Abstract
Background
Necrotizing fasciitis is a life-threatening infectious disease with rapidly progressive involvement of the affected site. Because of the high mortality rate of this disease, early diagnosis, surgical exploration, and administration of appropriate antibiotics are necessary. The present study aimed to further review the changes in the clinical and microbiological characteristics of necrotizing fasciitis using patients' medical records from consecutive databases of 3 hospitals in Korea.
Materials and Methods
In this study, we retrospectively reviewed the medical records of patients with necrotizing fasciitis who were clinically diagnosed between May 2001 and February 2012 in 3 university hospitals in Korea. In total, the data of 83 patients were analyzed, including those of 20 patients from our previous study in 2006. An organism found in a blood culture or surgical specimen was regarded as a causative organism.
Results
Of the 83 patients, 68(81.9%) had community-acquired infections. Ninety microorganism species were indentifed by culture. Streptococcus was the most commonly identified pathogen. Non-fermentative gram-negative bacteria and Candida species have recently emerged, especially in immunocompromised hosts.
Conclusions
Gram-positive organisms are still the most common pathogens of necrotizing fasciitis. However in our study, various gram-negative bacteria with different levels of susceptibility to antibiotics, as well as Candida species, were responsible for the necrotizing fasciitis. Initial empirical antimicrobial agents for necrotizing fasciitis should be considered depending on the individual patient's condition.
Keywords: Necrotizing fasciitis, Skin and soft tissue infection
Introduction
Necrotizing fasciitis (NF) is a relatively rare but rapidly progressive soft tissue infection characterized by widespread fascial necrosis. Because of the high mortality rate of NF (6-76%) [1], early diagnosis, early surgical exploration, and appropriate antibiotics are necessary. Moreover, a local epidemiological investigation of the causative organism and its susceptibility to antibiotics should be performed. In 2006, we reported the clinical characteristics and causative organism of NF cases from 3 university hospitals in Korea [2].
The present study aimed to further review the changing clinical and microbiological characteristics of NF using consecutive database records of patients from 3 hospitals. In addition, we performed an analysis to determine the contributing factors to NF-related mortality.
Materials and Methods
We retrospectively reviewed the medical records of patients with NF who were clinically diagnosed between May 2001 and February 2012 in 3 university hospitals, which located in Seoul, Bucheon, and Cheonan. Patients who were readmitted for secondary treatment such as skin grafting were excluded.
In our previous study, we enrolled 22 patients with NF diagnosed between May 2001 and May 2005. In addition, we reviewed the medical records of 30 and 38 patients with NF diagnosed between June 2005 and April 2009, and between May 2009 and February 2012, respectively. Of the 90 patients, 7 were not subjected to analysis because they either discharged against medical recommendation or transferred to another hospital.
Therefore, the records of a total of 83 patients were analyzed. Age, sex, comorbidities, affected sites, clinical signs including bulla, microbiological findings in sterile specimens, antibiotics susceptibility, antibiotics used, type of surgery, clinical outcome, and cause of death were reviewed for each patient.
Hospital-acquired infection was defined according to the definition of the Centers for Disease Control and Prevention (CDC) [3], as the onset of signs or symptoms 48 h after hospital admission. The organism isolated from blood cultures or surgical specimens was regarded as the causative organism. If blood culture result was not available, aspiration culture results of a skin bulla specimen were used. Open pus culture results were not considered for microbiological analysis.
The time of performing initial curative surgical procedures such as incision and drainage, fasciotomy, or amputation was regarded as the starting time of operation.
Data are presented as mean [SD] for normally distributed variables and as median (interquartile range) or number (%) of subjects for categorical variables. Comparisons of the baseline and disease characteristics between the patients who died and those who survived were performed using the Pearson Chi-square test, Fisher exact test, or Student t-test according to the type of variable. To determine the risk factors for outcome, we performed a multiple logistic regression analysis. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). A 2-sided P-value of < 0.05 was considered statistically significant.
Results
Of the 83 patients included in this study, 59 (71%) were men, with a mean age of 58 years. There were 68 (81.9%) patients with community-acquired infection. The most common comorbidity among the patients was diabetes mellitus (n = 38; 45.8%), followed by chronic liver disease (n = 22; 26.5%), and chronic kidney disease (n = 22; 26.5%). The most frequently affected site was the lower extremities (n = 42; 50.6%). Thirty-one patients presented with bulla at the time of diagnosis. In the records of 5 patients, there was no information regarding the occurrence of bulla. Septic shock, severe sepsis, and sepsis occurred in 23 (27.7%), 12 (14.5%), and 12 (14.5%) patients, respectively (Table 1).
