Abstract
Obesity markedly increases the risk of hypertension and cardiovascular disease, which may be related to activation of the sympathetic nervous system (SNS). Sympathetic overactivity directly and indirectly contributes to blood pressure (BP) elevation in obesity, including stimulation of the renin–angiotensin–aldosterone system (RAAS). The adipocyte-derived peptide leptin suppresses appetite, increases thermogenesis, but also raises SNS activity and BP. Obese individuals exhibit hyperleptinemia but are resistant to its appetite-suppressing actions. Interestingly, animal models of obesity exhibit preserved sympathoexcitatory and pressor actions of leptin, despite resistance to its anorexic and metabolic actions, suggesting selective leptin resistance. Disturbance of intracellular signaling at specific hypothalamic neural networks appears to underlie selective leptin resistance. Delineation of these pathways should lead to novel approaches to treatment. In the meantime, treatment of obesity–hypertension has relied on antihypertensive drugs. Although sympathetic blockade is mechanistically attractive in obesity–hypertension, in practice its effects are disappointing because of adverse metabolic effects and inferior outcomes. On the basis of subgroup analyses of obese patients in large randomized clinical trials, drugs such as diuretics and RAAS blockers appear superior in preventing cardiovascular events in obesity–hypertension. An underused alternative approach to obesity–hypertension is induction of weight loss, which reduces circulating leptin and insulin, partially reverses resistance to these hormones, decreases sympathetic activation and improves BP and other risk factors. Though weight loss induced by lifestyle is often modest and transient, carefully selected pharmacological weight loss therapies can produce substantial and sustained antihypertensive effects additive to lifestyle interventions.
Keywords: mechanisms, obesity, sympathetic, therapy
The incidence of obesity has increased dramatically, particularly in developed countries, such as the United States and Japan.1,2 Obesity accelerates atherosclerosis and increases coronary and stroke morbidity and mortality3 Most of these are mediated by cardiovascular risk factors, especially systolic hypertension.4 The prevalence of hypertension in middle-aged obese humans (body mass index >30 kgm−2) is 40–50%.5 There are substantial data suggesting that activation of the sympathetic nervous system (SNS) has a key role in obesity–hypertension. We review evidence linking obesity and the SNS, pathways by which the SNS elevates blood pressure (BP), mechanisms for SNS activation in obesity and therapeutic implications.
OBESITY AND SYMPATHETIC ACTIVATION
In animal models, obese dogs fed a high-fat diet develop sodium retention and hypertension. Renal denervation prevents both, indicating that renal nerves have an critical role in obesity-related hypertension.6 There are also data that sympathoactivation precedes development of obesity in animal models prone to adiposity. BP, renal tissue norepinephrine content and renin expression are increased in the obesity-prone offspring of obese rats.7 In human obesity, increased circulating catecholamines have been demonstrated.8 Moreover, there is an augmented SNS contribution for maintenance of arterial pressure in obese compared with lean normotensive and hypertensive subjects.9,10
However, these data do not permit elucidation of region-specific sympathetic tone, nor the mechanisms by which BP is elevated by the SNS. Increased sympathetic neural drive in obese humans has been demonstrated in skeletal muscle (microneurography) and kidney (norepinephrine spillover),11,12 suggesting that vasoconstriction and renal mechanisms are both involved. But there is also evidence that sympathetic activation in obesity is not generalized.12 There is also evidence that regionally increased sympathetic nerve activity (SNA) does not always translate into direct vascular effects.
Peripheral sympathetic vascular tone in obesity
Many studies have demonstrated increased SNA to skeletal muscle (mSNA) in human obesity.11,13 However, the function of this elevated mSNA is unknown, though it is widely assumed that it contributes to vasoconstriction and thus obesity-related hypertension. We have tested the hypothesis that sympathetically mediated vascular tone is increased in humans with obesity. In agreement with prior studies, we showed that mSNA in upper and lower limbs was significantly higher (~40%) in obese compared with lean normotensive subjects.14 Interestingly, obese and lean subjects had similar vasoconstrictor responses to α-adrenoceptor agonism. Importantly, despite increased mSNA, vasodilatation of forearm resistance vessels to complete α-adrenergic blockade with intra-arterial phentolamine was completely normal in obese normotensive subjects.14 We subsequently demonstrated that forearm vasodilator responses to phentolamine were not increased in obese hypertensives compared with lean subjects. In fact, such responses were somewhat lower in obese hypertensive subjects than in lean hypertensives.15 In addition, weight loss did not reduce vasodilator responses to phentolamine in obese hypertensive subjects, despite reducing mSNA and arterial pressure. This is opposite of what would be expected if obesity-related sympathoactivation directly increases limb vascular tone through activation of α-adrenergic receptors on resistance-artery vascular smooth muscle cells.16,17
How can this perplexing and apparently contradictory finding of dissociation between vascular tone and sympathetic outflow be explained? First, it is possible that endothelial dysfunction could lead to increased vascular tone that could augment an initially decreased phentolamine-induced vasodilatation; however, dilatation to isoproterenol (a selective β2-adrenoreceptor agonist) and nitroprusside (nitric oxide donor) was not reduced in obese subjects.14 Second, sustained elevation of mSNA in obesity could lead to downregulation of α-adrenergic receptors and reduction of sympathetic vascular tone; however, there is preserved α-adrenergic vasoconstrictor sensitivity in obese subjects.14 Third, the modest changes in SNA observed in obesity (~40%) may not be sufficient to increase vascular tone and may subserve other functions (that is, metabolic). The last explanation has some corroborating evidence in studies of single-fiber versus multiple-fiber sympathetic nerve discharge patterns in obesity. Although obese normotensive and hypertensive subjects both exhibit increased multifiber mSNA, obese subjects do not show increased firing activity of single SNA fibers compared with lean controls.13 This might suggest that additional sympathetic neurons are ‘recruited’ in obesity, but that they do not subserve vasoconstriction. Clearly, increased multifiber sympathetic discharge rate could be derived from sympathetic neurons innervating muscle or fat, rather than blood vessels. These may mediate metabolic functions, such as lipid oxidation18 or thermogenesis. Thus, our work and that of others suggest that inferences regarding vascular tone drawn from multifiber recordings of mSNA may be misleading in obesity, and that increased SNA does not necessarily translate into increased vascular tone in obesity. Importantly, because data from systemic SNS blockade indicate an increased sympathetic contribution to BP and vascular resistance in obesity,9,10 other pressor actions of SNA must be having a role. Such actions would presumably be able to increase BP without increases in vascular smooth muscle contraction via binding of norepinephrine to α-adrenoreceptors.
Links between the sympathetic and renin–angiotensin–aldosterone systems
It is tempting to implicate the actions of the SNS on sodium balance, as the resistance-vessel-independent mechanism contributes to obesity-related hypertension. However, acute sympathetic blockade causes an exaggerated decrease in BP and systemic vascular resistance in obesity–hypertension before changes in sodium balance can occur.9 An intriguing possibility is that increased SNA activates the renin–angiotensin–aldosterone system (RAAS). Classically, the SNS rapidly activates the RAAS through β-adrenoceptor-mediated release of renin from the renal juxtaglomerular apparatus. Activation of the RAAS by the SNA may also occur slowly through upregulation of adipocyte angiotensinogen (AGT) generation (Figure 1). For instance, dietary carbohydrate activates white adipose tissue SNA, and significantly increases adipose AGT gene expression, which is abolished by sym-pathectomy.19 Biological responses to β-adrenergic stimulation in adipose cells are mediated by intracellular cyclic AMP, which stimulates AGTmRNA levels and protein.20 Obese subjects have evidence of circulating and tissue RAAS activation, and weight loss attenuates this.21–27 Thus, although the direct vascular effects of sympathetic activation in obesity may have been overestimated, indirect effects on renal sodium retention, increased cardiac output and activation of the RAAS may have been underestimated (Figure 1). This would explain the apparently contradictory finding of dissociation between vascular tone and sympathetic outflow, and have implications for the treatment of obesity-related hypertension.
