Abstract
Pupillometry is a non-invasive technique, based on well-established neurophysiologic principles, that can be utilized to objectively characterize pathophysiologic demyelinating and neurodegenerative changes involving the pupillary reflex pathway. In animal models of human disorders, pupillometry derived reflex metrics could potentially be used to longitudinally monitor disease activity and responses to pharmacotherapies. These investigations would have important implications for translational initiatives focused on the identification and application of novel neuroprotective and restorative treatments for human diseases such as multiple sclerosis. Here, we have established normal reference values for various pupillary reflex metrics across different mouse strains. Ultimately, we anticipate that this new data will help to catalyze unique lines of inquiry using pupillometry methods.
Keywords: Pupillometry, Mouse models, Experimental autoimmune encephalomyelitis, Multiple sclerosis
1. Introduction
Pupillometry has long been utilized in humans to measure the pupil diameter and its dynamic changes. Demyelination, neurodegeneration, or synaptic impairment in the peripheral or central pupillary reflex pathways can be detected and quantified. This technique is most widely employed in the diagnosis and paraclinical monitoring of autonomic nervous system disorders (Pozzessere et al. 1996; Bitsios et al. 1996; Prettyman et al. 1997; O'Neill et al. 1996; O'Neill et al. 1998). However, it has also been used diagnostically and experimentally in pharmacological studies (Bitsios et al. 1999; Brown et al. 2004; Knaggs et al. 2004), sleep disorders (O'Neill et al. 1998; Wilhelm et al. 2001; McLaren et al. 2002; Merritt et al. 2004), fatigue (Morad et al. 2000), and pain medicine (Barvais et al. 2003; Donaldson et al. 2003; Tegeder et al. 2003).
The potential role of pupillometry in the diagnostic work-up and monitoring of neuroinflammatory and neurodegenerative diseases has perhaps been under-utilized and is only now being explored. Multiple sclerosis (MS) is the most common acquired inflammatory demyelinating disorder (Steinman 2001). The animal model of MS, experimental autoimmune encephalomyelitis (EAE), has been used for many decades to develop and test novel pharmacotherapies (Steinman and Zamvil 2006). The clinical evaluation of animals is currently the only metric that allows the scientist a longitudinal assessment of experimental animals. This clinical disability metric is limited to the motor function and is quantified by a nominal scale (Zamvil and Steinman 1990). Quantification of inflammation, demyelination, and neurodegeneration can only be assessed cross sectionally after sacrificing animals and performing a histopathological evaluation.
Pupillometry has the potential to assess demyelinating and neurodegenerative events in the optic nerve and upper brainstem of mice with EAE and other animal models of human disorders. This technique may prove especially useful to identify defects in the pupillary reflex pathway in genetically-altered animals, and in monitoring the effects of pharmacotherapies over time. Here, we tested the safety and feasibility of the procedure, and we established normal reference values for pupillary reflex metrics in different mouse strains.
2. Methods
2.1. Animals
Experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. A minimum of five male and five female C57BL/6, B10.PL, and SJL/J mice between the ages of 8 and 12 weeks were evaluated. The animals were fully acclimated to the research environment, had unrestricted access to food and water and were maintained in a 12hour light-dark cycle. For testing, mice were lightly sedated with intraperitoneal injection of ketamine (40 mg/ml), xylazine (3 mg/ml), and acepromazine (1.5 mg/ml) (Mukherjee and Vernino 2007). Alternate forms of sedation (isoflurane or pentobarbital) had been noted to cause impairment of pupillary responses. Other investigators have reported that anesthesia with low dose nitrous oxide (30%), intraperitoneal ketamine (up to 45 mg/kg) or xylazine (up to 17 mg/kg) has no effect on the mouse pupil size or the magnitude of the pupillary light reflex (Pennesi et al. 1998; Grozdanic et al. 2002; Aleman et al. 2004).
