Abstract
Pyogenic sacroiliitis is a rare osteoarticular infection, occurring most frequently in children and young adults. Diagnosis of the disease is challenging because of a general lack of awareness of the disease and its nonspecific signs and symptoms. Staphylococcus aureus is the most common causative bacteria in pyogenic sacroiliitis. Methicillin-resistant S. aureus (MRSA) has typically been considered a hospital-associated pathogen; however, community-acquired (CA)-MRSA infections are becoming increasingly common in Korea. We report the first domestic case of acute pyogenic sacroiliitis with abscess and bacteremia caused by CA-MRSA. The pathogen carried the type IV-A staphylococcal cassette chromosome mec (SCCmec) without the Panton-Valentine leukocidin (PVL) gene, and was identified as sequence type (ST) 72 by multilocus sequence typing.
Keywords: Community-acquired infections, Methicillin-resistant Staphylococcus aureus, Pyogenic sacroiliitis
Introduction
Infection of the sacroiliac joint, also known as pyogenic sacroiliitis, is a rare condition, representing only 1-2% of all cases of septic arthritis. The majority of pyogenic sacroiliitis cases are caused by Staphylococcus aureus [1]. The risk factors for pyogenic sacroiliitis include intravenous drug abuse, pregnancy, trauma, and infection of other organ systems [2]. Since 2007, methicillin-resistant S. aureus (MRSA) has emerged as a cause of pyogenic sacroiliitis, and cases with community-acquired (CA)-MRSA as the causative pathogen have been reported [3-5]. At present, there is no consensus regarding the optimal duration of antimicrobial therapy for patients with pyogenic sacroiliitis caused by CA-MRSA [6-7]. Moreover, most studies of CA-MRSA-associated pyogenic sacroiliitis have been conducted in young children or adolescents; there are limited data available for adult patients [3-5]. Six cases of pyogenic sacroiliitis have been reported in Korea, most often in patients with risk factors such as a recent invasive procedure or pregnancy. However, no case of CA-MRSA pyogenic sacroiliitis in a patient without risk factors has been reported in Korea. Similarly, no such cases have been reported in adults in other countries. In most patients with septic arthritis caused by CA-MRSA, intravenous antibiotics are switched to oral trimethoprim-sulfamethoxazole (TMP-SMX) or clindamycin. However, in our case, a fluoroquinolone was used for oral treatment. We report a case of an adult patient with acute pyogenic sacroiliitis caused by CA-MRSA with no known risk factor, which was effectively cured by surgery and antibiotic therapy. A review of literature is included.
Case Report
A 57-year-old woman had severe right sacral-area pain and heating sensation that had begun 2 days before admission. She did not receive any medical treatment for her symptoms; she eventually visited the emergency room because of worsening of pain. The patient had no history of diabetes, hypertension, or pulmonary tuberculosis, but had received a hysterectomy 19 years ago as a treatment for uterine myoma. She had no history of hospital admission, surgery, hemodialysis, or medical prosthesis insertion in the last 10 years. The patient did not drink alcohol or smoke, had not been exposed to any trauma, and had not travelled to a foreign country in the previous 5 years. At the time of admission she was residing at home alone, was unemployed, and had not been in contact with any healthcare professionals or hospitalized patients in her recent past.
Her vital signs were mostly normal: her blood pressure was 110/60 mmHg, her heart rate was 82 beats per minute, and her respiration rate was 20 breaths per minute. However, the patient was febrile (38.9℃). Physical examination revealed tenderness in the right sacral area and limitation in flexion of the right hip joint due to severe pain. Lung sounds were clear without any wheezing or crackles, and her heartbeat was regular without any murmur. Her abdomen was free of tenderness or rebound tenderness, and hepatosplenomegaly was not detected. Neurological examinations revealed normal lower extremity motor and sensory function. Peripheral blood examination showed a white blood cell (WBC) count of 13,000/mm3 (neutrophils 77.3%, lymphocytes 12.9%, monocytes 9.6%), a hemoglobin level of 12.7 g/dL, a platelet count of 196,000/mm3, an erythrocyte sedimentation rate of 53 mm/hr, and a C-reactive protein level of 18.05 mg/dL. Blood chemistry revealed a total protein level of 7.0 g/dL, an albumin level of 4.1 g/dL, a total bilirubin level of 3.2 mg/dL, an aspartate aminotransferase level of 30 IU/L, an alanine aminotransferase level of 25 IU/L, an alkaline phosphatase level of 125 IU/L, a blood urea nitrogen/creatine level of 11.4/0.4 mg/dL, a sodium/potassium level of 134/3.9 mEq/L, and a calcium/phosphorus level of 9.2/3.8 mg/dL. Urinalysis was normal. Chest and abdomen radiographs were normal, and echocardiography to rule out infective endocarditis was also normal. Hip magnetic resonance imaging (MRI) was performed, and revealed a 1.8 × 3.0 cm abscess anterior to the right sacroiliac joint, accompanied by infective arthritis with adjacent myositis (Fig. 1).