Table 1.
Comparison of the baseline characteristics of patients with necrotizing fasciitis who survived and those who died
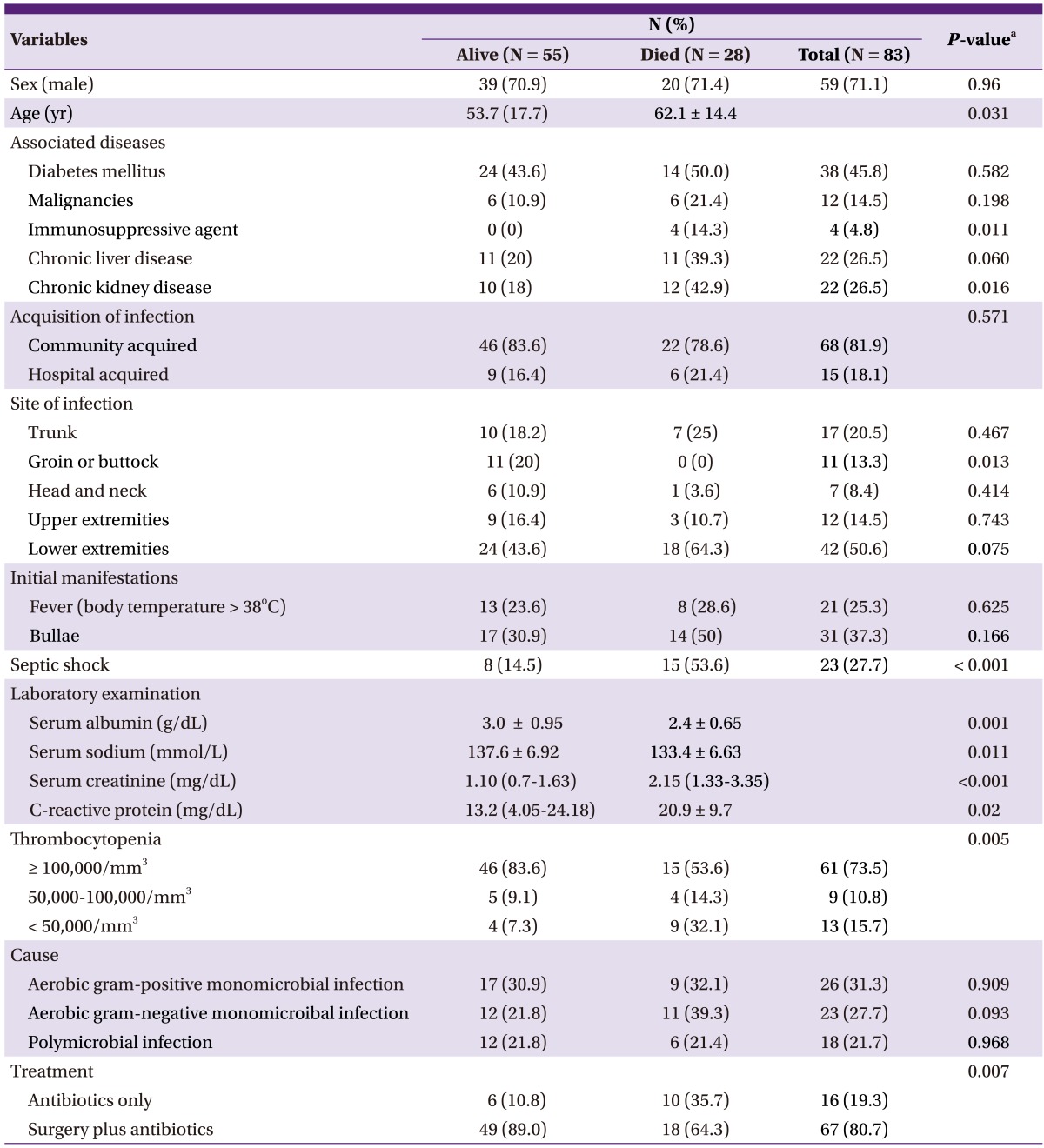
The data are expressed as number of patients (%) or mean [SD], unless otherwise indicated.
aThe P-values were calculated using the Pearson Chi-square test, Fisher exact test, or Student t-test, as appropriate.