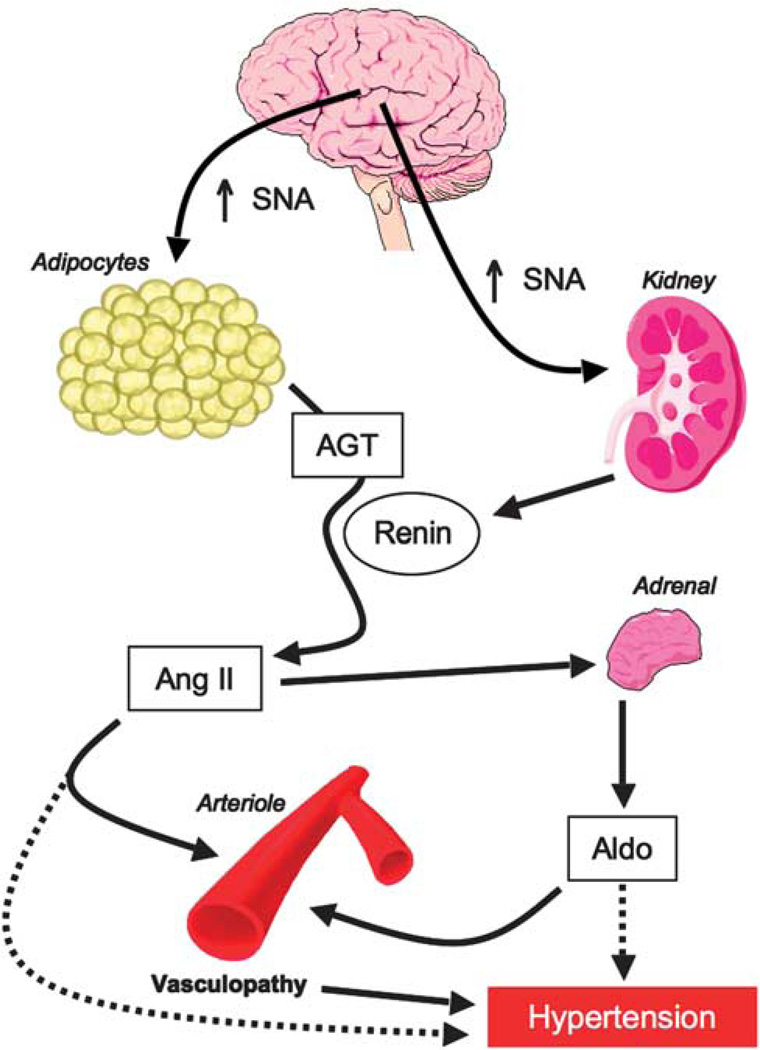
Figure 1.
Possible pathways for activation of the renin–angiotensin– aldosterone system in obesity. Obesity-related increases in sympathetic nerve activity can acutely release activated renin from the kidney, and more chronically though stimulation of adipose-tissue angiotensinogen generation. Angiotensin II (Ang II) then stimulates aldosterone (Aldo) production from the adrenal cortex. Both Ang II and Aldo can then have deleterious effects on blood vessels and blood pressure.
MECHANISMS BY WHICH OBESITY INCREASES SNA
There are multiple neurohumoral mechanisms by which obesity can activate the SNS. Neural mechanisms include direct activation of the SNA in response to activation of higher cerebral centers by hunger or feeding, and renal afferent nerve activation mediated by perirenal fat accumulation and kidney compression. Among humoral mediators, the preponderance of evidence suggests a particularly important role for leptin in the development of obesity-induced hypertension (see below). This review focuses on leptin, but we briefly discuss some other mediators first, including insulin, glucocorticoids, endocannabinoids, ghrelin and adiponectin.
Obesity and hypertension are both associated with increasing insulin resistance and hyperinsulinemia. The effects of insulin on BP control are multifactorial, including a direct antinatriuretic action and sympathetic activation. In a series of landmark papers, Mark and colleagues at our institution demonstrated that hyperinsulinemia causes sympathetic activation in humans.28 Though hypertensive or elderly subjects exhibit resistance to the glucose uptake (and vasodilator) effects of insulin29,30, they have preserved insulin-mediated sympathoactivation (that is, selective insulin resistance). One could therefore hypothesize that as obesity develops, hyperinsulinemia could contribute to sympathetic activation. We have also shown in rodents that central nervous system administration of insulin increases sympathetic nerve outflow to the hind limb, brown adipose tissue, kidney and adrenal gland.31 Importantly, regulation of sympathetic outflow to brown adipose tissue is mediated by neuronal intracellular activation of mitogen-activated protein kinase, whereas outflow to the hind limb is mediated by phosphoinositol kinase activation. Thus, it could be possible in the future to develop therapeutic interventions to prevent insulin-induced sympathetic activation.
Glucocorticoids are known to increase food intake, fat accumulation, insulin resistance and decrease energy expenditure.32 Glucocorticoids also cause hypertension via multiple mechanisms: activating the mineralocorticoid receptor in the kidney and perhaps via upregulation of the angiotensin receptors and alteration of sodium and calcium influx into cells or action on vascular smooth muscle.33,34 Importantly, suppression of corticotrophin releasing factor using prolonged dexamethasone administration inhibits the SNS in obese, although not lean, subjects, suggesting a role for hypophyseal corticotrophin releasing factor in the sympathoexcitation of obesity.35 However, such increased corticotrophin releasing factor could be secondary to changes in leptin levels or resistance.
Endocannabinoids are fatty-acid derivatives that activate cannabinoid receptors and alter food intake and metabolism.36 Endocannabinoid administration causes appetite initiation in mice.37 Obese human subjects have upregulation of the endocannabinoid system.38 The endocannabinergic system has a role in human hypertension, and cannabinoids and their analogs have hypotensive and depressor effects, acting both directly and indirectly on myocardium and vasculature.39 Spontaneously hypertensive rats exhibit reduced cannabinoid-1 receptor density, which appears to contribute to their elevated sympathetic tone.40 Rimonabant, a selective cannabinoid type 1 receptor blocker, was shown to modestly decrease BP. After 1 year of rimonabant treatment, there was a 1.1mmHg placebo-adjusted decrease in systolic BP.41 However, such decreases would be expected from any drug causing weight loss. Defective leptin signaling is associated with increased hypothalamic levels of endocannabinoids in obese mice and Zucker rats.42 When leptin was administered to normal and obese rats, it reduced endocannabinoids in the hypothalamus, indicating the endocannabinoid actions may be regulated by leptin.42 Thus, as with CRF, changes in endocannabinoids may be related to changes in leptin levels or sensitivity caused by obesity.
