Abstract
Corneal endothelial cells do not proliferative in vivo sufficiently to enable endothelial regeneration, and thus diseases of the corneal endothelium, which cause poor vision and discomfort, require treatment by transplantation of cadaveric donor corneal endothelial cells. The two major goals of any corneal transplant procedure are to restore vision and to promote longevity of the donor cornea by maintaining a healthy donor endothelial cell density. Over the last decade, the surgical treatment for endothelial disease has rapidly evolved toward endothelial keratoplasty, or selective tissue transplantation, and away from full-thickness penetrating keratoplasty (PK). While endothelial keratoplasty offers distinct advantages over PK in terms of visual outcomes and a smaller incision, the new surgical manipulations of the fragile donor tissue cause significant donor endothelial cell trauma. As a result, donor endothelial cell loss is much higher during the first month after Descemet stripping endothelial keratoplasty (DSEK) compared to after PK, and the primary (or more appropriately, iatrogenic) graft failure rate of 5% remains unacceptably high. Nevertheless, the rate of endothelial cell loss rapidly decreases beyond 6 months after DSEK, and thus endothelial cell loss at 5 years after DSEK appears to be lower than that at 5 years after PK. In the absence of primary (iatrogenic) graft failure, graft survival through 5 years after DSEK is similar to that after PK. Given the promising longer-term endothelial outcomes of DSEK, the quest for optimizing the visual outcomes has spurred interest in Descemet membrane endothelial keratoplasty (DMEK). While early results after DMEK suggest better visual outcomes than after DSEK, the technique needs to be simplified, and longer-term outcomes must show an advantage over DSEK with respect to vision, endothelial cell loss, and graft survival. DMEK also has a high rate of primary (iatrogenic) graft failure, and additional donor tissue wastage occurs when preparation of DMEK grafts is unsuccessful. This review discusses endothelial keratoplasty techniques and the associated endothelial outcomes.
Keywords: endothelial keratoplasty, penetrating keratoplasty, DSEK, DMEK, graft failure, endothelial cell density, endothelial cell loss, endothelium
Introduction
The corneal endothelium is a delicate monolayer of neural crest-derived cells that maintains corneal deturgescence and transparency. The endothelial cells do not proliferate to any significant extent in vivo, and therefore diseases of the corneal endothelium frequently result in morbidity by causing poor vision and discomfort. Our understanding of corneal endothelial cell biology has been dramatically advanced by Nancy Joyce, PhD, and her laboratory, not least with their efforts to determine how to stimulate proliferation of the senescent endothelial cells.
Despite the advances in the basic science mechanisms pertaining to endothelial cell biology and disease, the current treatment of endothelial disease remains surgical. In 2010, 42,000 corneal transplants were performed in the United States, and over half of these were for endothelial disease (EBAA, 2010). For decades, penetrating (full-thickness) keratoplasty (PK) was the only procedure for endothelial replacement. PK was first reported by Eduard Zirm in 1906 (Zirm, 1989), and subsequently refined by Ramon Castroviejo (Castroviejo, 1931, 1932a, 1932b). Since the late 1990s, the surgical treatment of endothelial disease has rapidly evolved, with the current treatment of choice being endothelial keratoplasty (EK); in 2010, 19,000 EK procedures were performed in the United States (EBAA, 2010). EK is an intraocular procedure in which posterior host corneal layers are replaced by posterior donor corneal layers to restore endothelial function. The advent of EK has heralded new techniques, new challenges, new complications, and new uncertainties for long-term outcomes.
This review discusses endothelial keratoplasty techniques and how they pertain to the integrity of the corneal endothelium. Outcomes with respect to endothelial cell loss and graft survival will be discussed primarily.
1. Keratoplasty for Endothelial Disease
Knowledge of the surgical techniques for EK is important for understanding the factors that cause endothelial cell loss. The EK era has generated new questions about the resilience of the corneal endothelium to surgical trauma and the resulting long-term effects on graft survival.
1.1 Penetrating keratoplasty
Penetrating keratoplasty was for years the only surgical treatment for corneal endothelial disease, but has now been superseded by EK (Patel, 2007). Nevertheless, PK will remain the treatment of choice or default for some eyes (Patel, 2011), and can be a successful vision restoring procedure for many patients (Patel et al., 2008; Price, FW et al., 1991). PK involves the replacement of full-thickness corneal host tissue with full-thickness corneal donor tissue (Figure 1). The donor tissue is cut by the surgeon with a trephine, resulting in inevitable endothelial cell loss at the cut edge; for 8 mm-diameter donor tissue trephinations, 6–9% of donor endothelial cells were damaged (Terry et al., 2009a; Terry et al., 2009b). The central host cornea is similarly excised with a circular trephine and the donor cornea is sutured to the host rim. Surgical manipulation of the donor tissue is minimal compared to EK techniques.
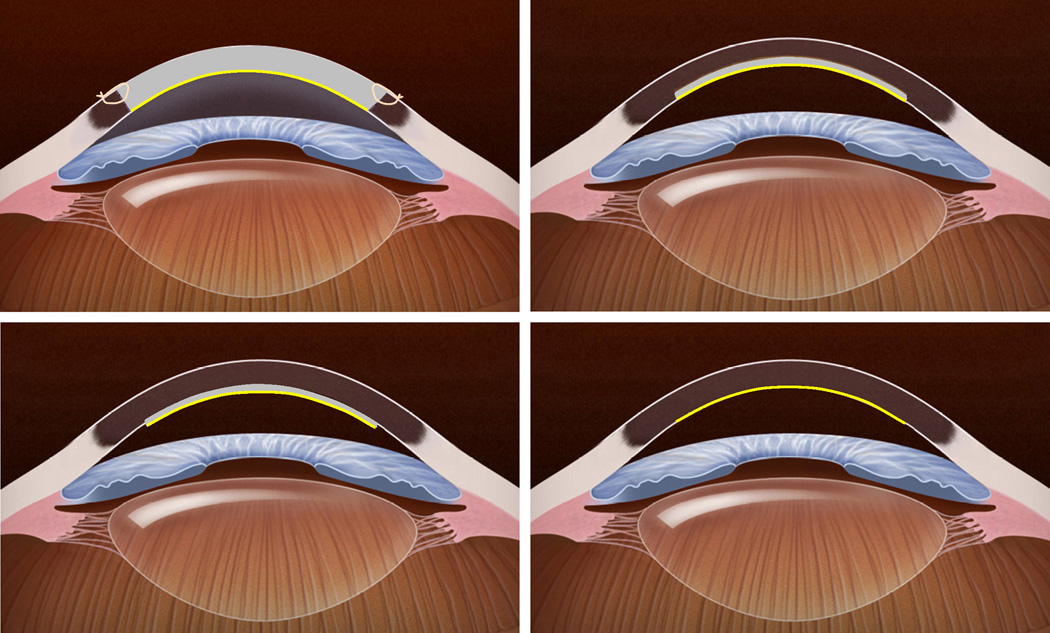
Figure 1. Schematic representation of different keratoplasty techniques for endothelial disease.