2.2. Pupillometry
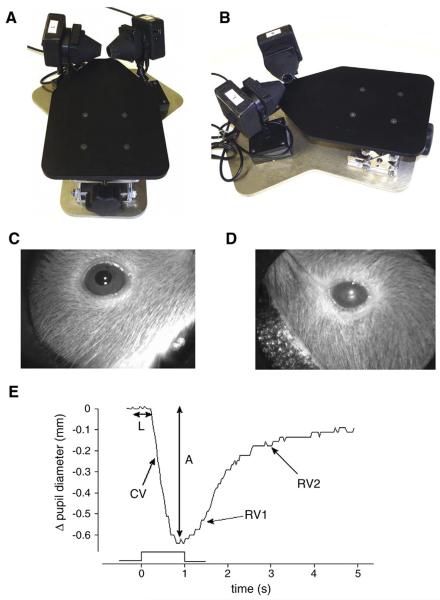
The pupillometry system is a research device developed by Neuroptics Inc. (San Clemente, CA), which can reliably capture and automatically measure the pupil diameter bilaterally and deliver controlled light stimuli (Fig. 1A and B). The system uses infrared cameras to capture (30 times per second) digital images of the pupils in darkness (Mukherjee and Vernino 2007). Pupil diameter is determined by threshold detection of the dark pupil and correction for distance of the pupil from the camera. After sedation, the mouse is positioned in the pupillometer (Fig. 1A and B) and allowed to dark adapt for at least 10min. While monitoring the pupil size, a time and intensity-calibrated light stimulus is presented to one or both eyes using a circumferential array of white light-emitting diodes. The light stimulus consists of a one second light presentation at intensity of 2, 32 or 125 μW. The raw data of pupil diameter from each eye is recorded for offline analysis. The amplified view of a healthy murine pupil is shown in Fig. 1C, whereas Fig. 1D depicts a mouse with cataract. Fig. 1E exemplifies murine pupil light reflex responses indicating measurement of key autonomic parameters.
Fig. 1.
A) Mouse pupillometer top-frontal view and B) top-lateral view. During the examination, the mouse sits in the center of the platform (not shown) with two adjustable pupil cameras focused on the eyes. Panel C) shows the amplified view of a healthy murine pupil, whereas panel D) depicts a C57BL/6 mouse with cataract. Similar observations were made in other mouse strains. Direct and consensual pupillary responses were mostly reproducible in mice with cataracts. However, light reflections from the clouded crystalline lenses commonly made it difficult to distinguish the outer border of the pupil from the iris. E) Example of mouse pupil light reflex responses indicating measurement of key autonomic parameters. The trace represents a normal pupil response to a 1s light flash. Onset latency (L) and response amplitude (A) are measured as shown. Constriction velocity (CV) represents the maximal downward slope of the response curve. The biphasic re-dilation response is shown with the initial re-dilation velocity (RV1) and slower second re-dilation velocity (RV2).
A custom program for analysis reads the raw pupil diameter data and determines baseline pupil size, onset latency, constriction velocity and response amplitude. To reject noise in the measurement, the first and second derivative of the raw data is calculated (representing instantaneous constriction velocity and pupil acceleration). Onset latency is detected at the point of maximum pupil acceleration. Maximum constriction velocity represents the largest downward slope (first derivative) of the response curve (in mm/s), and the maximum response is detected at the point of inflection from negative to positive constriction velocity. Pupil re-dilation in the dark typically shows a biphasic pattern with an initial re-dilation (thought to be mediated by release of parasympathetic input) and a second slower re-dilation (thought to be driven by sympathetic activation). Using the derivative of the pupil diameter, the re-dilation velocities can be determined. We did not investigate re-dilation parameters in detail in this study since the initial re-dilation velocity (RV1) does not provide additional pathophysiological information, and the slow secondary re-dilation velocity (RV2) was often contaminated by noise.
