.1161/01.str.19.7.915. [DOI] [PubMed] [Google Scholar]
- 3.Tegeler CH, Ratanakorn D. Physics and principles. In: Babikian VL, Wechsler LR, editors. Transcranial Doppler Ultrasonography. 2. Waltham, MA: Butterworth-Heinemann; 1999. pp. 3–11. [Google Scholar]
- 4.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17(5):913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall GA, Cook DJ, Fulgham JR, Oliver WC, Jr, Proper JA. The relationship between cerebral blood flow and transcranial Doppler blood flow velocity during hypothermic cardiopulmonary bypass in adults. Anesth Analg. 1996;82(6):1146–1151. doi: 10.1097/00000539-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31(7):1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 7.Vriens EM, Kraaier V, Musbach M, Wieneke GH, van Huffelen AC. Transcranial pulsed Doppler measurements of blood velocity in the middle cerebral artery: reference values at rest and during hyperventilation in healthy volunteers in relation to age and sex. Ultrasound Med Biol. 1989;15(1):1–8. doi: 10.1016/0301-5629(89)90125-7. [DOI] [PubMed] [Google Scholar]
- 8.Arnolds BJ, von Reutern GM. Transcranial Doppler sonography. Examination technique and normal reference values. Ultrasound Med Biol. 1986;12(2):115–123. doi: 10.1016/0301-5629(86)90016-5. [DOI] [PubMed] [Google Scholar]
- 9.Grolimund P, Seiler RW. Age dependence of the flowvelocity in the basal cerebral arteries—a transcranial Doppler ultrasound study. Ultrasound Med Biol. 1988;14(3):191–198. doi: 10.1016/0301-5629(88)90139-1. [DOI] [PubMed] [Google Scholar]
- 10.Babikian V, Wechsler L, editors. Transcranial Doppler Ultrasonography. 2. Waltham, MA: Butterworth Heinmann; 1999. [Google Scholar]
- 11.Macko RF, Ameriso SF, Akmal M, et al. Arterial oxygen content and age are determinants of middle cerebral artery blood flowvelocity. Stroke. 1993;24(7):1025–1028. doi: 10.1161/01.str.24.7.1025. [DOI] [PubMed] [Google Scholar]
- 12.Ameriso SF, Paganini-Hill A, Meiselman HJ, Fisher M. Correlates of middle cerebral artery blood velocity in the elderly. Stroke. 1990;21(11):1579–1583. doi: 10.1161/01.str.21.11.1579. [DOI] [PubMed] [Google Scholar]
- 13.Brass LM, Pavlakis SG, DeVivo D, Piomelli S, Mohr JP. Transcranial Doppler measurements of the middle cerebral artery. Effect of hematocrit. Stroke. 1988;19(12):1466–1469. doi: 10.1161/01.str.19.12.1466. [DOI] [PubMed] [Google Scholar]
- 14.Fiermonte G, Aloe Spiriti MA, Latagliata R, Petti MC, Giacomini P. Polycythaemia vera and cerebral blood flow: a preliminary study with transcranial Doppler. J Intern Med. 1993;234(6):599–602. doi: 10.1111/j.1365-2796.1993.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton HJ, Adams RJ, Zhao S, Johnston SC. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104(2):336–339. doi: 10.1182/blood-2004-02-0636. [DOI] [PubMed] [Google Scholar]
- 16.Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J Appl Physiol. 1996;81(3):1084–1095. doi: 10.1152/jappl.1996.81.3.1084. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension. 2010;56(2):268–273. doi: 10.1161/HYPERTENSIONAHA.110.152066. [DOI] [PubMed] [Google Scholar]
- 18.Doering TJ, Brix J, Schneider B, Rimpler M. Cerebral hemodynamics and cerebral metabolism during cold and warm stress. Am J Phys Med Rehabil. 1996;75(6):408–415. doi: 10.1097/00002060-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bisschops LL, van der Hoeven JG, Hoedemaekers CW. Effects of prolonged mild hypothermia on cerebral blood flow after cardiac arrest. Crit Care Med. 2012;40(8):2362–2367. doi: 10.1097/CCM.0b013e318255d983. [DOI] [PubMed] [Google Scholar]
- 20.Lupetin AR, Davis DA, Beckman I, Dash N. Transcranial Doppler sonography. Part 1. Principles, technique, and normal appearances. Radiographics. 1995;15(1):179–191. doi: 10.1148/radiographics.15.1.7899596. [DOI] [PubMed] [Google Scholar]
- 21.Martin PJ, Evans DH, Naylor AR. Measurement of blood flow velocity in the basal cerebral circulation: advantages of transcranial color-coded sonography over conventional transcranial Doppler. J Clin Ultrasound. 1995;23(1):21–26. doi: 10.1002/jcu.1870230105. [DOI] [PubMed] [Google Scholar]