Top Left, In penetrating keratoplasty, the full-thickness host cornea is replaced by a full-thickness donor cornea to provide donor endothelial cells (yellow). Sutures are required to secure the donor tissue to the host. Top Right, In deep lamellar endothelial keratoplasty, the posterior host corneal stroma, Descemet membrane and endothelium are excised through a large or small limbal incision and replaced with donor tissue consisting of similar layers. This provides donor endothelial cells (yellow) without corneal incisions or sutures, but introduces an irregular, manually dissected lamellar interface between the donor tissue and host corneas. Bottom Left, In Descemet stripping endothelial keratoplasty, host Descemet membrane and endothelium are stripped from the host posterior corneal stroma, and replaced with donor tissue consisting of posterior stroma, Descemet membrane and endothelium. This provides donor endothelial cells (yellow) without an anterior corneal incision or corneal sutures. Because the donor tissue is usually prepared with a mechanical microkeratome, and the posterior stromal surface is not disrupted, the lamellar interface between donor tissue and host is regular, but the procedure adds a variable thickness of stromal tissue. Bottom Right, In Descemet membrane endothelial keratoplasty, host Descemet membrane and endothelium are stripped from the host posterior corneal stroma, and selectively replaced with donor Descemet membrane and endothelium (yellow). This anatomically mimics the native cornea without requiring an anterior corneal incision, but manipulation of the thin, delicate donor membrane can be technically challenging.
The PK technique is relatively simple compared to lamellar surgical techniques. Nevertheless, good visual outcomes require meticulous wound construction and tissue apposition, though ultimately a high spherocylindrical correction or rigid contact lens might be required to treat high or irregular astigmatism induced by the irregular shape of the anterior graft surface (Crawford et al., 1986; McLaren et al., 2009; Riddle et al., 1998). In addition, PK can be associated with ocular surface complications (Mannis et al., 1997; Meyer and Bobb, 1980; Thompson et al., 2003), suture-related infections (Christo et al., 2001; Forstot et al., 1975), and in rare cases, devastating expulsive hemorrhage intraoperatively or postoperatively (Price, FW et al., 1994; Purcell et al., 1982).
1.2 Posterior lamellar keratoplasty/deep lamellar endothelial keratoplasty
The modern era of EK began in the late 1990s when Melles described a posterior lamellar keratoplasty procedure to replace host endothelium, Descemet membrane, and posterior stroma, with donor tissue of a similar configuration (Figure 1) (Melles et al., 1998; Melles et al., 1999). With modifications, Terry introduced the procedure to the United States as deep lamellar endothelial keratoplasty (DLEK) (Terry and Ousley, 2001a). DLEK was slowly adopted because it required manual stromal lamellar dissection of the host and donor tissue, and was therefore technically challenging. Nevertheless, it was the first successful implementation of EK, and the procedure was advantageous over PK because there were no anterior corneal incisions or sutures, resulting in a predictable spherocylindrical correction for best vision and preventing suture-related complications, and it was performed through a smaller limbal incision (9 mm) providing better postoperative globe integrity in the event of postoperative trauma (Patel et al., 2008; Terry and Ousley, 2001b, 2005a). The anatomy of the virgin cornea was mimicked because the procedure typically provided a relatively continuous posterior corneal surface at the graft-host junction, similar to that after PK (Figure 1).
As experience with posterior lamellar keratoplasty/DLEK mounted, it became the first opportunity to transition EK from a large (9 mm) limbal incison to a smaller (5–6 mm) limbal incision, which was ideal for tectonic reasons (Melles et al., 2002a; Terry and Ousley, 2005b). However, reducing the size of the incision heralded new and significant surgical manipulations of the thin posterior donor lamella and therefore the donor endothelium (Patel, 2007; Terry et al., 2007). For placement into the eye, the donor tissue, which typically had a diameter of 8–9 mm, was folded, inserted through the small incision with forceps, unfolded in the anterior chamber, and positioned against the dissected recipient stromal bed. These additional manipulations, and the striae created in Descemet membrane by folding (Bahn et al., 1986), were, and still remain, factors associated with increased donor endothelial cell loss (see below).
1.3 Descemet stripping endothelial keratoplasty
Endothelial keratoplasty became more rapidly adopted with the realization that host Descemet membrane and endothelium could be easily stripped from the posterior stroma (Heindl et al., 2008; Melles et al., 2004), and that the donor lenticule could adhere to posterior stroma without dissecting a recipient bed (Figure 1) (Price, FW and Price, 2005, 2006). This eliminated the need for the technically challenging manual lamellar dissection of the host tissue, and stripping of Descemet membrane was facilitated in Fuchs dystrophy because the Descemet membrane is thicker than normal (Ahmed et al., 2010; Bourne et al., 1982). Shortly thereafter, manual lamellar stromal dissection of the donor tissue was also simplified by preparation of the tissue with semi-automated mechanical microkeratomes (Gorovoy, 2006; Price, FW and Price, 2006). As a result, Descemet stripping endothelial keratoplasty (DSEK) became the procedure of choice, and remains the most popular EK technique at this time. Rapid adoption of DSEK was enabled in part by the simpler technique compared to DLEK, and in part by eye banks preparing and distributing pre-cut donor lenticules (Price, MO et al., 2008). DSEK is an additive procedure because the host Descemet membrane and endothelium are replaced by donor stroma of variable thickness, Descemet membrane and endothelium (Ahmed et al., 2010) (Figure 1). The donor stroma acts as a carrier for the thin Descemet membrane and donor endothelium and thus aids handling of the tissue.
DSEK was initially performed through a small (5 mm) limbal incision (Price, FW and Price, 2005), and with minimal disruption of the anterior corneal surface, thus maintaining all the advantages of EK over PK. The small incision required surgical manipulation of the donor tissue for insertion and positioning into the anterior chamber (Patel, 2007). In contrast to DLEK, in which the dissected recipient stromal bed facilitated adhesion of the donor tissue to the host, in DSEK, the donor tissue was apposed to the smooth posterior stromal surface by an intracameral air bubble. As a result, the earliest and most frequent (15% of cases (Lee, WB et al., 2009)} complication of DSEK was, and still is, donor tissue dislocation, which typically necessitates a further injection of intracameral air and repositioning of the donor tissue (Price, FW and Price, 2006).
The new surgical challenges associated with DSEK increased the risk of endothelial cell damage during the procedure. The most challenging aspect of DSEK, especially for novice surgeons, has been the insertion and positioning of the donor tissue. The original technique was forceps insertion of folded donor tissue into the anterior chamber (Price, FW and Price, 2005). With increasing popularity, a variety of insertion techniques were adopted, including pull-though and push-through methods (Balachandran et al., 2009; Macsai and Kara-Jose, 2007; Mehta et al., 2007; Vajpayee et al., 2006; Van Cleynenbreugel et al., 2008). Incisions also became as small as 3 mm, which required either multiple folds or compression of the donor tissue for insertion (Price, MO et al., 2010). In human corneas ex vivo, a 5 mm limbal incision was associated with 18 to 20% endothelial cell damage, regardless of the method of insertion, whereas a 3 mm incision was associated with 30% cell loss (Terry et al., 2009a). Endothelial cell damage was highest (56% cell loss) when the donor tissue was compressed through a 3 mm incision without folding (Terry et al., 2009a). While forceps insertion of folded tissue remains acceptable, several insertion devices have been designed to help deliver the donor tissue into the anterior chamber (Busin et al., 2008; Khor et al., 2010). Intraocular lens cartridges have also been used to deliver the donor tissue with decreased endothelial cell loss compared to forceps insertion in human corneas ex vivo (Kuo et al., 2008).
Aside from surgical trauma related to the insertion method and incision size, other aspects of the DSEK technique contribute to cell damage. Intracameral air is associated with endothelial cell damage (Eiferman and Wilkins, 1981; Lee, DA et al., 1991; Tsubota et al., 1988) with as much as 10% cell loss (Hong et al., 2009). Laboratory simulations of donor tissue manipulation within the anterior chamber have not been reported, but simulation of anterior chamber collapse (i.e. iridolenticular touch) was associated with 55% cell loss (Lee, WB et al., 2007).