Radiological imaging (magnetic resonance imaging, computed tomography, or ultrasonography) was performed in 66.2% (55/83) of the patients. Of these patients, 52.7% (29/55) had NF-positive results, of which 34.9% (29/83) were confirmed by positive imaging study results.
Sixty-eight patients (81.9%) had a microbiologically defined infection. Monomicrobial infection was confirmed in 50 patients (73.5%), and polymicrobial infection was confirmed in 18 patients (26.5%). Twenty-three organisms were isolated from blood cultures, and 59 from surgical specimens. Gram-positive bacteria (GPB) were found in 45 patients (54.2%), and gram-negative bacteria (GNB) in 40 patients (48.2%). Escherichia coli was the most frequently identified pathogen (n = 13, 14.4%; extended-spectrum beta-lactamase [ESBL]-producing microbes: 30.8%), followed by Staphylococcus aureus (n = 12, 13.3%; methicillin-resistant Staphylococcus aureus [MRSA]: 41.6%), and Streptococcus pyogenes (n = 12, 12%; penicillin susceptibility: 100%; Table 1). None of the Streptococcus pyogenes infection cases was hospital acquired. However, 2 patients with Streptococcus pyogenes infection had a history of intramuscular injection or acupuncture, which fits the definition of health-care-associated infection. The GPB-to-GNB ratio in the present study was similar to that in our previous study (Table 2).
Table 2.
Comparison of the causative organisms of necrotizing fasciitis
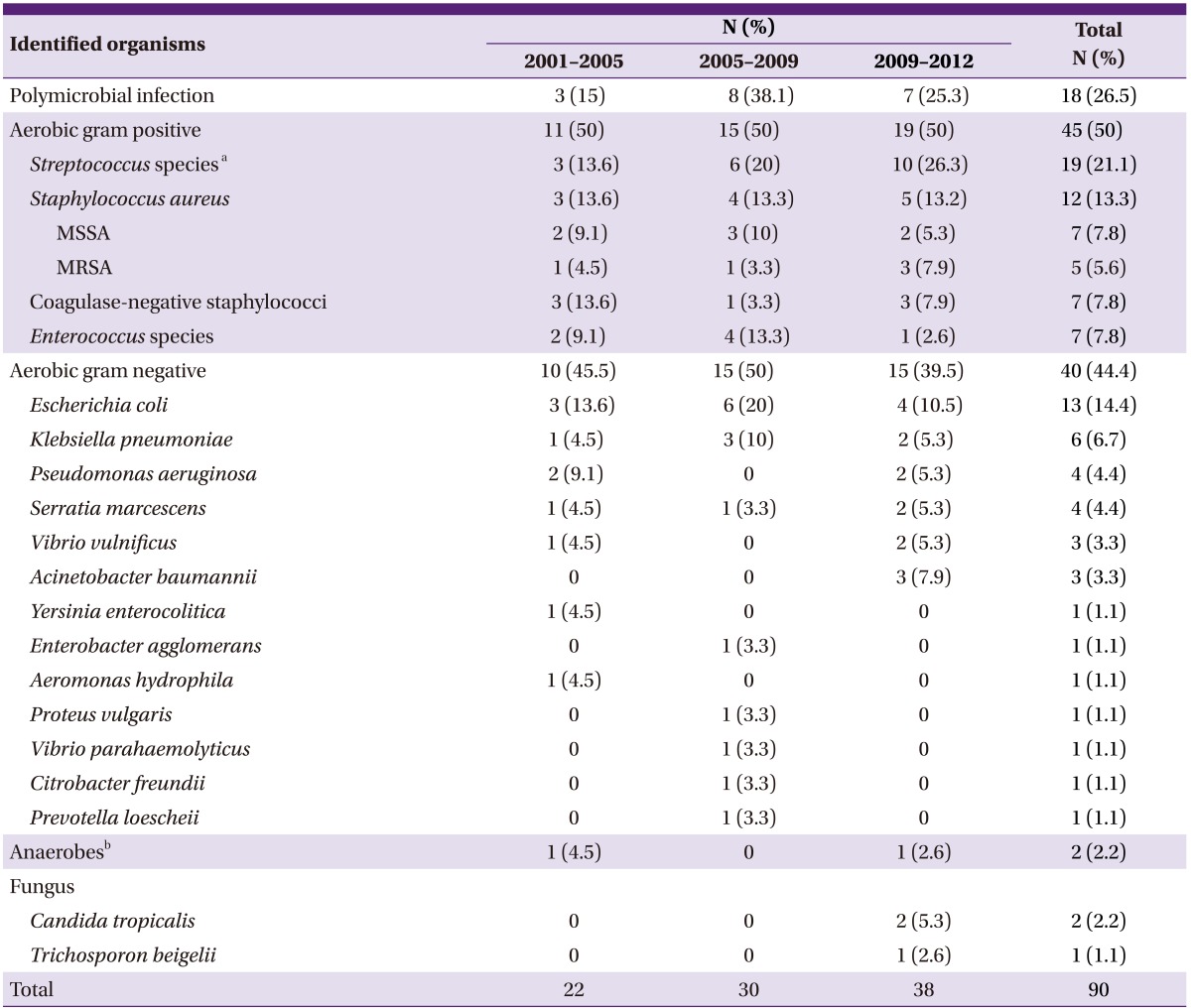
MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
aStreptococcus pyogenes, Streptococcus agalactiae, viridans group streptococci, Streptococcus milleri group, and group F streptococci.