- 22.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 23.Arnolds BJ, von Reutern GM. Transcranial Doppler sonography. Examination technique and normal reference values. Ultrasound Med Biol. 1986;12(2):115–123. doi: 10.1016/0301-5629(86)90016-5. [DOI] [PubMed] [Google Scholar]
- 24.Hennerici M, Rautenberg W, Sitzer G, Schwartz A. Transcranial Doppler ultrasound for the assessment of intracranial arterial flow velocity—Part 1. Examination technique and normal values. Surg Neurol. 1987;27(5):439–448. doi: 10.1016/0090-3019(87)90251-5. [DOI] [PubMed] [Google Scholar]
- 25.Spencer MP, Whisler D. Transorbital Doppler diagnosis of intracranial arterial stenosis. Stroke. 1986;17(5):916–921. doi: 10.1161/01.str.17.5.916. [DOI] [PubMed] [Google Scholar]
- 26.Alexandrov AV, Sloan MA, Wong LK, et al. American Society of Neuroimaging Practice Guidelines Committee. Practice standards for transcranial Doppler ultrasound: part I—test performance. J Neuroimaging. 2007;17(1):11–18. doi: 10.1111/j.1552-6569.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Tegeler CH, Babikian VL, Gomes CR, editors. Neurosonology. St. Louis, MO: Mosby-Year Book; 1996. [Google Scholar]
- 28.Ringelstein EB, Kahlscheuer B, Niggemeyer E, Otis SM. Transcranial Doppler sonography: anatomical landmarks and normal velocity values. Ultrasound Med Biol. 1990;16(8):745–761. doi: 10.1016/0301-5629(90)90039-f. [DOI] [PubMed] [Google Scholar]
- 29.Fish P. Physics and Instrumentation of Diagnostic Medical Ultrasound. Hoboken, NJ: Wiley; 1990. [Google Scholar]
- 30.Verlhac S. Transcranial Doppler in children. Pediatr Radiol. 2011;41 (Suppl 1):S153–S165. doi: 10.1007/s00247-011-2038-y. [DOI] [PubMed] [Google Scholar]
- 31.Sloan MA, Alexandrov AV, Tegeler CH, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academyof Neurology. Neurology. 2004;62 (9):1468–1481. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 32.Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: A systematic review. Stroke. 2001;32 (10):2292–2298. doi: 10.1161/hs1001.097108. [DOI] [PubMed] [Google Scholar]
- 33.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis bymeans of angiography and blood velocity measurements. Acta Neurochir (Wien) 1989;100(1–2):12–24. doi: 10.1007/BF01405268. [DOI] [PubMed] [Google Scholar]
- 34.Tsivgoulis G, Alexandrov AV, Sloan MA. Advances in transcranial Doppler ultrasonography. Curr Neurol Neurosci Rep. 2009;9(1):46–54. doi: 10.1007/s11910-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 35.Saqqur M, Zygun D, Demchuk A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007;35(5, Suppl):S216–S223. doi: 10.1097/01.CCM.0000260633.66384.FB. [DOI] [PubMed] [Google Scholar]
- 36.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 37.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27(11):1974–1980. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 38.Babikian VL, Feldmann E, Wechsler LR, et al. Transcranial Doppler ultrasonography: year 2000 update. J Neuroimaging. 2000;10 (2):101–115. doi: 10.1111/jon2000102101. [DOI] [PubMed] [Google Scholar]
- 39.Rasulo FA, De Peri E, Lavinio A. Transcranial Doppler ultrasonography in intensive care. Eur J Anaesthesiol Suppl. 2008;42 (42):167–173. doi: 10.1017/S0265021507003341. [DOI] [PubMed] [Google Scholar]
- 40.Camerlingo M, Casto L, Censori B, Ferraro B, Gazzaniga GC, Mamoli A. Transcranial Doppler in acute ischemic stroke of the middle cerebral artery territories. Acta Neurol Scand. 1993;88(2):108–111. doi: 10.1111/j.1600-0404.1993.tb04200.x. [DOI] [PubMed] [Google Scholar]
- 41.Zanette EM, Fieschi C, Bozzao L, et al. Comparison of cerebral angiography and transcranial Doppler sonography in acute stroke. Stroke. 1989;20(7):899–903. doi: 10.1161/01.str.20.7.899. [DOI] [PubMed] [Google Scholar]
- 42.Baumgartner RW, Mattle HP, Schroth G. Assessment of >/= 50% and < 50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke. 1999;30(1):87–92. doi: 10.1161/01.str.30.1.87. [DOI] [PubMed] [Google Scholar]