Ghrelin, a 28-amino-acid growth-hormone-releasing peptide secreted by the stomach, causes weight gain in rodents by increasing food intake.43 However, circulating ghrelin levels are decreased in obese humans, arguing against a central role for ghrelin in causation of common obesity.44,45 Importantly, ghrelin infusion decreases BP by 5–10mmHg,46 although it also increases SNS activity, perhaps through compensatory baroreflex activation.46 Untreated hypertensive subjects exhibit decreased circulating ghrelin concentrations.47 A polymorphism in the ghrelin gene is associated with increased risk of hypertension, although no ghrelin halotypes are associated with BP or body mass index.48 Thus, it seems plausible that the low ghrelin concentrations observed in common obesity could contribute to obesity-related hypertension. Interestingly, plasma ghrelin levels are elevated in subjects with Prader–Willi Syndrome, and could be a causative factor in the development of obesity.49 About 40% of adults with Prader–Willi syndrome develop hypertension, suggesting that elevated ghrelin levels are not sufficient to prevent obesity-related increases in BP.50
Adipocytes produce multiple peptides that may have a role in increased SNS activity. The most important are adiponectin and leptin. Others include visfatin and cytokines, such as tumor necrosis factor-α, but these have less robust evidence linking to hypertension. Adiponectin is a 247-amino-acid protein and a major cytokine abundantly synthesized by adipose tissue that regulates lipid and glucose metabolism. High levels of adiponectin improve insulin resistance, dyslipidemia and atherosclerosis. Adiponectin reduces efferent sympathetic nerve traffic to the kidney and BP in rodents.51 Higher circulating adiponectin levels are associated with lower BP in humans.52–54 Metabolic syndrome and hypertension are associated with low adiponectin levels.55,56 Importantly, subjects with low adiponectin levels exhibit cardiac sympathetic overactivity.57 Adiponectin gene polymorphisms are associated with the combination of metabolic syndrome and hypertension in Taiwanese subjects. Such association was not seen in subjects with hypertension alone or normotensive subjects with metabolic syndrome.58 Most recently, adiponectin has been shown to be an independent predictor of coronary heart disease in the Framingham offspring study.59,60
Leptin, a 167-amino-acid hormone, predominantly secreted by adipose tissue, inhibits feeding behavior and increases sympathetically mediated thermogenesis. Thus, leptin functions as an adipostat hormone that should maintain adipose tissue mass stability through a negative feedback mechanism (Figure 2). Leptin has multiple actions, including direct effects on the endocrine, vascular, hematopoietic, immune and musculoskeletal systems in addition to anorexia and thermogenesis. Leptin-dependent increases in SNA to brown adipose tissue promote thermogenic metabolism and contribute to leptin-induced weight loss.61 Leptin has also been shown to increase SNA to the kidneys, adrenal glands and hind limbs in rats.62 We and others have shown that chronic increases in systemic or central nervous system leptin concentrations increase BP in rodents, which is preventable by adrenergic receptor blockade.63–66 Leptin appears to have the most robust evidence linking it to the development of obesity-related hypertension, including rodent, epidemiological and genetic data. For example, the extremely rare cases of genetic leptin deficiency are not only associated with early severe obesity, but also with normal BP and orthostatic hypotension.67,68 Moreover, a leptin gene polymorphism has been linked to increased risk for developing hypertension independent of obesity.69 In addition, rodents with selective knockout of leptin receptors in hypothalamic proopiomelanocortin neurons exhibit obesity and insulin resistance but without the usual hypertension.70 Furthermore, epidemiological data suggest a clear link between leptin and BP, which is clearly independent of obesity.71 This association appears to be more apparent in children, which could suggest an initiating role for leptin.72 Importantly, 50–60% of the association between body mass index and BP pressure variance can be explained by variance in leptin levels.72,73 Together, these data suggest that leptin is a necessary intermediary in the development of SNS activation and hypertension, although other mediators could still contribute to lesser degrees.
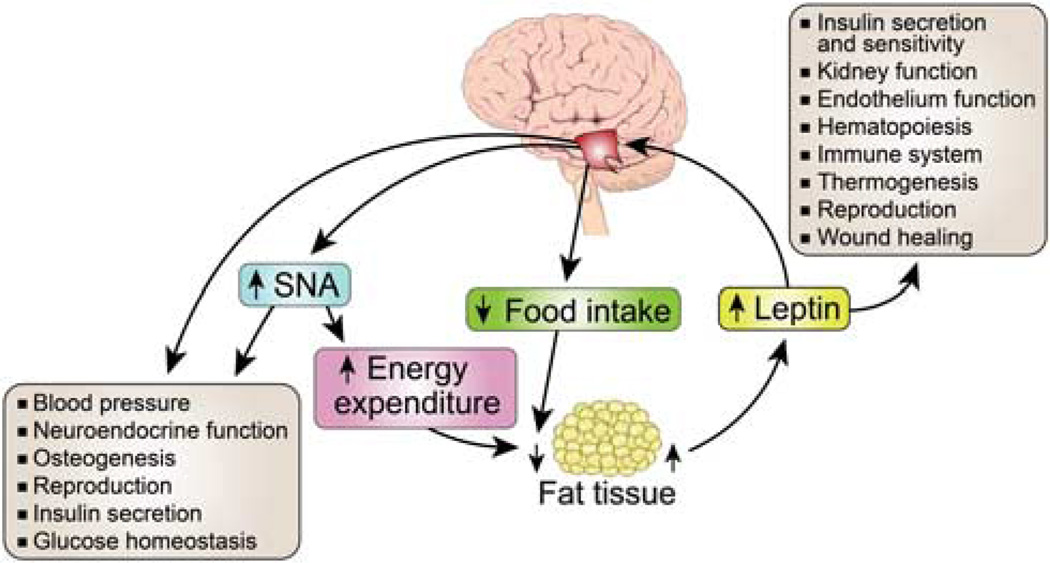
Figure 2.
Biological roles of leptin. Leptin is secreted by adipocytes and circulates in the blood in a concentration proportional to fat mass content. In addition to regulation of appetite, thermogenesis and body weight, leptin has multiple other biological actions. The binding of leptin to its receptor in the hypothalamus inhibits food intake and increases energy expenditure through stimulation of sympathetic nerve activity(SNA). Leptin has multiple other functions, either directly through action in peripheral tissues or through activation of thermogenic and cardiorenal SNA.
LEPTIN SIGNALING
Leptin actions occur mainly through activation of central nervous system pathways starting in the hypothalamus. The binding of leptin to its receptor (Lrb) leads to conformational changes, and promotes intracellular activation of Janus kinase 2, which phosphorylates the Src homology-2 domains of the signal transducer and activator of transcription protein type 3 (STAT3). Phosphorylated STAT3 dimerizes, enters the nucleus, binds to promoter regions and regulates gene expression. In addition to triggering the Janus kinase–STAT3 pathway, binding of leptin to LRb also activates phosphatidylinositol-3-kinase,74 mitogen-activated protein kinase75 and the ATP-sensitive K-channel (Figure 3). Activation of each of these pathways contributes to the anorexic effects of leptin.74–76
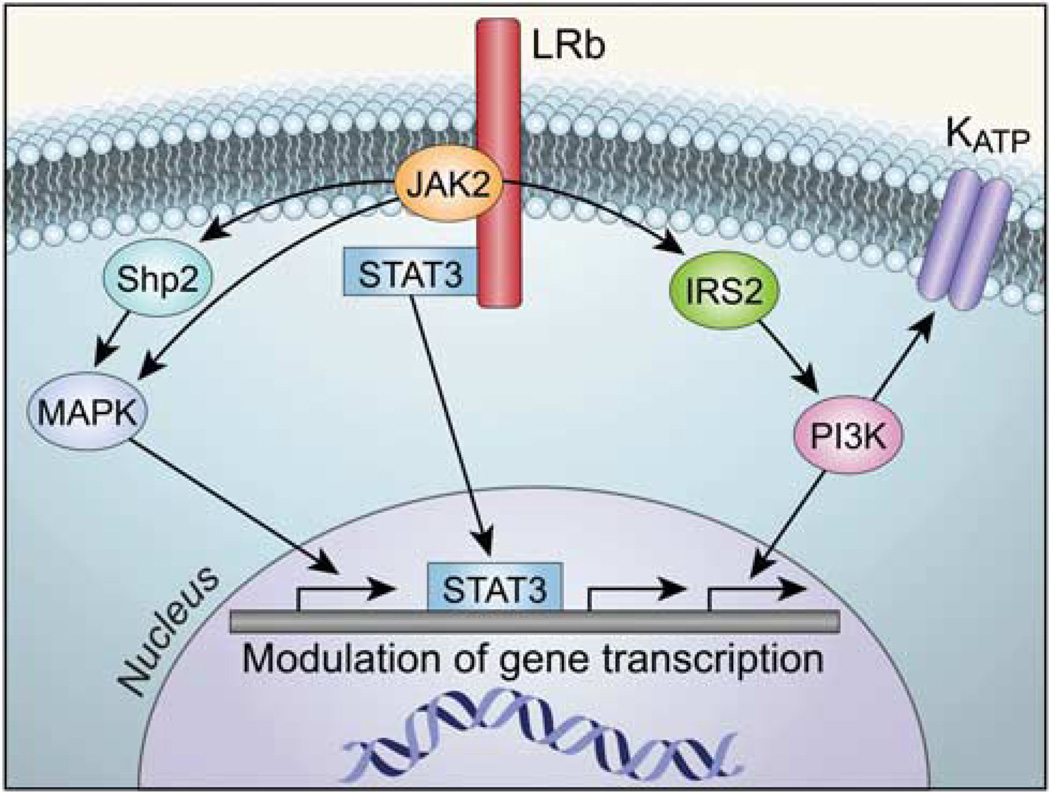
Figure 3.