Figure 1.
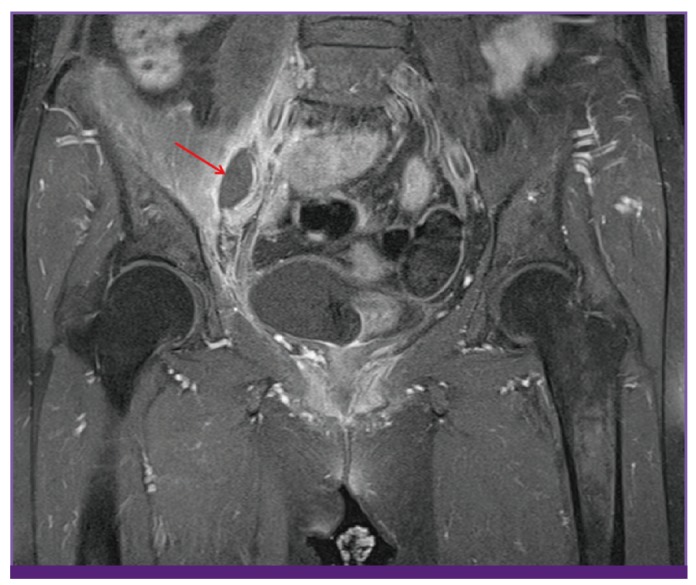
Contrast-enhanced T1-weighted magnetic resonance imaging of both hips shows infectious arthritis of the right sacroiliac joint associated with cellulitis and myositis with abscess formation (red arrow).
Peripheral blood and urine cultures were performed before initiating antibiotic therapy under the suspicion of infective myositis and arthritis. Ceftriaxone (2 g every 24 h) was given intravenously. The abscess was surgically removed, and S. aureus was identified in blood and pus cultures 5 days after hospitalization. Vitek2 AST-P601 card (BioMérieux, France) analysis demonstrated susceptibility to ciprofloxacin, fusidic acid, gentamicin, habekacin, linezolid, mupirocin, nitrofurantoin, quinupristin/dalfopristin, rifampin, teicoplanin, telithromycin, tetracycline, tigecycline, TMP-SMX, and vancomycin (MIC 1 g/mL). It was also revealed that the pathogen was resistant to clindamycin, erythromycin, oxacillin, and penicillin. The D-test for inducible clindamycin resistance was positive.
The cultured strain was tested for the staphylococcal cassette chromosome mec (SCCmec) gene and the Panton-Valentine leukocidin (PVL) gene. Multilocus sequence typing (MLST) for SCCmec was carried out with multiplex-polymerase chain reaction methodology using a specific primer set, following the method of Oliveira et al. [8]. The results showed that the strain was SCCmec type IV-A. that was negative for PVL (Fig. 2). MLST analysis, according to the method of Enright et al., was conducted by sequencing analysis of 7 housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) [9]. The analysis revealed ST 72. The MLST database (http://saureus.mlst.net) revealed a sequence type of 1-4-1-8-4-4-3 (ST 72). On the sixth day of admission, the antibiotic regimen was changed to vancomycin (1 g every 12 h) plus rifampin (600 mg every 24 h). Rifampin was stopped after 20 days, and thereafter vancomycin was switched to teicoplanin (400 mg every 12 h due to the emergence of neutropenia after 25 days) and combined with intravenous levofloxacin (750 mg every 24 h). The latter antibiotic therapy was continued for an additional 10 days. The patient was discharged after her symptoms had subsided and her blood test results normalized. The patient was prescribed oral levofloxacin (750 mg every 24 h for 1 week) upon discharge. The patient did not show additional symptoms, and levofloxacin was continued for an additional 10 days on an outpatient basis. Treatment was stopped after a total of 8 weeks of antibiotic therapy, and no disease recurrence was reported after 1 year of follow-up.