1.3 Descemet membrane endothelial keratoplasty
The additive nature of DSEK has been suggested to impair visual outcomes (Letko et al., 2011), and has thus spurred interest in selectively replacing host Descemet membrane and endothelium with donor Descemet membrane and endothelium alone to maintain normal corneal thickness and posterior curvature (Figure 1) (Dapena et al., 2009; Melles et al., 2002b; Melles et al., 2006). This procedure, called Descemet membrane endothelial keratoplasty (DMEK), is the latest form of EK offered at only a few centers worldwide. The adoption of DMEK has been slower than that of DSEK because of its increased technical difficulty, and, to date, the paucity of outcomes data that show a clear advantage over DSEK. The two new challenges that DMEK poses over DSEK are preparation of the donor tissue without wastage, and insertion, manipulation and adhesion of the thin membrane while minimizing damage to the donor endothelium.
Donor tissue preparation for DMEK has ranged from careful manual dissection (Laaser et al., 2011; Melles et al., 2006; Price, MO et al., 2009) to more complicated hybrid techniques (Busin et al., 2010; McCauley et al., 2009; Studeny et al., 2010). Manual dissection relies on the Descemet membrane forming a roll to aid with orientation of the endothelial side of the membrane, and subsequent insertion into the eye through an intraocular lens cartridge or other device (Bachmann et al., 2010; Dapena et al., 2011; Price, MO et al., 2009). Although cell loss as low as 3.4% has been reported with manual dissection and insertion in human corneas ex vivo (Melles et al., 2002b), tearing of the donor Descemet membrane during preparation can render the tissue unusable for surgery (Melles et al., 2006). Difficult tissue preparation by experienced surgeons was reported for 17% of grafts, with 8% of grafts becoming unusable (Price, MO et al., 2009). To improve manipulation of the thin donor Descemet membrane, hybrid donor tissue preparation techniques are being developed in which peripheral donor stroma is retained for structural support while exposing the central Descemet membrane (Busin et al., 2010; McCauley et al., 2009; Studeny et al., 2010). In the hybrid technique, Descemet membrane is cleaved from the posterior stroma by injection of air, which can also result in perforation; this technique has been associated with 30% donor tissue wastage (Shah et al., 2009), although with more experience, the wastage rate decreases to 5% (Studeny et al., 2010).
Insertion and manipulation of the donor tissue in DMEK is more difficult than with DSEK, resulting in more frequent donor tissue dislocations, higher endothelial cell loss, and increased early graft failure (Ham et al., 2008; Laaser et al., 2011; Price, MO et al., 2009). Although the donor tissue can be stained with trypan blue to aid visualization and orientation (Bachmann et al., 2010; Melles et al., 2006), grafts are often oriented incorrectly with the endothelial cell surface apposed to recipient stroma (Ham et al., 2008). A “no-touch” technique has been described to minimize endothelial trauma (Dapena et al., 2011), and grafts with peripheral stromal support will be easier to manipulate, but there are no laboratory data assessing cell damage with these methods.
For DMEK to become widely adopted, the procedure will require minimal tissue wastage, donor tissue preparation by eye banks, a simple insertion technique, and few dislocations. In addition, the clinical outcomes of DMEK will need to supersede those of DSEK.
2. Endothelial Outcomes of Keratoplasty
The success of corneal transplantation has traditionally been assessed by graft survival, which is the time to graft failure (Coster and Williams, 2005). Endothelial cell loss has also been frequently reported, although cell loss might not always indicate overall loss of endothelial function (Lass et al., 2010). Visual outcomes were difficult to assess in the era of PK, but are gaining more importance as EK predominates (Patel, 2011). Keratoplasty outcomes should be interpreted with caution, especially when comparing different studies, because of variability in surgeon experience and techniques, and a lack of standardization for defining and measuring the outcomes. Furthermore, keratoplasty techniques have evolved so rapidly that sufficient data might not be available to rigorously determine some outcomes.
2.1 Endothelial cell loss
When comparing endothelial cell density (ECD) data between studies, it is important to know which endothelial analysis techniques were used and whether the microscopes were independently calibrated (McCarey et al., 2008). A few studies report microscope calibration for preoperative donor endothelial cell data for accurate comparison to postoperative data acquired with a different microscope (Bourne et al., 1994a; Cornea Donor Study Investigator Group et al., 2008b); however, most studies report the preoperative donor ECD as measured by the eye bank and assume an accurate calibration. In addition, because endothelial cell data after keratoplasty are derived from surviving grafts (failed grafts often cannot be measured), endothelial cell loss is typically underestimated.
2.1.1 Physiologic endothelial cell loss
Human corneal endothelial cells undergo little mitosis in vivo, although they do retain the ability to proliferate in vitro (Engelmann et al., 1988; Senoo and Joyce, 2000). As a result, endothelial cells are lost at a rate of 0.6% per year during adult life (Bourne et al., 1997), a rate that is low enough for most endothelia to function normally over a lifetime. Physiologic endothelial cell loss represents the death of stressed, senescent cells (Joyce et al., 2009), although the exact mechanism of cell death is not fully understood. The rate of endothelial cell loss increases after surgical procedures of the anterior segment, including after cataract surgery (Armitage et al., 2003; Bourne et al., 1994b).
2.1.2 Penetrating keratoplasty
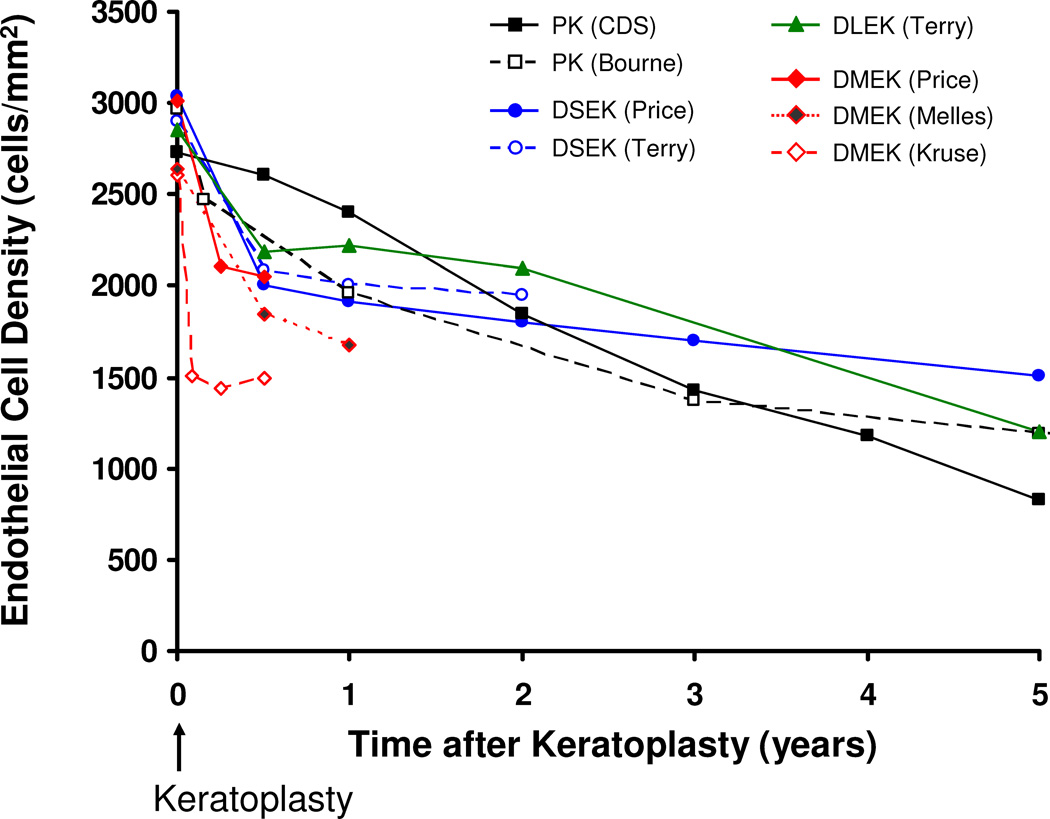
During the first decade after PK, the annual rate of donor endothelial cell loss is 4–8% (Ing et al., 1998), but thereafter, the mean rate of cell loss approaches that of normal corneas, although individual grafts vary widely from the mean (Patel et al., 2010; Patel et al., 2005). In a large PK series by one surgeon (Bourne), endothelial cell loss from preoperative donor ECD was 34% at 1 year, 59% at 5 years, and 74% at 20 years (Figure 2) (Patel et al., 2010). Although this series included PKs for any recipient diagnosis, cell loss was similar between PK for keratoconus and for Fuchs dystrophy (Patel et al., 2010; Patel et al., 2005), and similar to other results (Sellevoll et al., 2009). The Specular Microscopy Ancillary Study to the Cornea Donor Study examined endothelial cell loss after PK for endothelial disease only and found a median cell loss of 70% at 5 years (Figure 2) (Cornea Donor Study Investigator Group et al., 2008b); this study has been extended to determine the effect of donor age on cell loss at 10 years.