Molecular mechanisms involved in leptin receptor signaling in the hypothalamus. Leptin modulates gene transcription via the activation of signal transducer and activator of transcription 3 (STAT3) proteins, phosphoinositol 3 kinase (PI3K) and mitogen-activated protein kinase (MAPK). The PI3K pathway is also involved in modulation of neuronal firing rate via activation of membrane potassium–ATP channels (KATP). IRS2, insulin receptor substrate type 2; JAK2, Janus kinase 2; LRb, long splice variant leptin receptor isoform; Shp2, sulfhydryl-domain containing protein tyrosine phosphatase.
In the hypothalamus, leptin promotes the expression of proopiomelanocortin and thus α-melanocyte-stimulating hormone, and suppresses the expression of agouti-related protein.77 In peripheral tissues, leptin activates transcription of genes encoding for enzymes that participate in lipid metabolism, such as carnitine palmitoyltransferase 1, acetyl coenzyme A carboxylase, acyl-coenzyme A oxidase and fatty acid synthase.78
SELECTIVE LEPTIN RESISTANCE
Obese individuals exhibit hyperleptinemia due to an increase in adipocyte mass, but remain obese, indicating leptin resistance. Indeed, both human studies and experimental murine models of polygenic obesity have demonstrated resistance to the anorexic and weight-lowering effects of leptin. If leptin contributes to hypertension, then at least some of its actions on the SNS would have to be preserved in the face of such resistance (selective leptin resistance). There is increasing evidence that this indeed occurs. For example, monogenic obese Agouti mice (Ay) exhibit resistance to the metabolic effects of leptin (that is, less satiety and less weight loss), but have relative preservation of leptin-induced renal sympathoactivation.79,80 Thus, leptin resistance in the Ay mouse is selective and confined to certain metabolic actions of leptin, whereas the hormone’s sympathetic actions remain unaffected. Importantly, selective leptin resistance is not restricted to Ay mice with monogenic obesity.
In our laboratory, C57BL/6J mice with diet-induced obesity responded poorly to the satiety and lipopenic effects of chronic leptin administration.75 Similar to previous results with Ay mice, the renal sympathetic response to either systemic or central neural administration of leptin was similar in polygenic obese and control mice, despite metabolic leptin resistance in the obese. Interestingly, leptin-induced SNA to brown adipose tissue and skeletal muscle was also blunted in obese mice. The preservation of leptin-induced sympathetic activation to the kidneys might predispose obese animals to hypertension, whereas the inhibition of leptin-dependent sympathoactivation to the brown adipose tissue and skeletal muscle could theoretically impair thermogenic metabolism and aggravate obesity (Figure 4). Importantly, the pressor effect of leptin was preserved in diet-induced obese mice, despite metabolic leptin resistance. Obesity induced by 10 weeks of a high-fat diet increased mean BP by about 10mmHg in conscious mice implanted with radiotelemetry probes.81 Furthermore, systemic leptin treatment for 12 days increased mean BP by about 10mmHg in both lean and obese animals, demonstrating preserved pressor actions of leptin in animals resistant to the anorexic and thermogenic effects of leptin. Together, these results raise the attractive hypothesis that renal sympathoexcitatory effects of leptin could contribute to hypertension despite metabolic leptin resistance (Figure 3).
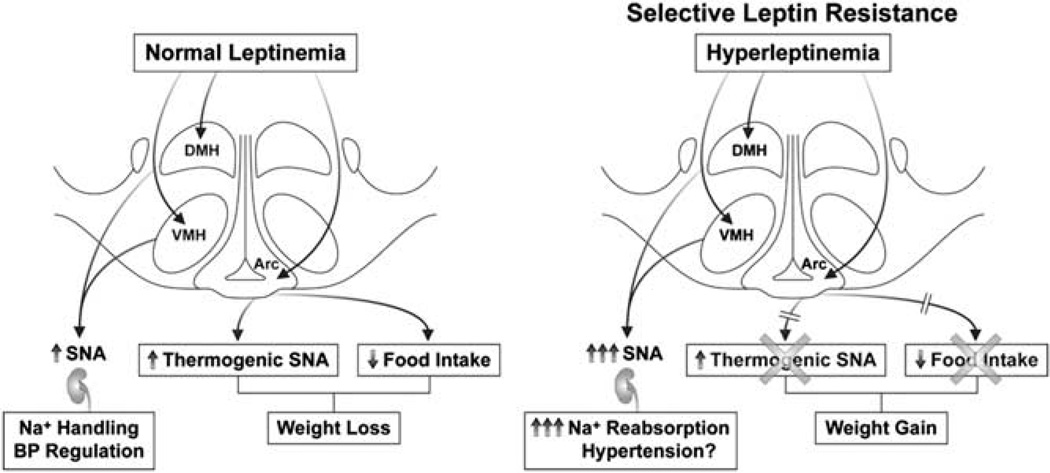
Figure 4.
Mechanisms of selective leptin resistance. In lean conditions, leptin acts in the hypothalamus to decrease food intake and increase thermogenesis, as well as increase sympathetic nerve activity (SNA) to non-thermogenic organs (left). Increasing evidence suggests that these actions can be dissociated in obesity (right), with resistance to the anorexic and thermogenic effects of leptin (mediated through the arcuate nucleus) but with preservation of cardiorenal sympathoactivation (mediated through medial hypothalamic nuclei (VMH and DMH)). This phenomenon might explain, in part, how hyperleptinemia could be accompanied by obesity (partial loss of appetite and metabolic actions of leptin) but still contribute to sympathetic overactivity and hypertension because of preservation of the sympathetic actions of leptin to some organs involved in blood pressure regulation (for example, the kidney). Arc, arcuate nucleus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus.
MECHANISMS OF SELECTIVE LEPTIN RESISTANCE
Experimental evidence indicates that leptin-dependent sympathetic outflow acting on metabolism and the cardiovascular system do not share common neuronal or molecular mechanisms. We have demonstrated that although intracerebral administration of leptin increases brown adipose tissue and renal SNA simultaneously, co-treatment with a melanocortin-4 receptor antagonist inhibits only sympathoactivation to brown adipose tissue, preserving leptin-activated sympathetic response to the kidneys.82 In addition, mice without the gene for the melanocortin-4 receptor exhibit decreased leptin-induced renal sympathoactivation.74 On the other hand, leptin-dependent SNA to thermogenic brown adipose tissue is inhibited by blockade of corticotrophin-releasing factor.63 These results indicate that sympathetic output to thermogenic brown adipose tissue is conveyed through hypothalamic pathways distinct from those that mediate sympathetic modulation of cardiorenal function. We have also shown that baroreflex activation suppresses leptin-induced SNA to the kidney, but that leptin-induced sympathetic output to thermogenic tissue is not inhibited by the baroreflex.83 These results indicate that cardiorenal sympathetic regulation—but not thermogenesis—is influenced by baroreflex signals; confirming the independence of the cardiorenal and thermogenic sympathetic effects of leptin.