- 43.Fieschi C, Argentino C, Lenzi GL, Sacchetti ML, Toni D, Bozzao L. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neurol Sci. 1989;91(3):311–321. doi: 10.1016/0022-510x(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 44.Demchuk AM, Christou I, Wein TH, et al. Accuracy and criteria for localizing arterial occlusion with transcranial Doppler. J Neuroimaging. 2000;10(1):1–12. doi: 10.1111/jon20001011. [DOI] [PubMed] [Google Scholar]
- 45.Eggers J, Koch B, Meyer K, König I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53(6):797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 46.Alexandrov AV. Ultrasound enhancement of fibrinolysis. Stroke. 2009;40(3 Suppl):S107–S110. doi: 10.1161/STROKEAHA.108.530931. [DOI] [PubMed] [Google Scholar]
- 47.Bor-Seng-Shu E, de Nogueira RC, Figueiredo EG, Evaristo EF, Conforto AB, Teixeira MJ. Sonothrombolysis for acute ischemic stroke: a systematic review of randomized controlled trials. Neurosurg Focus. 2012;32(1):E5. doi: 10.3171/2011.10.FOCUS11251. [DOI] [PubMed] [Google Scholar]
- 48.Camerlingo M, Casto L, Censori B, Servalli MC, Ferraro B, Mamoli A. Prognostic use of ultrasonography in acute non-hemorrhagic carotid stroke. Ital J Neurol Sci. 1996;17(3):215–218. doi: 10.1007/BF01995686. [DOI] [PubMed] [Google Scholar]
- 49.Baracchini C, Manara R, Ermani M, Meneghetti G. The quest for early predictors of stroke evolution: can TCD be a guiding light? Stroke. 2000;31(12):2942–2947. doi: 10.1161/01.str.31.12.2942. [DOI] [PubMed] [Google Scholar]
- 50.Kushner MJ, Zanette EM, Bastianello S, et al. Transcranial Doppler in acute hemispheric brain infarction. Neurology. 1991;41(1):109–113. doi: 10.1212/wnl.41.1.109. [DOI] [PubMed] [Google Scholar]
- 51.Toni D, Fiorelli M, Zanette EM, et al. Early spontaneous improvement and deterioration of ischemic stroke patients. A serial study with transcranial Doppler ultrasonography. Stroke. 1998;29 (6):1144–1148. doi: 10.1161/01.str.29.6.1144. [DOI] [PubMed] [Google Scholar]
- 52.Molina CA, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32(5):1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 53.Wijnhoud AD, Koudstaal PJ, Dippel DW. The prognostic value of pulsatility index, flowvelocity, and their ratio, measured with TCD ultrasound, in patients with a recent TIA or ischemic stroke. Acta Neurol Scand. 2011;124(4):238–244. doi: 10.1111/j.1600-0404.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- 54.Derdeyn CP, Khosla A, Videen TO, et al. Severe hemodynamic impairment and border zone—region infarction. Radiology. 2001;220(1):195–201. doi: 10.1148/radiology.220.1.r01jl09195. [DOI] [PubMed] [Google Scholar]
- 55.Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20 (6):735–740. doi: 10.1161/01.str.20.6.735. [DOI] [PubMed] [Google Scholar]
- 56.Molina CA, Alexandrov AV, Demchuk AM, Saqqur M, Uchino K, Alvarez-Sabín J CLOTBUST Investigators. Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke. 2004;35(1):151–156. doi: 10.1161/01.STR.0000106485.04500.4A. [DOI] [PubMed] [Google Scholar]
- 57.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 58.Jordan LC, Casella JF, Debaun MR. Prospects for primary stroke prevention in children with sickle cell anaemia. Br J Haematol. 2012 Jan;:9. doi: 10.1111/j.1365-2141.2011.09005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams RJ, Brambilla D Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. . Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353(26):2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 60.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr. 2010;157(3):479–484. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markus H, Pereira A, Cloud G. Stroke Medicine. 1. New York: Oxford University Press; 2010. [Google Scholar]
- 62.Molloy J, Markus HS. Asymptomatic embolization predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke. 1999;30(7):1440–1443. doi: 10.1161/01.str.30.7.1440. [DOI] [PubMed] [Google Scholar]
- 63.Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36(5):971–975. doi: 10.1161/01.STR.0000162717.62684.40. [DOI] [PubMed] [Google Scholar]