Figure 2. Comparison of donor endothelial cell loss by keratoplasty technique.
Donor endothelial cell loss in the early postoperative period is higher after endothelial keratoplasty than after penetrating keratoplasty (PK). At 2 years, cell loss from preoperative is comparable between PK, deep lamellar endothelial keratoplasty (DLEK) with a 9 mm incision, and Descemet stripping endothelial keratoplasty (DSEK). At 5 years, cell loss after DSEK appears to be lower than that after PK. Only data from larger series were included for clarity, and thus data for Descemet membrane endothelial keratoplasty (DMEK) are only shown through 12 months. When endothelial cell densities were not reported, the data were estimated from the reported endothelial cell loss. The primary surgeon for each series is indicated; CDS, Cornea Donor Study.
Endothelial cell density in the early months after PK is predictive of late endothelial graft failure, whereas predicting late endothelial failure from preoperative ECD varies (Lass et al., 2010; Patel et al., 2010). This variable relationship indicates that endothelial cell function cannot be predicted simply by measuring ECD; 14% of clear grafts had an ECD <500 cells/mm2 in the Specular Microscopy Ancillary Study (Lass et al., 2010).
2.1.3 Deep lamellar endothelial keratoplasty
In the largest reported series of DLEK, Terry et al. found that endothelial cell loss from preoperative ECD was 25% at 6 months, 26% at 1 year, and 37% at 2 years after surgery (Figure 2) (Terry et al., 2007). Cell loss at 2 years was higher with small incision (5 mm) DLEK than with large incision (9 mm) DLEK (Terry et al., 2007), and was the first clear indication that incision size affected cell loss; the effect of incision size in DLEK was also found in other studies (Fillmore et al., 2010; van Dooren et al., 2007).} At 5 years after DLEK in Terry’s series, the large incision group had 60% cell loss from preoperative (Figure 2) (personal communication with Mark Terry on 4/10/11), no worse than cell loss after PK. However, in a smaller series of small incision DLEK, endothelial cell loss was 43% at 1 year, and 62% at 4 years (Mashor et al., 2010). The longest reported follow-up for DLEK is 10 years, with 79% endothelial cell loss in 15 surviving grafts (van Dijk et al., 2011).
2.1.4 Descemet stripping endothelial keratoplasty
There are several reports of endothelial cell changes after DSEK, but few reports beyond 12 months of follow-up. The American Academy of Ophthalmology recently published a comprehensive analysis of outcomes after DSEK, including endothelial cell loss (Lee, WB et al., 2009). In the larger series of DSEK, endothelial cell loss was high in the early postoperative period with 28% to 35% cell loss at 6 months, 31% to 36% at 12 months, and 31% to 41% cell loss at 24 months (Figure 2) (Price, MO and Price, 2008; Terry et al., 2011). Price et al. have reported the longest follow-up after DSEK with cell loss of 44% at 3 years and 54% at 5 years (Figure 2) (Price, MO et al., 2011). Thus, despite the high initial endothelial cell loss after DSEK compared to after PK, the rate of cell loss declines rapidly after 6 months (Price, MO et al., 2011; Terry et al., 2011). In fact, at 5 years after DSEK, endothelial cell loss was lower than that at 5 years after PK in the Cornea Donor Study (Cornea Donor Study Investigator Group et al., 2008b; Price, MO et al., 2011). The reason for the apparent decline in cell loss after 6 months is unknown, and longer follow-up is needed to determine if this trend will persist. In a prospective study at Mayo Clinic, endothelial cell loss was highest in the first month after DSEK, with a slower rate of cell loss through 3 years (author’s unpublished data), similar to that reported by Price et al (Price, MO et al., 2011).
Several factors increase endothelial cell loss after DSEK, including smaller incisions (Price, MO et al., 2010), longer incisions (Price, MO and Price, 2008), larger area of tissue compression by forceps (Price, MO and Price, 2008), and donor tissue dislocation (Price, MO and Price, 2008). Factors unassociated with endothelial cell loss include donor tissue storage time (Price, MO et al., 2011; Terry et al., 2011), graft diameter (Price, MO et al., 2011; Terry, 2009), preoperative ECD (Terry et al., 2008), manual versus microkeratome preparation of the donor tissue (Price, MO and Price, 2008), and the use of pre-cut versus surgeon-cut tissue (Price, MO et al., 2008). Early cell loss after DSEK using graft delivery devices, which do not require tissue folding or cause tissue compression, appeared to be lower in small, non-randomized series (Busin et al., 2008; Khor et al., 2010).
2.1.5 Descemet membrane endothelial keratoplasty
With DMEK in its infancy, there are only a few reports of early endothelial cell loss after this procedure. Price et al. found 30% cell loss at 3 months, and 32% at 6 months (Price, MO et al., 2009), whereas Laaser et al. found approximately 42% cell loss at 1, 3, and 6 months (Figure 2) (Laaser et al., 2011).. The longest follow-up to date is for 7 eyes that had 34% cell loss at 2 years, although in the same but expanded series, cell loss was 36% at 1 year (Figure 2) (Ham et al., 2009b).
2.2 Graft Survival
The definition of graft survival, or more specifically, that of graft failure, varies between studies (Patel, 2011). Graft failure can be divided into primary failure, which is either early (primary donor failure) or late (late endothelial failure (Bell et al., 2000; Nishimura et al., 1999)), and secondary failure, in which there is a precipitating cause of failure, such as rejection or infection. Of note, primary donor failure after EK is better termed iatrogenic graft failure because most are caused by the surgical manipulation of donor tissue (Lee, WB et al., 2009).
2.2.1 Penetrating keratoplasty
The cumulative probability of developing graft failure and late endothelial failure after PK for any indication was 30% and 13%, respectively, at 20 years (Patel et al., 2010). Graft failure rates vary with the indication for PK, with grafts for keratoconus being less likely to fail from any cause or from late endothelial failure than grafts for endothelial disease (Patel et al., 2010; Patel et al., 2005). Similar results have been found in other large series (Thompson et al., 2003; Williams et al., 2008).