In addition, the anorexic and lipopenic effects of leptin appear to be mediated by neural networks relayed through the arcuate nucleus (a collection of neurons in the mediobasal hypothalamus), whereas the sympathetic and cardiovascular actions of leptin appear to be associated with neuronal activation of the ventromedial and dorsomedial hypothalamus (Figure 3).84 It has been proposed that the arcuate nucleus, being less insulated from the systemic circulation by the blood–brain barrier than other hypothalamic areas, would be particularly influenced by serum leptin and other circulating factors.85 Indeed, at the molecular level, STAT3-dependent signaling pathways in the arcuate nucleus are depressed in murine models of diet-induced obesity, possibly due to increased expression of inhibitory SOCS3, whereas other hypothalamic and extrahypothalamic nuclei remain leptin sensitive.86 Activation of mitogen-activated protein kinase might be an alternative arcuate neuron signaling pathway that is altered in the obese state; pharmacological inhibition of mitogen-activated protein kinase mimics the pattern of selective leptin resistance, namely, loss of the anorexic and thermogenic effects of leptin with preservation of renal sympathoactivation.87
Another molecular candidate mechanism for leptin resistance is protein-tyrosine phosphatase 1B (PTP1B). In vitro evidence indicates that PTP1B regulates leptin-dependent signaling by dephosphorylation of Janus kinase 2 and STAT3.88 Additionally, PTP1B reduces expression of STAT3 mRNA.89 In vivo studies in PTP1B-deficient mice show increased sensitivity to the weight-loss effects of leptin.90 Treatment with a PTP1B inhibitor improves leptin-dependent suppression of food intake in leptin-resistant ageing rats.91 Nevertheless, unlike SOCS3, dysregulation of hypothalamic PTP1B has not been demonstrated in experimental models of obesity.85
Another possible pathway to explain leptin resistance would be via increased endoplasmic reticulum stress and unfolded protein response in the hypothalamus.92 Mice with pharmacologically induced endoplasmic reticulum stress show severe leptin resistance and augmented obesity on a high-fat diet. One of the main regulators of unfolded protein response is X-box binding protein-1, although modulators for X-box binding protein-1 are poorly understood. A severe defect in X-box binding protein-1 translocation to the nucleus is seen in leptin-deficient obese ob/ob mice, and provides a mechanism for development of endoplasmic reticulum stress.93
LEPTIN AND OBESITY-RELATED HYPERTENSION IN HUMANS
Most obese individuals are leptin resistant and exhibit compensatory hyperleptinemia from high adipose tissue mass. If the concept of selective leptin resistance holds in obese humans, it would be plausible that hyperleptinemia contributes to obesity-related sympathoactivation and hypertension, despite resistance to leptin’s anorexic effects. Indeed, epidemiological studies have shown positive correlations between leptin and BP. Circulating leptin correlates with BP and hypertension in multiple populations, including white Europeans, Hispanic and Japanese. It is important to bear in mind that these are association studies, which do not necessarily prove a causal link between leptin and BP. However, several reports have demonstrated associations between circulating leptin and BP, independent of obesity, for example in lean subjects94, and after correction for body mass index,95,96 waist circumference96 and other measures of adiposity.94–99
Human studies demonstrating unequivocal effects of leptin on the SNS have not been conducted. However, renal norepinephrine spillover is significantly correlated with leptinemia in overweight/ obese men (correlation coefficient=0.63; P<0.01).100 In apparent contradiction, another study showed that leptin administration to normal-weight individuals did not change plasma or urinary catecholamine levels, although these are poor indicators of systemic sympathetic activity.101 However, leptin given for weight maintenance reverses cardiac and renal sympathetic suppression caused by weight loss.102 Larger studies in which exogenous leptin was administered to obese individuals have been conducted, but no detailed evaluation of sympathetic activity or BP responses were reported.
THERAPEUTIC IMPLICATIONS
Optimal therapy for obesity–hypertension is not yet clear, in part because its mechanisms are not fully elucidated. Blockade of the SNS would be attractive in theory based on the mechanistic data presented above. Our discussion of therapy will focus on two questions. First, are there specific classes of antihypertensive medications that work better or have fewer side effects in obesity-hypertension? Second, what are the BP effects of weight loss interventions, whether lifestyle, surgical or pharmacological?
Antihypertensive pharmacotherapy in obesity–hypertension
There are no data to suggest enhanced BP-lowering efficacy for specific classes of antihypertensive drugs in obese compared with lean subjects, with the possible exception of combined alpha- and beta-adrenoceptor blockade. However, it is clear whether non-adrenergic blocking agents have outcome advantages in hypertension, and these persist in obese subjects with hypertension. In addition, some antihypertensive classes (particularly antiadrenergics) have metabolic side effects that may be particularly undesirable in obesity.
β-Adrenoceptor blockers exercise antihypertensive effects by decreasing cardiac output and renin activity. Though these mechanisms would seem to be ideal for obesity–hypertension, β-blockers have disadvantages. Though β-blockers were shown to reduce stroke compared with placebo in hypertension,103 they have also been shown to be inferior to angiotensin AT1 receptor blocker (ARB)-and calcium channel blocker (CCB)-based regimens in reducing events in the Losartan Intervention for Endpoint Reduction in Hypertension Study and Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) trials.104,105 Importantly, β-blockers prevented significantly fewer events than these other classes in subgroup analyses restricted to obese subjects.105,106 The inferiority of β-blockers may relate to several factors, including weight gain (3–4 kg on long-term use), type 2 diabetes, low high-density lipoprotein and high triglycerides.103,107 β-Blockers also lessen exercise tolerance and cause erectile dysfunction. Some of these side effects of β-blockers may be ameliorated by the so-called ‘vasodilator’ β-blockers (nebivolol and carvedilol); however, these agents lack outcome trials.108,109 Because β-blockers have inferior outcomes in obese subjects, and possess metabolic and other side effects that are particularly undesirable in obesity, this class should not be used as first-line therapy.
α-adrenoreceptor antagonists reduce BP through blockade of sympathetic vasoconstrictor tone on arterioles. It has been argued that because obesity is associated with increased sympathetic outflow, blockade of peripheral sympathetic tone would be advantageous. However, as discussed earlier, we have shown that complete forearm α-adrenergic receptor blockade causes similar vasodilatation in obese and lean subjects, with and without hypertension.14,110 These data would suggest that there is no physiological rationale for using α-blockade alone in obesity–hypertension. Combined α- and β-blockade has been shown to reduce mean arterial BP to a greater extent in obese than in lean hypertensive subjects (−15 versus −7mmHg), though systolic and diastolic BP differences were not significant.10 However, unlike β-blockers, α-blockers have never been shown to reduce cardiovascular events compared with placebo. In addition, an α-blocker-based regimen using doxazosin was inferior to a thiazide-diuretic-based regimen using chlorthalidone in reduction of cardiovascular events and heart failure in the large Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) study,111 even though ~30% also received a β-blocker.112 α-Blockers remained inferior to chlorthalidone in subgroup analyses restricted to (mainly obese) subjects with metabolic syndrome or glucose intolerance in the ALLHAT study, with 20–40% more cardiovascular events and strokes.113,114 This inferiority occurred despite the fact that α-blockers improve both dyslipidemia and glycemia.111 The adverse effect profile of α-blockers includes orthostatic hypotension, headache, edema and flushing. These do not appear to be specifically worse in obese subjects. Given the lack of a physiological rationale for therapy, no evidence for benefit versus placebo and more cardiovascular events in obese subjects than thiazides, α-adrenoceptor antagonism should be avoided in obese hypertensive patients.
The centrally acting antihypertensives clonidine, methyldopa and moxonidine reduce sympathetic outflow by stimulating brainstem α-adrenoreceptors and imidazoline receptors. They reduce BP by decreasing vascular resistance and cardiac output. Moxonidine improves lipids and glucose in subjects with metabolic syndrome.115 There is no evidence that these agents reduce cardiovascular events in patients with obesity or hypertension. Adverse effects, including weight gain, impotence, depression, sedation and rebound hypertension, limit their use. Given the lack of evidence for benefits on outcomes, and their adverse effect profiles, these centrally acting agents should not be used in obesity–hypertension, unless other classes have been exhausted.