- 64.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111(17):2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 65.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9 (7):663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363(9413):915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 67.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 68.Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol. 2004;559(Pt 3):965–973. doi: 10.1113/jphysiol.2004.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19(4):197–211. doi: 10.1007/s10286-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 70.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011;196(2):221–237. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23(5):668–673. doi: 10.1161/01.str.23.5.668. [DOI] [PubMed] [Google Scholar]
- 72.Mancini M, De Chiara S, Postiglione A, Ferrara LA. Transcranial Doppler evaluation of cerebrovascular reactivity to acetazolamide in normal subjects. Artery. 1993;20(4):231–241. [PubMed] [Google Scholar]
- 73.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005;98(1):151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- 74.Sorond FA, Lipsitz LA. Aging and the cerebral microvasculature: clinical implications and potential therapeutic interventions. In: Masoro E, Austad S, editors. Handbook on the Biology of Aging. 7. London: Academic Press; 2011. pp. 347–371. [Google Scholar]
- 75.Kelley RE, Chang JY, Suzuki S, Levin BE, Reyes-Iglesias Y. Selective increase in the right hemisphere transcranial Doppler velocity during a spatial task. Cortex. 1993;29(1):45–52. doi: 10.1016/s0010-9452(13)80210-9. [DOI] [PubMed] [Google Scholar]
- 76.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100(1):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 77.Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38(4):225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan KH, Miller JD, Dearden NM. Intracranial blood flow velocity after head injury: relationship to severity of injury, time, neurological status and outcome. J Neurol Neurosurg Psychiatry. 1992;55 (9):787–791. doi: 10.1136/jnnp.55.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trabold F, Meyer PG, Blanot S, Carli PA, Orliaguet GA. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004;30(1):108–112. doi: 10.1007/s00134-003-2057-8. [DOI] [PubMed] [Google Scholar]
- 80.Ract C, Le Moigno S, Bruder N, Vigué B. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 2007;33 (4):645–651. doi: 10.1007/s00134-007-0558-6. [DOI] [PubMed] [Google Scholar]
- 81.Rudolph JL, Sorond FA, Pochay VE, et al. Cerebral hemodynamics during coronary artery bypass graft surgery: the effect of carotid stenosis. Ultrasound Med Biol. 2009;35(8):1235–1241. doi: 10.1016/j.ultrasmedbio.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Halsey JH, McDowell HA, Gelmon S, Morawetz RB. Blood velocity in the middle cerebral artery and regional cerebral blood flow during carotid endarterectomy. Stroke. 1989;20(1):53–58. doi: 10.1161/01.str.20.1.53. [DOI] [PubMed] [Google Scholar]
- 83.Halsey JH, McDowell HA, Gelman S. Transcranial Doppler and rCBF compared in carotid endarterectomy. Stroke. 1986;17(6):1206–1208. doi: 10.1161/01.str.17.6.1206. [DOI] [PubMed] [Google Scholar]
- 84.Keage HA, Churches OF, Kohler M, et al. Cerebrovascular function in aging and dementia: a systematic review of transcranial Doppler studies. Dement Geriatr Cogn Dis Extra. 2012;2(1):258–270. doi: 10.1159/000339234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabayan B, Jansen S, Oleksik AM, et al. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev. 2012;11 (2):271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Purandare N, Burns A, Morris J, Perry EP, Wren J, McCollum C. Association of cerebral emboli with accelerated cognitive deterioration in Alzheimer’s disease and vascular dementia. Am J Psychiatry. 2012;169(3):300–308. doi: 10.1176/appi.ajp.2011.11010009. [DOI] [PubMed] [Google Scholar]