The 10-year failure rate of first PKs for Fuchs dystrophy varies from 10–20% (Ing et al., 1998; Pineros et al., 1996; Thompson et al., 2003), and is lower than that for pseudophakic or aphakic corneal edema (Ing et al., 1998; Thompson et al., 2003). The Cornea Donor Study, which examined graft survival after PK for endothelial disease, found that 14% of grafts failed by 5 years (Cornea Donor Study Investigator Group et al., 2008a), and the failure rate for Fuchs’ dystrophy (7%) was lower than that for pseudophakic or aphakic corneal edema (Sugar et al., 2009). The primary donor and late endothelial failure rates in the Cornea Donor Study were <1% and approximately 4%, respectively, at 5 years (Cornea Donor Study Investigator Group et al., 2008a).
2.2.2 Deep lamellar endothelial keratoplasty
Graft survival data for DLEK are sparse and relatively short-term. Primary donor failure occurred in 1 to 8% of eyes (Fillmore et al., 2010; Mashor et al., 2010; Terry et al., 2007), higher than that after PK, and was more likely iatrogenic than attributable to the donor tissue. The rate of late endothelial failure was 1% at 2 years (Terry et al., 2007), 27% at 4 years (Mashor et al., 2010), and 14% at 10 years, (van Dijk et al., 2011) in three separate studies.
2.2.3 Descemet stripping endothelial keratoplasty
Primary or iatrogenic graft failure is much higher after DSEK than after PK; the reported rates vary (Lee, WB et al., 2009), although higher rates are likely to be associated with surgeon inexperience. In the larger DSEK series, and thus those by surgeons with the most experience, Terry had no primary graft failures (Terry et al., 2008), and Price reported a 3.5% failure rate (Price, FW and Price, 2006); most other studies report the rate to be less than 10% with an average of 5% (Lee, WB et al., 2009).
The rate of late endothelial failure after DSEK is difficult to ascertain because there are few reports with more than 2 years of follow-up. At five years, Price et al. found that 2.4% of grafts failed because of late endothelial failure and concluded that the overall graft failure rate for DSEK was similar to that after PK in the Cornea Donor Study (Price, MO et al., 2011). Although the rate of late endothelial failure after DSEK is similar to that after PK and poor visual outcomes might become a more common cause of graft failure (Letko et al., 2011; Price, MO et al., 2011), Price et al. did not include the primary/iatrogenic graft failures in their 5-year analysis (Price, MO et al., 2011), and these were in fact the most common cause of graft failure through 5 years (Price, FW and Price, 2006).
2.2.4 Descemet membrane endothelial keratoplasty
The primary or iatrogenic graft failure rate in initial reports of DMEK is 6 to 8% (Ham et al., 2009a; Price, MO et al., 2009), and is in addition to 8% donor tissue wastage during preparation (Price, MO et al., 2009). Longer follow-up is required to determine late endothelial failure rates.
3. Visual Outcomes
The initial experience and results of DSEK have provided optimism for long-term graft survival and for the potential of restoring normal vision. The major advantages of EK over PK for vision are better uncorrected visual acuity and a predictable postoperative refractive error (Chen et al., 2008; Patel et al., 2009; Price, FW and Price, 2005). Although PK can provide a similar rate of visual recovery as DSEK (Patel et al., 2009), visual recovery is considered to be quicker, and quality of vision better, after DSEK than after PK. Nevertheless, quality of vision is not restored to normal (Seery et al., 2011a), and attention has focused on the variable thickness of the donor tissue in DSEK (Ahmed et al., 2010) and to mismatch in curvature of the graft and host that might increase optical aberrations (Seery et al., 2011b). This has spurred the current interest in DMEK, which offers the potential for improved visual outcomes (Price, MO et al., 2009). Nevertheless, the success of DMEK will also require that endothelial cell loss and graft survival to be similar to or better than those after DSEK. In addition, changes in the anterior cornea as a result of chronic endothelial dysfunction might also affect visual outcomes in some cases, irrespective of the method of endothelial replacement (Hecker et al., 2011; Patel et al., 2009).
4. Future Prospects for Treating Endothelial Disease
Future treatments for endothelial disease would ideally avoid the need for surgery, and might include pharmacologic agents to stimulate endothelial cell proliferation (Joyce and Harris, 2010; Okumura et al., 2011; Okumura et al., 2009) or function (Hatou et al., 2010), or therapies to slow or halt disease progression. Until such novel therapies become available, keratoplasty will remain the mainstay of treatment, and efforts should continue to improve keratoplasty techniques and outcomes. Most important at the present time are methods to protect the donor endothelium during EK to reduce the high rate of primary/iatrogenic graft failure (Fuchsluger et al., 2011). In addition, a better understanding of the mechanisms of chronic endothelial cell loss and senescence (Joyce et al., 2011) might help improve graft longevity. Transplantation of cultured corneal endothelial cells should be further developed to help expand the donor pool (Hitani et al., 2008; Koizumi et al., 2007; Mimura et al., 2007; Patel et al., 2009). While the prospects for future treatment of endothelial disease are vast, they all require continued understanding of endothelial cell biology in normal and transplanted corneas, and improved knowledge of the mechanisms of disease, and we are indebted to Nancy Joyce for setting this precedent.
Acknowledgments
Role of the Funding Source
The funding sources had no involvement in the study design, collection, analysis and interpretation of data, in the writing of the report or in the decision to submit for publication.
Dr. Patel is supported by: National Institutes of Health (EY 19339), Bethesda, MD; Research to Prevent Blindness (unrestricted departmental grant, and as Olga Keith Wiess Scholar), New York, NY; and Mayo Foundation, Rochester, MN.
Footnotes
Financial Disclosure: None.