CCBs reduce the influx of ionic calcium across voltage-gated L-type calcium channels in arterial smooth muscle and myocardial cells, thereby decreasing peripheral vascular resistance. Some also possess negative inotropic and chronotropic effects. In the ASCOT trial, an amlodipine-based CCB regimen improved high-density lipoprotein cholesterol, weight, triglycerides, creatinine and glucose compared with a β-blocker-based regime. The CCB-based regimen also prevented more cardiovascular events and induced less diabetes than the atenolol-based regimen, including in analyses restricted to patients with obesity–hypertension.105 However, a detailed subgroup analysis of the ALLHAT study showed that metabolic syndrome patients (n=23 077) treated with amlodipine had a substantial and significant 25–50% higher risk of heart failure than those treated with chlorthalidone.113 The excess risk from amlodipine was higher for black subjects, but still elevated in non-blacks.113 Amlodipine was similar to chlorthalidone in terms of preventing stroke, cardiovascular events and renal failure in metabolic syndrome patients.113 Adverse effects of CCBs include flushing, headache and pedal edema that are no worse in obese patients, and they lack metabolic side effects that would be problematic in obesity. Given the evidence base, CCBs would seem to have advantages in obese patients, though perhaps secondary to chlorthalidone.
Drugs that act on RAAS include angiotensin-converting enzyme inhibitors (ACEIs), ARBs, direct renin inhibitors and aldosterone receptor antagonists. Such drugs lower BP through multiple mechanisms, including reductions in vascular resistance and natriuresis. In obese hypertensives, treatment with an ACEI, ARB or direct renin inhibitor has been shown to have identical BP-lowering efficacy as compared with treatment with a CCB, thiazide or β-blocker.109,116–119 In terms of cardiovascular outcomes, combined CCB and ACEI treatment was superior to combined β-blocker and diuretic in the ASCOT trial, even in analyses restricted to obese patients. However, subgroup analysis in the ALLHAT trial showed that ACEI (without CCB) was inferior to thiazide in prevention of heart failure in metabolic syndrome patients (n=23077), with 20–49% more events depending on race.113 Moreover, ACEI was significantly inferior to thiazide in preventing combined cardiovascular disease events in metabolic syndrome patients (10–20% more events). Black patients with metabolic syndrome (n=7327) did particularly bad with ACEI compared with thiazide treatment for all comparisons, but especially in terms of stroke (37% more) and end-stage renal disease (70% more events).113 Adverse effects caused by ACEIs (but not ARBs) include cough and angioedema; these are not exaggerated in obesity, but are more frequent in black patients. Relevant to obesity, ACEI and ARB appear to prevent type 2 diabetes mellitus120 and provide renoprotection in patients with diabetes. On the basis of animal studies, aldosterone has been implicated as a potential contributing factor in obesity–hypertension. Unblinded observational studies suggest substantial benefit of aldosterone receptor antagonists in resistant hypertensives with obesity; however, randomized comparisons are few and do not show appreciable additional efficacy.121 Visceral obesity does appear to predict a better response to aldosterone blockade.122 In general, drugs that block RAAS do not appear to be superior to thiazide diuretics for treatment of obesity–hypertension, though they may offer some advantages in non-black patients with, or at high risk of developing, type 2 diabetes mellitus.
Diuretics work by reducing intravascular volume and cardiac output. Thiazide diuretics are the most commonly used, and chlorthalidone appears to be superior to hydrochlorothiazide.123 All diuretic classes stimulate the SNS and RAAS. Antihypertensive efficacy is similar for hydrochlorothiazide versus ACEI or ARB in obese hypertensive patients.109,116 In the ALLHAT study, patients with metabolic syndrome had significantly fewer cardiovascular events with chlorthalidone compared with α-blocker (which had 18–37% more events) and ACEI (which had 10–24% more events); chlorthalidone was also superior to ACEI and CCB in prevention of heart failure.113 Thiazide diuretics do worsen cholesterol, glucose, uric acid and cause hypokalemia. Worsening glycemia, insulin resistance and hypertriglyceridemia are more likely to occur in obese compared with lean patients when hydrochlorothiazide is given.124 Nonetheless, chlorthalidone has been shown to have such clear outcome advantages over alternative classes in patients with metabolic syndrome that it probably should be preferred for first-line therapy, especially in blacks. Clearly, it is very important to regularly monitor potassium, lipids, urate and glucose if thiazides are used.
In summary, antihypertensive treatment in obesity should be guided by evidence from large-scale outcome trials, though informed by adverse effect profiles. Unfortunately, such an approach fails to support the use of adrenergic blockade (whether α, β or centrally acting). Outcome trials support the use of chlorthalidone as the first-line therapy in obesity–hypertension without type 2 diabetes. Second-line therapies would include CCB or ACEI/ARB. Black patients with obesity–hypertension should receive chlorthalidone and then a CCB before a RAAS blocker (and an ARB over an ACEI), based on outcomes and adverse events. Patients with obesity–hypertension and diabetes should receive an ACEI or ARB early, probably with chlorthalidone. β-Blockers do appear to confer some benefits but less than the classes above, so they should be considered as the fourth-line therapy. Obese patients with resistant hypertension may benefit from an aldosterone receptor antagonist. Centrally acting agents and α-adrenoceptors should be considered as the last-line therapy. However, it is important to realize that none of these antihypertensive drugs addresses the central causation of obesity–hypertension, namely, increased adipose tissue mass.
Antihypertensive effects of weight loss interventions
Given that obesity increases BP by multiple mechanisms, including hyperleptinemia, hyperinsulinemia and changes in renal afferent nerve signaling, weight loss can be thought of as a pathophysiologically targeted intervention for obesity–hypertension. Weight loss reduces circulating leptin and insulin concentrations, and improves leptin and insulin resistance. These improvements should lead to a decrease in SNA and BP. We review changes in BP with different weight loss interventions below. In order to aid assessment of potential weight-independent BP effects, we have calculated changes in BP for different interventions corrected to a standardized 5% weight loss (Table 1).
Table 1.