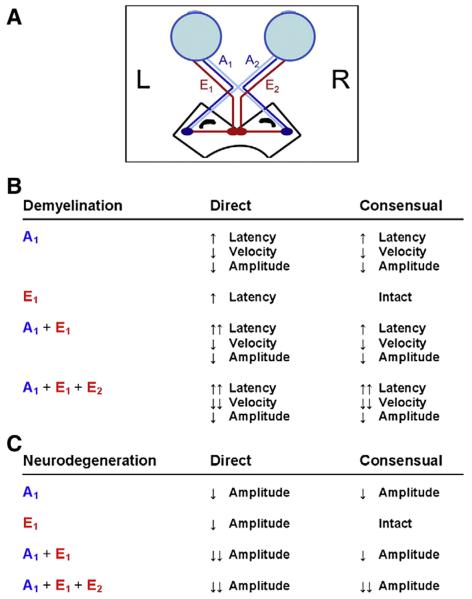
Since the absolute magnitude of the change in pupil diameter depends, in part, on the initial pupil diameter, we express the response amplitude as a ratio between the maximal change in pupil size and the initial pupil diameter. This ratio, or relative peak constriction amplitude, is expressed as a percent. This metric is the most commonly used single quantitative measure of the pupillary light reflex (Fotiou et al., 2000). The pupillary reflex pathway and expected outcomes as a result of an afferent or efferent lesion are shown in Fig. 2A and B.
Fig. 2.
A) The pupillary reflex pathway. The direct afferent (A1) and efferent (E1) pathways, as well the consensual afferent (A2) and efferent (E2) pathways are shown. B) Expected outcomes from demyelinating or neurodegenerative lesions involving the direct or consensual afferent and efferent pathways with regard to the latency, constriction velocity and amplitude of a papillary response.
2.3. Statistical analysis
The distribution of datapoints within cohorts was analyzed with distribution tables. Student T test was utilized to compare the means of two groups. The means of multiple groups were compared by analysis of variance (ANOVA). Data are expressed as mean±standard deviation. P-values<0.05 were considered significant. Prisms for Windows v. 4.03 (GraphPad Software Inc., La Jolla, CA) were used for statistical analyses.
3. Results
3.1. Safety and feasibility of pupillometry
Baseline values for unstimulated pupil diameter, latency, constriction velocity, dilation velocity, and amplitude were obtained in C57BL/6 (H-2b), B10.PL (H-2u), and SJL/J (H-2s) mice. These animal strains are commonly utilized in EAE experiments (Goverman et al. 1993; Stuve et al. 2002; Stuve et al. 2006). Pupillometry was found to be a safe, reproducible method. There were no morbidity or mortality associated with the procedure. Training of personnel took less than 8h and the apparatus and software were found to be easy to use and intuitive. Inter-examiner results showed no significant variations (data not shown).
We were unable to obtain pupillometry baseline values for SJL/J mice. SJL/J mice are congenitally blind, and the absence of pupillary responses may suggest a defect in the anterior visual pathway. In addition, the contrast between pupil and iris was below the detection limit of the pupillometer used. Thus, subtle pupillary responses would have gone undetected. Similar difficulties are to be expected in other mouse strains with similar pigmentation that are occasionally utilized in EAE experiments, including in BALB/C mice (Lyons et al. 2002).
3.2. Baseline pupillometry values in C57BL/6 and B10.PL mice
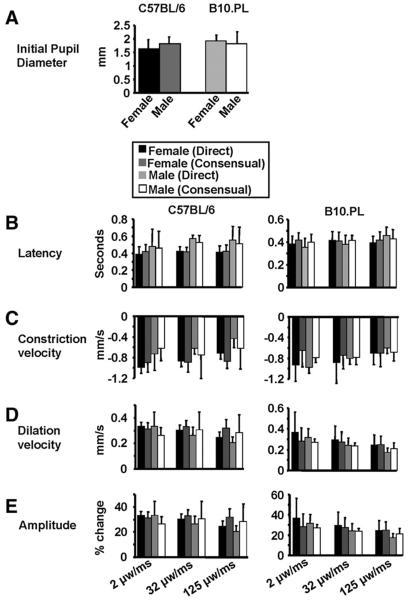
There was no significant difference between male and female C57BL/6 and B10.PL mice with regard to the unstimulated pupil diameter (Fig. 3A), latency, constriction velocity, dilation velocity, and amplitude (data not shown). We also did not observe any pupillary asymmetry at rest in any of the mouse strains that were examined (data not shown). Consequently, male and female animals and left and right eyes were pooled for all subsequent experiments. As shown in Fig. 3A–E, the values obtained for unstimulated pupil diameter, as well as latency, constriction velocity, dilation velocity, and amplitude captured after a light stimulus consisting of a one second light presentation at an intensity of 2, 32 or 125 μW were very similar and evenly distributed in each group, and very likely present valid baseline standards for future experiments.