bClostridium subterminale and anaerobic gram-positive bacilli: not included in the gram-positive group.
Of the 4 cases of ESBL-producing Escherichia coli, 2 were hospital acquired, 1 was health-care associated, and 1 was community acquired. However, the patient with community-acquired infection had a history of intramuscular injection. Of the 5 cases of MRSA infection, 2 were hospital acquired, 2 were health-care associated, and 1 was of an unknown cause.
Of the 2 cases of infection with Candida species, 1 was health-care associated and occurred in a patient with a pressure sore due to paraplegia; the patient visited the hospital because the sore worsened despite self-medication. MRSA was confirmed by surgical specimen analysis, and the presence of Candida tropicalis and Streptococcus sanguis was confirmed by blood culture. The patient was treated with antibiotics, including amphotericin B for candidemia. The other 2 patients with confirmed Candida species infection had a history of liver transplantation and immunosuppressive agent use. Unfortunately, acute transplant rejection occurred, leading to general edema that caused the NF on the patient's trunk and leg. Candida tropicalis was isolated from the surgical specimen. The patient was treated with empirical antibiotics, including amphotericin B but eventually died.
A total of 67 patients underwent surgery, and amputation was performed in 5. Two amputation procedures were performed initially, and the other 3 were performed after simple fasciotomy or debridement. Sixteen patients did not undergo surgery because of rapid deterioration leading to death in 10 of the patients and a relatively mild disease progression in the other 6 patients. Seven of the 10 patients who died without surgery developed septic shock, and the other 3 had severe sepsis. The patients who did not undergo surgery had a significantly higher mortality rate than those who underwent surgery (62.5% vs. 26.8%; P = 0.007).
Of the 83 patients, 55 survived and 28 died (in-hospital mortality: 33.7%) due to NF (n = 23; 27.7%), pneumonia during hospitalization (n = 3; 3.6%), and progression of malignancy (n = 2; 2.4%).
Age, chronic kidney disease, septic shock, hyponatremia, thrombocytopenia (< 50,000/mm3), hypoalbuminemia, high C-reactive protein level, and surgery were significantly associated with mortality (Table 3).
Table 3.
Univariate analysis of risk factors of death in necrotizing fasciitis
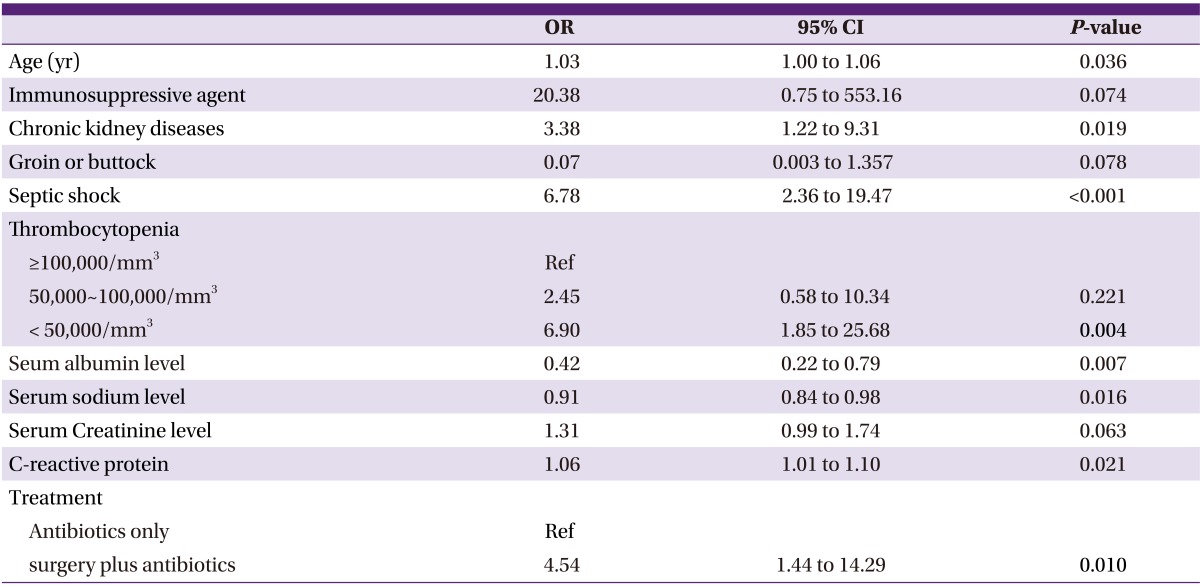
OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
According to previous studies, the cumulative mortality rate of NF is 24% (6-76%) [4]. In medical practice, the clinician's judgment is an important element in diagnosis [5]. However, NF is not easy to diagnose because of its ambiguous clinical manifestation.