Selected controlled clinical trials of weight loss interventions and their effect on BP
| Subjects (active/control) |
Duration (months) |
Daily dose (mg) |
Weight, Δ (%) |
Placebo-adjusted weight Δ (%) |
SBP Δ (mmHg) |
Placebo-adjusted SBP Δ (mmHg) |
SBP Δ per 5% weight loss (mm Hg) |
|
|---|---|---|---|---|---|---|---|---|
| Lifestyle | ||||||||
| Neter et al.130 | 4874 | 6 | NA | −5.8 | −4.4 | −3.8 | ||
| DPP: intensive150 | 1079/1082 | 38 | NA | −5.9 | −5.8 | −3.3 | −2.7 | −2.3 |
| LOOK AHEAD136 | 2570/2575 | 48 | NA | −4.7 | −3.6 | −4.6 | −1.3 | −1.8 |
| Restrictive surgery | ||||||||
| Sjöström et al.137 | 156/627 | 120 | NA | −13.2 | −14.8 | +2.1 | −2.3 | −0.8 |
| O’Brien et al.151 | 40/40 | 24 | NA | −21.6 | −16.1 | − 14.2 | −4.8 | −1.5 |
| Malabsorptive surgery | ||||||||
| Hofso et al.152 | 76/63 | 12 | NA | −30 | −22.0 | −14.0 | −4.0 | −0.9 |
| Sjöström et al.137 | 34/627 | 120 | NA | −25 | −26.6 | −4.7 | −6.8 | −1.3 |
| Sibutramine | ||||||||
| SCOUT138 | 4906/4898 | 41 | 10 | −1.8 | −1.8 | +1.4 | +2.0 | |
| Erondu et al.153 | 100/101a | 6 | 10 | −6.0 | −4.2 | +2.1 | +2.0 | +2.4 |
| Phentermine | ||||||||
| OB301154 | 326a | 6 | 15 | −5.8 | −4.3 | −3.3 | −1.7 | −2.0 |
| Kang et al.155 | 74 | 3 | 30 | −9.3 | −7.4 | −1.0 | +2.0 | +1.3 |
| Diethylpropion | ||||||||
| Cercato et al.156 | 37/32 | 6 | 12 | −9.8 | −6.6 | −4.4 | +2.7 | +2.1 |
| Bupropion | ||||||||
| Anderson et al.157 | 105/112 | 6 | 400 | − 10.1 | −5.1 | − 1.7 | +0.9 | +0.9 |
| Orlistat | ||||||||
| Sjöström et al.158 | 343/340 | 12 | 360 | −10.2 | −4.1 | −2.0 | −3.0 | −3.7 |
| XENDOS159 | 1640/1637 | 48 | 360 | −5.3 | −2.6 | −4.9 | −1.5 | −2.9 |
| Miles et al.160 | 250/254 | 12 | 360 | −4.6 | −2.9 | −2.1 | −1.7 | −2.9 |
| Erondu et al.153 | 99/101a | 6 | 360 | −4.8 | −3.4 | −1.4 | −1.5 | −2.2 |
| GLP-1 agonists | ||||||||
| Liraglutide: Astrup et al.142 | 95/98 | 5 | 3 | −7.4 | −4.5 | −6.9 | −3.1 | −3.4 |
| LEAD-6, liraglutide161 | 233 | 6 | 1.8 | −3.5 | −2.5 | −3.6 | ||
| LEAD-6, exenatide161 | 231 | 6 | 0.02 | −3.1 | −2.0 | −3.2 | ||
| Topiramate | ||||||||
| Bray et al.162 | 50/48 | 6 | 192 | −8.2 | −4.6 | −8.4 | −6.7 | −7.3* |
| Wilding et al.133 | 215/215 | 12 | 192 | −9.1 | −7.4 | −5.7 | −6.1 | −4.1 |
| Tonstad et al.163 | 53/56 | 6 | 192 | −6.5 | −4.6 | −9.7 | −4.8 | −5.2* |
| Stenlof et al.164 | 77/78 | 9 | 192 | −9.1 | −6.6 | −7.6 | −5.6 | −4.2 |
| Toplak et al.143 | 105/100 | 6 | 192 | −6.5 | −4.8 | −4.4 | −4.0 | −4.2 |
| Zonisamide | ||||||||
| Gadde et al.165 | 20/17 | 8 | 427 | −9.4 | −7.6 | −6.8 | −5.4 | −3.6 |
| Pramlintide | ||||||||
| Aronne et al.147 | 36/25 | 6 | 360 | −3.7 | − 1.5 | −4.5 | −1.0 | −3.3 |
| Lorcaserin | ||||||||
| Smith RS et al.131 | 1538/1499 | 12 | 20 | −5.8 | −3.7 | − 1.4 | −0.6 | −0.8 |
| Metformin | ||||||||
| DPP: metformin150 | 1073/1082 | 38 | 1700 | −2.2 | −2.1 | −0.3 | +0.3 | +0.7 |
| Top+ Phen | ||||||||
| Gadde et al.134 | 981/979 | 12 | 92/15 | −9.9 | −8.5 | −5.6 | −3.2 | −1.9 |
| Bup+Nal | ||||||||
| Greenway et al.135 | 581/583 | 12 | 360/32 | −6.1 | −4.8 | −0.1 | +1.8 | +1.9 |
Abbreviations: BP, blood pressure; Bup, bupropion; CI, confidence interval; DPP, diabetes prevention program; GLP-1, glucagon-like peptide-1; LEAD-6, liraglutide effect and action in diabetes; NA, not available; Nal, naltrexone; Phen, phentermine; SBP, systolic BP; XENDOS; Xenical in the Prevention of Diabetes in Obese Subjects.
The last column shows BP changes with weight loss adjusted for the degree of weight loss (to facilitate comparison of weight loss-independent effects on BP. The standard comparator for this parameter is derived from the first study listed (Netter et al.), a meta-analysis of lifestyle interventions lasting at least for 6 months). From this study, the calculated 95% confidence limits for change in SBP per 5% weight loss from lifestyle intervention are −2.5 to −5.1 mm Hg. BP reductions that are less than the lower 95% CI (that is, significantly less than that expected from degree of weight loss) are marked in italics. BP reductions that are more than the upper 95% CI (that is, significantly more than expected from the degree of weight loss) are marked in bold and with an.
Note that even longer-term lifestyle studies have an effect on BP that is less than would be predicted from short-term studies, even after controlling for less weight loss.
These trials had multiple active drugs. Subject numbers include only those randomized to placebo and the relevant drug.
Lifestyle interventions for weight loss include diet, behavior and exercise modification. Experimental weight gain increases SNA by ~20% in lean subjects.125 Multiple studies have shown that dietinduced weight loss reduces SNA by 14–34%126–129 and norepinephrine spillover by about 25%,129 with no additive effect of exercise. In a large meta-analysis, short-term (6 month) interventions reduced weight by ~6% and systolic BP by 4.4mmHg, with a change in SBP of −3.8 mm Hg per 5% weight loss (Table 1; 95% confidence interval −2.5 to −5.1).130 The main disadvantage of lifestyle interventions is lack of sustainability, unless extremely intensive interventions are performed. The longer-term ‘failure’ rate for lifestyle interventions (defined as <5% weight loss at 12 months) ranges from 68 to 84% in carefully controlled trials.131–135 The BP benefit of long-term lifestyle interventions is disappointing not only because of diminished weight loss, but also because the BP benefit diminishes when corrected for the degree of weight loss (that is, −3.8 versus −1.8 mm Hg per 5% weight loss for 6 versus 48-month studies; Table 1). Weight loss through lifestyle change also improves glycemia and lipids (usually by under 10%), though this diminishes with time too. For example, after 4 years, the Look AHEAD (Action for Health in Diabetes) Trial showed very modest improvements in high-density lipoprotein cholesterol (+3%) and glycemia (−2%), and low-density lipoprotein cholesterol, triglycerides and diastolic BP actually increased, compared with standard support.136
Bariatric surgery includes restrictive (gastric stapling and laparoscopic gastric banding) and malabsorptive procedures (Roux-en-Y gastric bypass). Restrictive surgery has been shown to reduce weight by 15% and BP by ~3.5mmHg (~1mmHg per 5% weight loss; Table 1). Malabsorptive surgery has been shown to reduce weight by 25% and BP by ~5mmHg (also ~1mmHg per 5% weight loss). Surgery substantially improves glycemia and also lipids (usually by 10–20%) depending on weight loss. Disadvantages include the risk of death and morbidity (infection and gastrointestinal) from surgery and modest weight regain (20% of weight lost regained after 10 years).137 In general, bariatric surgery almost always reduces BP beyond what is achieved with lifestyle change. However, the relative antihypertensive effect is significantly less than expected from the degree of weight loss (Table 1).
Pharmacotherapy for weight loss has diverse effects on BP, which are additive to those of lifestyle intervention. Stimulants such as phentermine, sibutramine, diethylpropion and bupropion reduce weight through appetite suppression. They all share an action to increase central nervous system synaptic catecholamine concentrations. Such agents reduce weight by 2–7%, but tend to increase BP compared with lifestyle intervention (+1 to +2 mmHg per 5% weight loss; Table 1). Heart rate also increases, suggesting sympathetic activation and propensity to cardiac arrhythmias. Importantly, in the Sibutramine Cardiovascular Outcome Trial, obese subjects on sibutramine had a 15% increase in cardiovascular events.138 This occurred despite modest (< 5%) improvements in lipids and glycemia, suggesting that small BP increases can offset benefits on other risk factors. Such stimulant agents should not be used to treat obesity, especially when combined with hypertension.
Orlistat is a reversible intestinal lipase inhibitor and blocks about 30% of dietary fat absorption. We have shown that orlistat decreases sympathetic nerve traffic by 20% in obese normotensive139 and by 26% in obese hypertensive subjects.140 Most patients have sustained weight loss with orlistat, with a ‘failure’ rate (defined as < 5% weight loss at 12 months) of 51% compared with 77% for placebo.132 Overall, orlistat reduces weight by ~3% and BP by ~2 mm Hg, additional to what is achieved with lifestyle intervention. Adjusted BP change of −3 mm Hg per 5% weight loss is proportional (though additive) with what would be expected from lifestyle intervention (Table 1). Orlistat has particular benefits on low-density lipoprotein cholesterol and glycemia (10 and 7% reductions) and reduces risk of developing diabetes. However, it can worsen high-density lipoprotein cholesterol, and cause gastrointestinal symptoms if there is high-fat intake.