Fig. 3.
A) The initial unstimulated pupil diameter in female and male C57BL/6 mice and B10.PL mice is shown. B) Latency, C) constriction velocity, D) dilation velocity, and E) amplitude are depicted for C57BL/6 mice and B10.PL mice captured after a light stimulus consisting of a one second light presentation at an intensity of 2, 32 or 125 μW. Standard deviations are shown.
4. Discussion
This is the first assessment of normal values of direct and consensual pupillary responses in different mouse strains. Our study shows that this technique is feasible in small rodents. Specifically, we showed that mice can be sedated in a safe manner with agents that have no effect on pupillary responses. The greatest advantage of pupillometry is the fact that evaluations can be performed repeatedly and frequently, thus allowing for the longitudinal assessment of CNS disease activity, and the monitoring of therapeutic interventions.
The mouse strains that we evaluated are frequently utilized to conduct EAE experiments. They differ by the amount of pigmentation, one biological factor that can potentially impact the detection of changes in pupil size. Notwithstanding the demonstration of the feasibility of this method in mice, the values that were established in our study will provide a useful control for future EAE studies. Particularly, they will help to identify and quantify the degree of inflammation and neurodegeneration in the pupillary reflex pathway, and dissect the effects of pharmacotherapies on either component of EAE. A similar role for pupillometry can be envisioned in patients with MS, where sensitive, easy-to-use, and inexpensive detection assays are in demand in the context of clinical trials and in clinical practice.
Acknowledgements
This work was supported by a Start-up Grant from the Dallas VA Research Corporation, a New Investigator Award from VISN 17 from the Department of Veterans Affairs, a Merit Award from the Department of Veterans Affairs, and a grant from the Viragh Foundation to Dr. Stüve. Dr. Vernino was supported by NIH P50NS32352 and Department of Veterans Affairs contract number VA549-P-0027 awarded and administered by the Dallas, TX VA Medical Center. The content of this paper does not necessarily reflect the position or the policy of the U.S. government. No official endorsement should be inferred.
References
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, Cideciyan AV. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- Barvais L, Engelman E, Eba JM, Coussaert E, Cantraine F, Kenny GN. Effect site concentrations of remifentanil and pupil response to noxious stimulation. Br. J. Anaesth. 2003;91:347–352. doi: 10.1093/bja/aeg178. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Prettyman R, Szabadi E. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing. 1996;25:432–438. doi: 10.1093/ageing/25.6.432. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw CM. Comparison of the effects of venlafaxine, paroxetine and desipramine on the pupillary light reflex in man. Psychopharmacology (Berl) 1999;143:286–292. doi: 10.1007/s002130050949. [DOI] [PubMed] [Google Scholar]
- Brown SM, Khanani AM, McCartney DL. The effect of daily use of brimonidine tartrate on the dark-adapted pupil diameter. Am. J. Ophthalmol. 2004;138:149–151. doi: 10.1016/j.ajo.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Donaldson GW, Chapman CR, Nakamura Y, Bradshaw DH, Jacobson RC, Chapman CN. Pain and the defense response: structural equation modeling reveals a coordinated psychophysiological response to increasing painful stimulation. Pain. 2003;102:97–108. doi: 10.1016/s0304-3959(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Fotiou F, Fountoulakis KN, Goulas A, Alexopoulos L, Palikaras A. Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin. Physiol. 2000 Sep;20(5):336–347. doi: 10.1046/j.1365-2281.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Grozdanic S, Sakaguchi DS, Kwon YH, Kardon RH, Sonea IM. Characterization of the pupil light reflex, electroretinogram and tonometric parameters in healthy rat eyes. Curr. Eye Res. 2002;25:69–78. doi: 10.1076/ceyr.25.2.69.10156. [DOI] [PubMed] [Google Scholar]