In 1977, Gialinano et al. [6] classified NF into 2 subtypes: type I, a polymicrobial infection (usually caused by a combination of gram-positive cocci, gram-negative rods, and anaerobes), and type II, a monomicrobial infection (caused by Streptococcus and/or Staphylococcus aureus). A polymicrobial infection (type I) is often diagnosed in immunocompromised patients and usually occurs in the perineum and trunk area. In contrast, a monomicrobial infection (type II) usually affects otherwise healthy, young, immunocompetent patients and is typically limited to the extremities. A recent clinical classification distinguished 4 NF types: types I (70-80%, polymicrobial/synergistic), II (20% of cases; usually monomicrobial), III (gram-negative monomicrobial, including marine-related organisms), and IV (fungal) [7]. In our study, monomicrobial infections were more common. Of the 18 patients with confirmed polymicrobial infection, 4 acquired the infection during hospitalization and 8 had diabetes. Furthermore, 2 patients had a malignancy, and 6 had a chronic liver disease. In a recent multi-center study of community-acquired NF in Korea, monomicrobial infections were more common than polymicrobial infections [8].
Streptococcus species, especially Streptococcus pyogenes, are still common pathogens of NF. Moreover, E.coli is the most common pathogen among GNB. This was also reported in the above-mentioned multicenter study [8]. In our study, despite that GPB-to-GNB ratio was almost similar during whole study period, the causative organisms became more diverse and included fungus and non-fermentative GNB like Acinetobacter baumannii, as well as highly resistant organisms such as ESBL-producing E.coli and MRSA recently. Infections caused by community-associated MRSA were recently reported to be a health issue in North America [9]. However, such outbreaks have not been encountered in Korea yet.
The recommendation of the Infectious Diseases Society of America (IDSA) for early surgical treatment was based on the fact that the limited role of antibiotic treatment alone by the thrombogenic nature of microvessels [10-12]. Scheduled debridements at intervals of 6-48 hours should be performed until no further necrosis or infected tissue is seen. Most experts suggest continuation of antibiotic therapy until no additional surgical debridement is needed and the patient is no longer manifesting signs of systemic inflammation [11].
The 2011 IDSA guidelines recommend penicillin and clindamycin for NF caused by group A streptococci. In addition, treatment of polymicrobial infections should include antimicrobial agents effective against aerobes and anaerobes [5].
In our previous report of the clinical characteristics of NF, we indicated an increase in the number of cases of NF caused by third-generation cephalosporin resistant GNB; therefore, the use of antibiotics against Pseudomonas aeruginosa is recommended [2]. In the present study, the incidence rates of third-generation cephalosporin resistant GNB and ciprofloxacin-resistant GNB were 17.5% (7 cases) and 12.5% (5 cases). Therefore, on the basis of these results, the issue of GNB resistance to third-generation cephalosporin should be considered when dealing with NF caused by GNB. As noted above, the causative organisms of NF are becoming more diverse; therefore, we should consider each patient's individual condition before administering antibiotics.
In summary, Streptococcus species are still the most common causative pathogen of NF. However, diverse organisms, including GNB and Candida species, have been reported to cause NF as well. Owing to the complexity of the causative organisms, Candida species or highly resistant bacteria such as Acinetobacter baumannii have recently emerged as causative agents especially in immunocompromised hosts. Judicious consideration of the most appropriate antimicrobial therapy is necessary in such cases.