References
- Ahmed KA, McLaren JW, Baratz KH, Maguire LJ, Kittleson KM, Patel SV. Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Am. J. Ophthalmol. 2010;150:490–497. doi: 10.1016/j.ajo.2010.05.011. Epub 2010 Aug. [DOI] [PubMed] [Google Scholar]
- Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest. Ophthalmol. Vis. Sci. 2003;44:3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- Bachmann BO, Laaser K, Cursiefen C, Kruse FE. A method to confirm correct orientation of Descemet membrane during Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2010;149:922–925. doi: 10.1016/j.ajo.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Bahn CF, Grosserode R, Musch DC, Feder J, Meyer RF, MacCallum DK, Lillie JH, Rich NM. Effect of 1% sodium hyaluronate (Healon) on a nonregenerating (feline) corneal endothelium. Invest. Ophthalmol. Vis. Sci. 1986;27:1485–1494. [PubMed] [Google Scholar]
- Balachandran C, Ham L, Birbal RS, Wong T-H, van der Wees J, Melles GRJ. Simple technique for graft insertion in Descemet-stripping (automated) endothelial keratoplasty using a 30-gauge needle. J Cataract Refract Surg. 2009;35:625–628. doi: 10.1016/j.jcrs.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Bell KD, Campbell RJ, Bourne WM. Pathology of late endothelial failure: late endothelial failure of penetrating keratoplasty: study with light and electron microscopy. Cornea. 2000;19:40–46. doi: 10.1097/00003226-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Bourne WM, Johnson DH, Campbell RJ. The ultrastructure of Descemet's membrane. III. Fuchs' dystrophy. Arch Ophthalmol. 1982;100:1952–1955. doi: 10.1001/archopht.1982.01030040932013. [DOI] [PubMed] [Google Scholar]
- Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am. J. Ophthalmol. 1994a;118:185–196. doi: 10.1016/s0002-9394(14)72898-3. [DOI] [PubMed] [Google Scholar]
- Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994b;101:1014–1022. doi: 10.1016/s0161-6420(94)31224-3. [DOI] [PubMed] [Google Scholar]
- Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest. Ophthalmol. Vis. Sci. 1997;38:779–782. [PubMed] [Google Scholar]
- Busin M, Bhatt PR, Scorcia V. A modified technique for Descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008;126:1133–1137. doi: 10.1001/archopht.126.8.1133. [DOI] [PubMed] [Google Scholar]
- Busin M, Patel AK, Scorcia V, Galan A, Ponzin D. Stromal support for Descemet's membrane endothelial keratoplasty. Ophthalmology. 2010;117:2273–2277. doi: 10.1016/j.ophtha.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Castroviejo R. New method of corneal transplantation: final report. Proc Staff Meet Mayo Clinic. 1931;6:668. [Google Scholar]
- Castroviejo R. Keratoplasty. An historical and experimental study, including a new method. Part I. Am. J. Ophthalmol. 1932a;15:825–828. [Google Scholar]
- Castroviejo R. Keratoplasty. An historical and experimental study, including a new method. Part II. Am. J. Ophthalmol. 1932b;15:905–916. [Google Scholar]
- Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Descemet-stripping automated endothelial keratoplasty: six-month results in a prospective study of 100 eyes. Cornea. 2008;27:514–520. doi: 10.1097/ICO.0b013e3181611c50. [DOI] [PubMed] [Google Scholar]
- Christo CG, van Rooij J, Geerards AJ, Remeijer L, Beekhuis WH. Suture-related complications following keratoplasty: a 5-year retrospective study. Cornea. 2001;20:816–819. doi: 10.1097/00003226-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Cornea Donor Study Investigator Group. Gal RL, Dontchev M, Beck RW, Mannis MJ, Holland EJ, Kollman C, Dunn SP, Heck EL, Lass JH, Montoya MM, Schultze RL, Stulting RD, Sugar A, Sugar J, Tennant B, Verdier DD. The effect of donor age on corneal transplantation outcome results of the cornea donor study. Ophthalmology. 2008a;115:620–626. doi: 10.1016/j.ophtha.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea Donor Study Investigator Group. Lass JH, Gal RL, Dontchev M, Beck RW, Kollman C, Dunn SP, Heck E, Holland EJ, Mannis MJ, Montoya MM, Schultze RL, Stulting RD, Sugar A, Sugar J, Tennant B, Verdier DD. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular microscopy ancillary study results. Ophthalmology. 2008b;115:627–632. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am. J. Ophthalmol. 2005;140:1112–1122. doi: 10.1016/j.ajo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Crawford GJ, Stulting RD, Waring GO, 3rd, Van Meter WS, Wilson LA. The triple procedure. Analysis of outcome, refraction, and intraocular lens power calculation. Ophthalmology. 1986;93:817–824. doi: 10.1016/s0161-6420(86)33673-x. [DOI] [PubMed] [Google Scholar]
- Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK--the thinner the better? Curr Opin Ophthalmol. 2009;20:299–307. doi: 10.1097/ICU.0b013e32832b8d18. [DOI] [PubMed] [Google Scholar]
- Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K, Melles GRJ. Standardized "no-touch" technique for Descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2011;129:88–94. doi: 10.1001/archophthalmol.2010.334. [DOI] [PubMed] [Google Scholar]
- EBAA. Eye Bank Association of America. Annual statistical report. 2010 [Google Scholar]
- Eiferman RA, Wilkins EL. The effect of air on human corneal endothelium. Am. J. Ophthalmol. 1981;92:328–331. doi: 10.1016/0002-9394(81)90520-1. [DOI] [PubMed] [Google Scholar]
- Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1988;29:1656–1662. [PubMed] [Google Scholar]
- Fillmore PD, Sutphin JE, Goins KM. Visual acuity, refractive error, and endothelial cell density 6 and 12 months after deep lamellar endothelial keratoplasty. Cornea. 2010;29:601–606. doi: 10.1097/ICO.0b013e3181c11de4. [DOI] [PubMed] [Google Scholar]
- Forstot SL, Abel R, Jr, Binder PS. Bacterial endophthalmitis following suture removal after penetrating keratoplasty. Am. J. Ophthalmol. 1975;80:509–512. doi: 10.1016/0002-9394(75)90217-2. [DOI] [PubMed] [Google Scholar]
- Fuchsluger TA, Jurkunas U, Kazlauskas A, Dana R. Anti-apoptotic gene therapy prolongs survival of corneal endothelial cells during storage. Gene Ther. 2011 doi: 10.1038/gt.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25:886–889. doi: 10.1097/01.ico.0000214224.90743.01. [DOI] [PubMed] [Google Scholar]
- Ham L, van der Wees J, Melles GR. Causes of primary donor failure in descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2008;145:639–644. doi: 10.1016/j.ajo.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Ham L, Dapena I, van Luijk C, van der Wees J, Melles GR. Descemet membrane endothelial keratoplasty (DMEK) for Fuchs endothelial dystrophy: review of the first 50 consecutive cases. Eye (Lond) 2009a;23:1990–1998. doi: 10.1038/eye.2008.393. [DOI] [PubMed] [Google Scholar]
- Ham L, van Luijk C, Dapena I, Wong TH, Birbal R, van der Wees J, Melles GR. Endothelial cell density after descemet membrane endothelial keratoplasty: 1- to 2-year follow-up. Am J Ophthalmol. 2009b;148:521–527. doi: 10.1016/j.ajo.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Hatou S, Yamada M, Akune Y, Mochizuki H, Shiraishi A, Joko T, Nishida T, Tsubota K. Role of insulin in regulation of Na+−/K+-dependent ATPase activity and pump function in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2010;51:3935–3942. doi: 10.1167/iovs.09-4027. [DOI] [PubMed] [Google Scholar]
- Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in Fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129:555–561. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl LM, Hofmann-Rummelt C, Schlotzer-Schrehardt U, Kruse FE, Cursiefen C. Histologic Analysis of Descemet Stripping in Posterior Lamellar Keratoplasty. Arch Ophthalmol. 2008;126:461–464. doi: 10.1001/archophthalmol.2007.75. [DOI] [PubMed] [Google Scholar]
- Hitani K, Yokoo S, Honda N, Usui T, Yamagami S, Amano S. Transplantation of a sheet of human corneal endothelial cell in a rabbit model. Mol. Vis. 2008;14:1–9. [PMC free article] [PubMed] [Google Scholar]