- Knaggs RD, Crighton IM, Cobby TF, Fletcher AJ, Hobbs GJ. The pupillary effects of intravenous morphine, codeine, and tramadol in volunteers. Anesth. Analg. 2004;99:108–112. doi: 10.1213/01.ANE.0000116924.16535.BA. [DOI] [PubMed] [Google Scholar]
- Lyons JA, Ramsbottom MJ, Trotter JL, Cross AH. Identification of the encephalitogenic epitopes of CNS proteolipid protein in BALB/c mice. J. Autoimmun. 2002;19:195–201. doi: 10.1006/jaut.2002.0619. [DOI] [PubMed] [Google Scholar]
- McLaren JW, Hauri PJ, Lin SC, Harris CD. Pupillometry in clinically sleepy patients. Sleep Med. 2002;3:347–352. doi: 10.1016/s1389-9457(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Merritt SL, Schnyders HC, Patel M, Basner RC, O'Neill W. Pupil staging and EEG measurement of sleepiness. Int. J. Psychophysiol. 2004;52:97–112. doi: 10.1016/j.ijpsycho.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Morad Y, Lemberg H, Yofe N, Dagan Y. Pupillography as an objective indicator of fatigue. Curr. Eye Res. 2000;21:535–542. [PubMed] [Google Scholar]
- Mukherjee S, Vernino S. Dysfunction of the pupillary light reflex in experimental autoimmune autonomic ganglionopathy. Auton. Neurosci. 2007;137:19–26. doi: 10.1016/j.autneu.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill WD, Oroujeh AM, Keegan AP, Merritt SL. Neurological pupillary noise in narcolepsy. J. Sleep. Res. 1996;5:265–271. doi: 10.1111/j.1365-2869.1996.00265.x. [DOI] [PubMed] [Google Scholar]
- O'Neill WD, Oroujeh AM, Merritt SL. Pupil noise is a discriminator between narcoleptics and controls. IEEE Trans. Biomed. Eng. 1998;45:314–322. doi: 10.1109/10.661156. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Lyubarsky AL, Pugh EN., Jr. Extreme responsiveness of the pupil of the dark-adapted mouse to steady retinal illumination. Invest. Ophthalmol. Vis. Sci. 1998;39:2148–2156. [PubMed] [Google Scholar]
- Pozzessere G, Valle E, Rossi P, Petrucci B, Ambrosini A, D'Alessio M, Pierelli F, Giacomini P. Pupillometric evaluation and analysis of light reflex in healthy subjects as a tool to study autonomic nervous system changes with aging. Aging (Milano) 1996;8:55–60. doi: 10.1007/BF03340116. [DOI] [PubMed] [Google Scholar]
- Prettyman R, Bitsios P, Szabadi E. Altered pupillary size and darkness and light reflexes in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 1997;62:665–668. doi: 10.1136/jnnp.62.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann. Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- Stuve O, Youssef S, Slavin AJ, King CL, Patarroyo JC, Hirschberg DL, Brickey WJ, Soos JM, Piskurich JF, Chapman HA, Zamvil SS. The role of the MHC class II transactivator in class II expression and antigen presentation by astrocytes and in susceptibility to central nervous system autoimmune disease. J. Immunol. 2002;169:6720–6732. doi: 10.4049/jimmunol.169.12.6720. [DOI] [PubMed] [Google Scholar]
- Stuve O, Youssef S, Weber MS, Nessler S, von Budingen HC, Hemmer B, Prod'homme T, Sobel RA, Steinman L, Zamvil SS. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J. Clin. Invest. 2006;116:1037–1044. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain. 2003;126:1092–1102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- Wilhelm B, Giedke H, Ludtke H, Bittner E, Hofmann A, Wilhelm H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J. Sleep. Res. 2001;10:1–7. doi: 10.1046/j.1365-2869.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]