Table 4.
Multivariate analysis of risk factors of death in necrotizing fasciitis
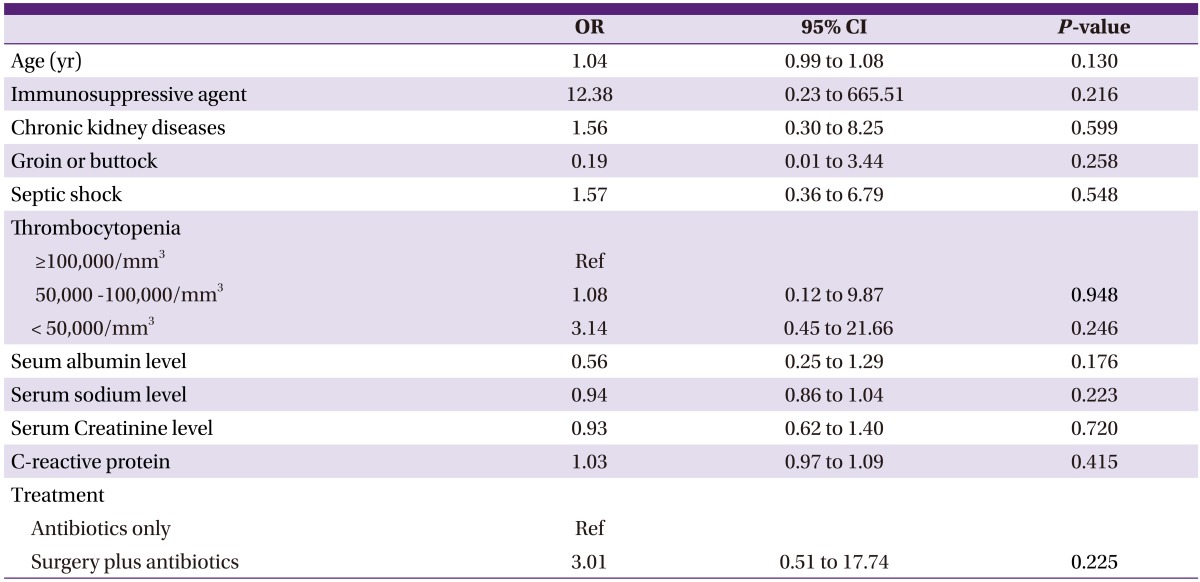
OR, odds ratio; 95% CI, 95% confidence interval.
References
- 1.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–563. doi: 10.1097/00000658-199505000-00013. discussion 563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MW, Kim TH, Choo EJ, Kang JH, Kim DW, Kim DK, Park SW, An JH, Yoon HG, Eo SJ, Lee GW, Lee YH, Lee JY, Cheon KI. Characteristics of necrotizing fasciitis in three university hospitals in Korea. Korean J Med. 2006;70:681–687. [Google Scholar]
- 3.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 4.Miller SA, Forrest JL. Translating evidence-based decision making into practice: appraising and applying the evidence. J Evid Based Dent Pract. 2009;9:164–182. doi: 10.1016/j.jebdp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 6.Guturu P, Shah V, Urrutia R. Interplay of tumor microenvironment cell types with parenchymal cells in pancreatic cancer development and therapeutic implications. J Gastrointest Cancer. 2009;40:1–9. doi: 10.1007/s12029-009-9071-1. [DOI] [PubMed] [Google Scholar]
- 7.Lenarz T, Lim H, Joseph G, Reuter G, Lenarz M. Central auditory prosthesis. HNO. 2009;57:551–562. doi: 10.1007/s00106-009-1944-x. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Choi SH, Kwak YG, Chung JW, Choo EJ, Kim KH, Yun NR, Lee S, Kwon KT, Cho JH, Kim NJ. Clinical characteristics and causative organisms of community-acquired necrotizing fasciitis. Infect Chemother. 2012;44:180–184. [Google Scholar]
- 9.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 10.Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15:467–473. doi: 10.1097/MCC.0b013e328332f6e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin D. Forensic age estimation in human skeletal remains: current concepts and future directions. Leg Med (Tokyo) 2010;12:1–7. doi: 10.1016/j.legalmed.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Tian B, Kempa TJ, Lieber CM. Coaxial group IIInitride nanowire photovoltaics. Nano Lett. 2009;9:2183–2187. doi: 10.1021/nl900858v. [DOI] [PubMed] [Google Scholar]