- Hong A, Caldwell MC, Kuo AN, Afshari NA. Air bubble-associated endothelial trauma in Descemet stripping automated endothelial keratoplasty. Am. J. Ophthalmol. 2009;148:256–259. doi: 10.1016/j.ajo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Zhu CC, Harris DL. Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2009;50:2116–2122. doi: 10.1167/iovs.08-3007. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Harris DL. Decreasing expression of the G1-phase inhibitors, p21Cip1 and p16INK4a, promotes division of corneal endothelial cells from older donors. Mol Vis. 2010;16:897–906. [PMC free article] [PubMed] [Google Scholar]
- Joyce NC, Harris DL, Zhu C. Age-Related Gene Response of Human Corneal Endothelium to Oxidative Stress and DNA Damage. Invest Ophthalmol Vis Sci. 2011;52:1641–1649. doi: 10.1167/iovs.10-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor W-B, Mehta JS, Tan DT-H. Descemet stripping automated endothelial keratoplasty with a graft insertion device: Surgical technique and early clinical results. Am. J. Ophthalmol. 2010;151:223–232. doi: 10.1016/j.ajo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Sakamoto Y, Okumura N, Okahara N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest. Ophthalmol. Vis. Sci. 2007;48:4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- Kuo AN, Harvey TM, Afshari NA. Novel delivery method to reduce endothelial injury in Descemet stripping automated endothelial keratoplasty. Am. J. Ophthalmol. 2008;145:91–96. doi: 10.1016/j.ajo.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Laaser K, Bachmann BO, Horn FK, Schlötzer-Schrehardt U, Cursiefen C, Kruse FE. Donor tissue culture conditions and outcome after Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2011 doi: 10.1016/j.ajo.2010.11.027. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Lass JH, Sugar A, Benetz BA, Beck RW, Dontchev M, Gal RL, Kollman C, Gross R, Heck E, Holland EJ, Mannis MJ, Raber I, Stark W, Stulting RD for the Cornea Donor Study Investigator Group. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128:63–69. doi: 10.1001/archophthalmol.2010.128.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Wilson MR, Yoshizumi MO, Hall M. The ocular effects of gases when injected into the anterior chamber of rabbit eyes. Arch Ophthalmol. 1991;109:571–575. doi: 10.1001/archopht.1991.01080040139045. [DOI] [PubMed] [Google Scholar]
- Lee WB, Sy HM, Holley GP, Edelhauser HF. Descemet's Stripping Automated Endothelial Keratoplasty (DSAEK): Intra-Operative Effects on the Donor Corneal Endothelium. Invest. Ophthalmol. Vis. Sci. 2007;48 ARVO E-Abstract #1131. [Google Scholar]
- Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet's Stripping Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Letko E, Price DA, Lindoso EMS, Price MO, Price FW., Jr Secondary graft failure and repeat endothelial keratoplasty after Descemet's stripping automated endothelial keratoplasty. Ophthalmology. 2011;118:310–314. doi: 10.1016/j.ophtha.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Macsai MS, Kara-Jose AC. Suture technique for Descemet stripping and endothelial keratoplasty. Cornea. 2007;26:1123–1126. doi: 10.1097/ICO.0b013e318124a443. [DOI] [PubMed] [Google Scholar]
- Mannis MJ, Zadnik K, Miller MR, Marquez M. Preoperative risk factors for surface disease after penetrating keratoplasty. Cornea. 1997;16:7–11. [PubMed] [Google Scholar]
- Mashor RS, Kaiserman I, Kumar NL, Sansanayudh W, Rootman DS. Deep lamellar endothelial keratoplasty: Up to 5-year follow-up. Ophthalmology. 2010;117:680–686. doi: 10.1016/j.ophtha.2009.12.039. [DOI] [PubMed] [Google Scholar]
- McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–16. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley MB, Price FW, Jr, Price MO. Descemet membrane automated endothelial keratoplasty: hybrid technique combining DSAEK stability with DMEK visual results. J Cataract Refract Surg. 2009;35:1659–1664. doi: 10.1016/j.jcrs.2009.05.034. [DOI] [PubMed] [Google Scholar]
- McLaren JW, Patel SV, Bourne WM, Baratz KH. Corneal wavefront errors 24 months after deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am. J. Ophthalmol. 2009;147:959–965. doi: 10.1016/j.ajo.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Mehta JS, Por Y-M, Beuerman RW, Tan DT. Glide insertion technique for donor cornea lenticule during Descemet's stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2007;33:1846–1850. doi: 10.1016/j.jcrs.2007.06.050. [DOI] [PubMed] [Google Scholar]
- Melles GR, Eggink FA, Lander F, Pels E, Rietveld FJ, Beekhuis WH, Binder PS. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618–626. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Melles GR, Lander F, Beekhuis WH, Remeijer L, Binder PS. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am. J. Ophthalmol. 1999;127:340–341. doi: 10.1016/s0002-9394(98)00324-9. [DOI] [PubMed] [Google Scholar]
- Melles GR, Lander F, Nieuwendaal C. Sutureless, posterior lamellar keratoplasty: a case report of a modified technique. Cornea. 2002a;31:325–327. doi: 10.1097/00003226-200204000-00018. [DOI] [PubMed] [Google Scholar]
- Melles GR, Lander F, Rietveld FJ. Transplantation of Descemet's membrane carrying viable endothelium through a small scleral incision. Cornea. 2002b;21:415–418. doi: 10.1097/00003226-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis) Cornea. 2004;23:286–288. doi: 10.1097/00003226-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006;25:987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- Meyer RF, Bobb KC. Corneal epithelium in penetrating keratoplasty. Am J Ophthalmol. 1980;90:142–147. doi: 10.1016/s0002-9394(14)74845-7. [DOI] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Usui T, Seiichi, Honda N, Amano S. Necessary prone position time for human corneal endothelial precursor transplantation in a rabbit endothelial deficiency model. Curr Eye Res. 2007;32:617–623. doi: 10.1080/02713680701530589. [DOI] [PubMed] [Google Scholar]
- Nishimura JK, Hodge DO, Bourne WM. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmology. 1999;106:1962–1965. doi: 10.1016/S0161-6420(99)90409-8. [DOI] [PubMed] [Google Scholar]
- Okumura N, Ueno M, Koizumi N, Sakamoto Y, Hirata K, Hamuro J, Kinoshita S. Enhancement on Primate Corneal Endothelial Cell Survival In Vitro by a ROCK Inhibitor. Invest. Ophthalmol. Vis. Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hirata K, Torii R, Hamuro J, Kinoshita S. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br. J. Ophthalmol. 2011 doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am. J. Ophthalmol. 2005;139:311–319. doi: 10.1016/j.ajo.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114:627–628. doi: 10.1016/j.ophtha.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Patel SV, McLaren JW, Hodge DO, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008;145:97–105. doi: 10.1016/j.ajo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Patel SV, Baratz KH, Hodge DO, Maguire LJ, McLaren JW. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–160. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- Patel SV, Bachman LA, Hann CR, Bahler CK, Fautsch MP. Human corneal endothelial cell transplantation in a human ex vivomodel. Invest. Ophthalmol. Vis. Sci. 2009;50:2123–2131. doi: 10.1167/iovs.08-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SV, Diehl NN, Hodge DO, Bourne WM. Donor risk factors for graft failure in a 20-year study of penetrating keratoplasty. Arch. Ophthalmol. 2010;128:418–425. doi: 10.1001/archophthalmol.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SV. Graft Survival After Penetrating Keratoplasty. Am. J. Ophthalmol. 2011;151:397–398. doi: 10.1016/j.ajo.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros O, Cohen EJ, Rapuano CJ, Laibson PR. Long-term results after penetrating keratoplasty for Fuchs' endothelial dystrophy. Arch. Ophthalmol. 1996;114:15–18. doi: 10.1001/archopht.1996.01100130013002. [DOI] [PubMed] [Google Scholar]