Figure 2.

SCCmec typing of isolated MRSA
(A) 100-bp marker, (B) patient's blood isolates, (C) SCCmec type II-positive control, (D) SCCmec type III-positive control, (E) SCCmec type IV-A-positive control, (F) negative control.
Discussion
Although Gram-positive bacteria including S. aureus and Streptococcus pyogenes are the most common causes of pyogenic sacroiliitis, there have been no reports of CA-MRSA as a pathogen in this disease in Korea, and few such infections with CA-MRSA have been reported in adult patients worldwide [2-3, 7]. Another Korean study has reported that skin and soft tissue infections account for most cases of CA-MRSA infection [10]. In Korea, case reports of CA-MRSA infection most often describe patients with pneumonia, acute pyelonephritis, pyogenic spondylitis, submandibular abscess, and perianal abscess. This is the first report of an adult patient with pyogenic sacroiliitis caused by CA-MRSA in Korea.
The diagnosis of sacroiliac joint infection is challenging, and may be delayed because of the non-specific clinical signs and symptoms [11, 12]. Delay in diagnosis may lead to various complications, such as abscess, metastatic infection, sepsis, joint disability, and even death [13]. Most patients with pyogenic infection of the sacroiliac joint show various symptoms such as fever, back pain, buttock pain, radiating pain, and limping gait, and therefore such patients may be misdiagnosed with septic arthritis of the hip, lumbar disc herniation, or pelvic abscess. Along with the problems caused by the non-specific symptoms, the anatomy of the sacroiliac joint may also complicate the diagnostic process [2]. Among the several imaging techniques available for the diagnosis of sacroiliac joint infection, bone scan and computed tomography may yield negative results. Therefore, timely rechecking or MRI is essential when clinical manifestations strongly suggest these diseases. Also, MRI has the advantage that it allows characterization of local abscess formation, which is important when making the decision on whether to conduct surgical intervention [4, 14]. In our case, the patient was immunocompetent. She had no risk factors, such as trauma, intravenous drug use, pregnancy, infective endocarditis, or infection at another site. However, we checked her MRI results immediately, since her initial condition was strongly suggestive of sacroiliac joint infection. Early diagnosis led to early surgical debridement, which might have contributed to the achievement of a successful outcome.
Mild skin and soft tissue infections caused by CA-MRSA do not require hospitalization and can be treated on an outpatient basis with oral antibiotics. Surgical intervention, such as incision and drainage, in addition to antibiotic therapy has been shown to aid clinical recovery when indicated in patients with bone and joint infection [15]. In our patient, vancomycin was used as the primary antibiotic therapy; however, surgical drainage of the abscess may have been effective in promoting early recovery in our patient. In this case, vancomycin was combined with rifampin, since the addition of rifampin in patients with MRSA bone and joint infections is recommended by the 2011 Practice Guidelines of the Infectious Diseases Society of America [6]. We added intravenous levofloxacin to the regimen because the efficacy of teicoplanin treatment had not been established in patients with MRSA bone and joint infection. After discharge, we switched the patient from intravenous to oral levofloxacin, due to the high penetration of fluoroquinolones into bone and joints [16]. The patient recovered after 6 weeks of intravenous antibiotic therapy and 2 weeks of oral antibiotic therapy. After pyogenic sacroiliitis treatment, some patients show joint space narrowing, subchondral sclerosis, cysts, erosions, ankylosis, or osteophytes [17]. However, our patient was cured without any post-treatment complications or recurrence.
The CA-MRSA strain cultured from blood and pus in our case was shown to express SCCmec type IV-A and ST72. The strain was negative for the PVL gene. These are the most common biomolecular results found in Korean CA-MRSA isolates, as previous reports have confirmed. The D-test for inducible clindamycin resistance was positive. This inducible clindamycin resistance is also a common finding in Korea [10, 18].
Pyogenic sacroiliitis is a rare disease, and not easily diagnosed. If pyogenic sacroiliitis is strongly suspected, early diagnosis using medical diagnostic imaging techniques (such as MRI) and accurate sampling are important in order to reveal the pathogen. To our knowledge, no other Korean report has been published of CA-MRSA causing musculoskeletal infection of the sacroiliac joint and abscess formation with bacteremia. The authors have therefore presented the diagnostic, therapeutic, and biomolecular analysis of a rare type of CA-MRSA infection, with a review of literature.
References
- 1.Hodgson BF. Pyogenic sacroiliac joint infection. Clin Orthop Relat Res. 1989:146–149. [PubMed] [Google Scholar]
- 2.Doita M, Yoshiya S, Nabeshima Y, Tanase Y, Nishida K, Miyamoto H, Watanabe Y, Kurosaka M. Acute pyogenic sacroiliitis without predisposing conditions. Spine (Phila Pa 1976) 2003;28:E384–E389. doi: 10.1097/01.BRS.0000092481.42709.6F. [DOI] [PubMed] [Google Scholar]
- 3.Taylor ZW, Ryan DD, Ross LA. Increased incidence of sacroiliac joint infection at a children's hospital. J Pediatr Orthop. 2010;30:893–898. doi: 10.1097/BPO.0b013e3181fbebe5. [DOI] [PubMed] [Google Scholar]
- 4.Wu MS, Chang SS, Lee SH, Lee CC. Pyogenic sacroiliitis--a comparison between paediatric and adult patients. Rheumatology (Oxford) 2007;46:1684–1687. doi: 10.1093/rheumatology/kem201. [DOI] [PubMed] [Google Scholar]
- 5.Molinos Quintana A, Morillo Gutiérrez B, Camacho Lovillo MS, Neth O, Obando Santaella I. Pyogenic sacroiliitis in children-a diagnostic challenge. Clin Rheumatol. 2011;30:107–113. doi: 10.1007/s10067-010-1549-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 7.Hermet M, Minichiello E, Flipo RM, Dubost JJ, Allanore Y, Ziza JM, Gaudin P, Thomas T, Dernis E, Glace B, Regnier A, Soubrier M. Infectious sacroiliitis: a retrospective, multicentre study of 39 adults. BMC Infect Dis. 2012;12:305. doi: 10.1186/1471-2334-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JS, Choe PG, Song KH, Cho JH, Kim SH, Bang JH, Lee CS, Park KH, Park KU, Shin S, Choi HJ, Kim ES, Kim DM, Lee MS, Park WB, Kim NJ, Oh MD, Kim EC, Kim HB, Choe KW. Multicenter study for frequency and clinical features of community-associated methicillin-resistant Staphylococcus aureus in Korea. Infect Chemother. 2006;38:325–333. [Google Scholar]
- 11.Schaad UB, McCracken GH, Jr, Nelson JD. Pyogenic arthritis of the sacroiliac joint in pediatric patients. Pediatrics. 1980;66:375–379. [PubMed] [Google Scholar]
- 12.Ford LS, Ellis AM, Allen HW, Campbell DE. Osteomyelitis and pyogenic sacroiliitis: A difficult diagnosis. J Paediatr Child Health. 2004;40:317–319. doi: 10.1111/j.1440-1754.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 13.Attarian DE. Septic sacroiliitis: the overlooked diagnosis. J South Orthop Assoc. 2001;10:57–60. [PubMed] [Google Scholar]
- 14.Klein MA, Winalski CS, Wax MR, Piwnica-Worms DR. MR imaging of septic sacroiliitis. J Comput Assist Tomogr. 1991;15:126–132. doi: 10.1097/00004728-199101000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 16.Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Haanpää M, Hannonen P, Kaira P, Laurikainen J, Möttönen TT, Oka M. Clinical sequelae and sacroiliac joint changes by computed tomography after recovery from septic sacroiliitis. Clin Rheumatol. 1989;8:197–201. doi: 10.1007/BF02030074. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007;60:1108–1114. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]