- Price FW, Whitson WE, Marks RG. Progression of visual acuity after penetrating keratoplasty. Ophthalmology. 1991;98:1177–1185. doi: 10.1016/s0161-6420(91)32136-5. [DOI] [PubMed] [Google Scholar]
- Price FW, Whitson WE, Ahad KA, Tavakkoli H. Suprachoroidal hemorrhage in penetrating keratoplasty. Ophthalmic Surg. 1994;25:521–525. [PubMed] [Google Scholar]
- Price FW, Price MO. Descemet's stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J. Refract. Surg. 2005;21:339–345. doi: 10.3928/1081-597X-20050701-07. [DOI] [PubMed] [Google Scholar]
- Price FW, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Price MO, Baig KM, Brubaker JW, Price FW. Randomized, prospective comparison of precut vs surgeon-dissected grafts for Descemet stripping automated endothelial keratoplasty. Am. J. Ophthalmol. 2008;146:36–41. doi: 10.1016/j.ajo.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Price MO, Price FW. Endothelial cell loss after Descemet stripping with endothelial keratoplasty: Influencing factors and 2-year trend. Ophthalmology. 2008;115:857–865. doi: 10.1016/j.ophtha.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Price MO, Giebel AW, Fairchild KM, Price FW. Descemet's membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Price MO, Bidros M, Gorovoy M, Price FW, Benetz BA, Menegay HJ, Debanne SM, Lass JH. Effect of incision width on graft survival and endothelial cell loss after Descemet stripping automated endothelial keratoplasty. Cornea. 2010;29:523–527. doi: 10.1097/ICO.0b013e3181c11e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MO, Fairchild KM, Price DA, Price FW. Descemet's stripping endothelial keratoplasty: Five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118:725–729. doi: 10.1016/j.ophtha.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Purcell JJ, Jr, Krachmer JH, Doughman DJ, Bourne WM. Expulsive hemorrhage in penetrating keratoplasty. Ophthalmology. 1982;89:41–43. doi: 10.1016/s0161-6420(82)34859-9. [DOI] [PubMed] [Google Scholar]
- Riddle HK, Jr, Parker DA, Price FW., Jr Management of postkeratoplasty astigmatism. Curr. Opin. Ophthalmol. 1998;9:15–28. doi: 10.1097/00055735-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Seery LS, McLaren JW, Kittleson KM, Patel SV. Retinal point-spread function after corneal transplantation for Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2011a;52:1003–1008. doi: 10.1167/iovs.10-5375. [DOI] [PubMed] [Google Scholar]
- Seery LS, Nau CB, McLaren JW, Baratz KH, Patel SV. Graft thickness, graft folds, and aberrations after Descemet stripping endothelial keratoplasty for Fuchs dystrophy. Am J Ophthalmol. 2011b doi: 10.1016/j.ajo.2011.05.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellevoll HB, Armitage WJ, Figueiredo MS, Figueiredo RSM, Figueiredo FC. Long-Term Outcomes of Corneal Transplantation. Invest. Ophthalmol. Vis. Sci. 2009;50 ARVO E-Abstract #2200. [Google Scholar]
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol. Vis. Sci. 2000;41:660–667. [PubMed] [Google Scholar]
- Shah AK, Shamie N, Davis-Boozer D, Terry MA. Descemet's Membrane Endothelial Keratoplasty (DMEK): A Comparison of Tissue Wastage Rates During Preparation of Donor Tissues Using the "Big Bubble" vs. "Scuba" Techniques. Invest. Ophthalmol. Vis. Sci. 2009;50 ARVO E-Abstract #640. [Google Scholar]
- Studeny P, Farkas A, Vokrojova M, Liskova P, Jirsova K. Descemet membrane endothelial keratoplasty with a stromal rim (DMEK-S) Br J Ophthalmol. 2010;94:909–914. doi: 10.1136/bjo.2009.165134. [DOI] [PubMed] [Google Scholar]
- Sugar A, Tanner JP, Dontchev M, Tennant B, Schultze RL, Dunn SP, Lindquist TD, Gal RL, Beck RW, Kollman C, Mannis MJ, Holland EJ. Recipient risk factors for graft failure in the Cornea Donor Study. Ophthalmology. 2009;116:1023–1028. doi: 10.1016/j.ophtha.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea. 2001a;20:239–243. doi: 10.1097/00003226-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ. Endothelial replacement without surface corneal incisions or sutures: topography of the deep lamellar endothelial keratoplasty procedure. Cornea. 2001b;21:14–18. doi: 10.1097/00003226-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty visual acuity, astigmatism, and endothelial survival in a large prospective series. Ophthalmology. 2005a;112:1541–1548. doi: 10.1016/j.ophtha.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ. Small-incision deep lamellar endothelial keratoplasty (DLEK): six-month results in the first prospective clinical study. Cornea. 2005b;24:59–65. doi: 10.1097/01.ico.0000133990.19027.a2. [DOI] [PubMed] [Google Scholar]
- Terry MA, Wall JM, Hoar KL, Ousley PJ. A prospective study of endothelial cell loss during the 2 years after deep lamellar endothelial keratoplasty. Ophthalmology. 2007;114:631–639. doi: 10.1016/j.ophtha.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Terry MA, Shamie N, Chen ES, Hoar KL, Phillips PM, Friend DJ. Endothelial keratoplasty: the influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell counts. Cornea. 2008;27:1131–1137. doi: 10.1097/ICO.0b013e3181814cbc. [DOI] [PubMed] [Google Scholar]
- Terry MA. Endothelial keratoplasty: a comparison of complication rates and endothelial survival between precut tissue and surgeon-cut tissue by a single DSAEK surgeon. Trans Am Ophthalmol Soc. 2009;107:184–191. [PMC free article] [PubMed] [Google Scholar]
- Terry MA, Saad HA, Shamie N, Chen ES, Phillips PM, Friend DJ, Holiman JD, Stoeger C. Endothelial keratoplasty: the influence of insertion techniques and incision size on donor endothelial survival. Cornea. 2009a;28:24–31. doi: 10.1097/ICO.0b013e318182a4d3. [DOI] [PubMed] [Google Scholar]
- Terry MA, Saad HA, Shamie N, Shah AK. Peripheral endothelial cell damage after trephination of donor tissue. Cornea. 2009b;28:1149–1152. doi: 10.1097/ICO.0b013e3181a87a28. [DOI] [PubMed] [Google Scholar]
- Terry MA, Shamie N, Straiko MD, Friend DJ, Davis-Boozer D. Endothelial keratoplasty: The relationship between donor tissue storage time and donor endothelial survival. Ophthalmology. 2011;118:36–40. doi: 10.1016/j.ophtha.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Thompson J, Robert W, Price MO, Bowers PJ, Price J, Francis W. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Laing RA, Chiba K, Kenyon KR. Effects of air and irrigating solutions on the corneal endothelium. Cornea. 1988;7:115–121. [PubMed] [Google Scholar]
- Vajpayee RB, Agarwal T, Jhanji V, Sharma N. Modification in descemet-stripping automated endothelial keratoplasty: "Hitch suture" technique. Cornea. 2006;25:1060–1062. doi: 10.1097/01.ico.0000225707.70135.34. [DOI] [PubMed] [Google Scholar]
- Van Cleynenbreugel H, Hillenaar T, Remeijer L. Graft insertion during Descemet-stripping automated endothelial keratoplasty: Pulling the graft inward. J Cataract Refract Surg. 2008;34:534–536. doi: 10.1016/j.jcrs.2007.12.029. [DOI] [PubMed] [Google Scholar]
- van Dijk K, Dapena I, Moutsouris K, Ham L, Nieuwendaal C, Melles GRJ. First DLEK series: 10-year follow-up. Ophthalmology. 2011;118 doi: 10.1016/j.ophtha.2010.10.006. 424-424.e423. [DOI] [PubMed] [Google Scholar]
- van Dooren BTH, Mulder PGH, Nieuwendaal CP, Beekhuis WH, Melles GRJ. Endothelial cell density after posterior lamellar keratoplasty: five- to seven-year follow-up. Am. J. Ophthalmol. 2007;144:471–473. doi: 10.1016/j.ajo.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian Corneal Graft Registry. Transplantation. 2008;86:1720–1724. doi: 10.1097/TP.0b013e3181903b0a. [DOI] [PubMed] [Google Scholar]
- Zirm EK. Eine erfolgreiche totale Keratoplastik (A successful total keratoplasty) Refract Corneal Surg. 1906;5:258–261. [PubMed] [Google Scholar]