Glucagon-like peptide-1 receptor agonists, such as liraglutide and exenatide, appear to reduce weight through effects on gastric emptying and possibly also increase fat oxidation.141 They decrease weight by ~ 4% and BP by ~ 3 mm Hg beyond what is achieved with lifestyle intervention alone (Table 1). Weight loss-adjusted BP change (−3.5 mm Hg per 5% weight loss) is additive and proportional to that of intensive short-term lifestyle intervention. Glucagon-like peptide-1 agonists reduce glycemia by about 10%, but do not alter lipids.142
Topiramate and zonisamide are both antiseizure drugs that reduce weight; they share carbonic anhydrase inhibition. They are the most efficacious drugs for obesity, decreasing weight by 5–8% and BP by ~7mmHg beyond lifestyle intervention alone (Table 1). Most patients exhibit sustained and significant weight loss on topiramate, with a ‘failure’ rate (defined as < 5% weight loss at 12 months) of 33% compared with 82% for placebo.133 Adjusted BP change for topiramate and zonisamide (−4 to −7mmHg per 5% weight loss) is additive to that from lifestyle intervention and disproportionately greater than expected from the degree of weight loss. Topiramate reduces glycemia by about 10%, but does not alter lipids.143
Lorcaserin is a centrally acting selective serotonin 2C receptor agonist, which reduces appetite. Most patients exhibit sustained and significant weight loss on lorcaserin, with a ‘failure’ rate (defined as <5% weight loss at 12 months) of 52% compared with 80% for placebo.131 Lorcaserin decreases weight by ~4% and BP by ~ 1 mm Hg beyond what is achieved with lifestyle intervention alone (Table 1). Weight loss-adjusted BP change (−1 mm Hg per 5% weight loss) is additive to lifestyle intervention, but significantly less than expected from the degree of weight loss. Lorcaserin produces extremely modest improvements in cholesterol and glycemia (1–2%).131
Pramlintide is a synthetic form of a pancreatic islet-derived polypeptide amylin that is co-secreted with insulin. Amylin reverses leptin resistance, reduces food intake, slows gastric emptying, attenuates postprandial glucagon secretion and reduces body weight in obese rodents.144,145 Pramlintide decreases weight by ~2% and BP by ~ 1 mm Hg beyond what is achieved with lifestyle intervention alone (Table 1). Weight loss-adjusted BP change (−3 mm Hg per 5% weight loss) is additive to lifestyle intervention, and commensurate with the degree of weight loss. Pramlintide reduces glycemia by ~5% and cholesterol by ~2%.146,147 The main disadvantages are the need for twice daily injections and nausea.
Metformin, a biguanide antihyperglycemic agent, works by increasing AMP-dependent protein kinase activity, stimulating fatty acid oxidation, decreasing hepatic glucose production and improving insulin sensitivity. It also has gastrointestinal side effects, including nausea and diarrhea that may contribute to weight loss. Metformin decreases weight by ~2%, but without appreciable BP reductions (Table 1). In fact, BP tends to be higher than expected from the degree of weight loss (+1 mm Hg per 5% weight loss). However, metformin produces substantial improvement in glycemia (−15%), and reduces triglycerides (−6%) and low-density lipoprotein cholesterol (−6%).148 In addition, metformin has been shown to reduce myocardial infarction and overall mortality in obese diabetic patients by ~ 30%.149
Several combination products have been tested for weight loss. Unfortunately, these have usually used at least one component that has a BP-elevating effect. For example, studies of topiramate and phentermine show a BP effect that is less than expected from the degree of weight loss (−2mmHg per 5% weight loss; Table 1), and certainly less than obtained with topiramate alone (~ 5 mm Hg per 5% weight loss). The combination of bupropion and naltrexone makes the already bad effect of bupropion on BP worse (Table 1).
CONCLUSION
In summary, obesity increases BP through multiple mechanisms, including sympathoactivation, increased activity of the RAAS and renal sodium retention. Hyperleptinemia combined with selective leptin resistance appears to have a critical role. Targeting specific mechanisms of leptin resistance is not yet possible. This has clinical implications for the treatment of obesity–hypertension. No specific antihypertensive class appears to have better BP-lowering efficacy in obesity–hypertension. However, α- and β-adrenoceptor blockers appear to be less likely to reduce cardiovascular events and cause problematic adverse events in metabolic syndrome. On the other hand, chlorthalidone and RAAS blockers appear to prevent more cardiovascular events in patients with metabolic syndrome, though potassium levels need to be monitored carefully.
Even before resorting to antihypertensive pharmacotherapy, weight loss interventions are warranted. Weight reduction addresses the underlying causes of obesity–hypertension, and often improves other comorbidities. Importantly, weight loss appears to reduce sympathetic activation in obesity. Lifestyle intervention should remain a cornerstone of treatment. However, lifestyle change is arduous for the patient and provider, and its effects are small and diminish greatly with time. Around 80% of patients fail to achieve meaningful weight loss at 1 year using lifestyle modification. Though surgery causes substantial weight loss, it can be fatal or disabling, and it has modest effects on hypertension. Thus, surgery may be best reserved for patients with diabetes or other complications of obesity. Carefully chosen weight loss pharmacotherapy offers great potential, with much lower failure rates than lifestyle. Some drugs have been shown to provide substantial weight loss and BP benefits, whereas others have undesirable BP consequences. Phentermine, sibutramine, diethylpropion, bupropion and the combination of bupropion and naltrexone have all been shown to increase BP regardless of weight loss. On the other hand, orlistat, carbonic anhydrase inhibitors (topiramate and zonisamide) and glucagon-like peptide-1 receptor agonists produce substantial BP reductions that are additive to lifestyle. In many cases, these are greater in degree than even the most intensive short-term lifestyle programs can achieve (especially for topiramate). In the long term, elucidation of the mechanisms of obesity–hypertension and the molecular signaling of leptin, insulin and glucagon-like peptide-1 should provide new approaches to obesity–hypertension.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.CDC. State-specific prevalence of obesity among adults—United States, 2009. MMWR. 2009;59:1–5. [Google Scholar]
- 2.Matshushita Y, Takahashi Y, Mizoue T, Inoue M, Noda M, Tsugane S. Overweight and obesity trends among Japanese adults: a 10-year follow-up of the JPHC Study. Int J Obes. 2008;32:1861–1867. doi: 10.1038/ijo.2008.188. [DOI] [PubMed] [Google Scholar]
- 3.Lapidus L, Bengtsson C, Bjorntorp P. The quantitative relationship between ‘the metabolic syndrome’ and abdominal obesity in women. Obes Res. 1994;4:372–377. doi: 10.1002/j.1550-8528.1994.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 6.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 7.Samuelson A, Morris A, Igosheva N, Kirk SL, Pombo JMC, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 8.Tuck ML. Obesity, the sympathetic nervous system and essential hypertension. Hypertension. 1992;19:I67–I77. doi: 10.1161/01.hyp.19.1_suppl.i67. [DOI] [PubMed] [Google Scholar]
- 9.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 10.Wofford MR, Anderson DC, Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of α- and β-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14:694–698. doi: 10.1016/s0895-7061(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 12.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 13.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 14.Agapitov AV, Correia MLG, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension. 2008;52:687–695. doi: 10.1161/HYPERTENSIONAHA.107.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia MLG, Agapitov AV, Siney CA, Mark AL, Haynes WG. Obesity does not increase sympathetic vascular tone in hypertensives. Am J Hypertens. 2005;2:195A. [Google Scholar]
- 16.Correia M, Agapitov A, Siney C, Mark A, Haynes W. Weight loss does not reduce sympathetic vascular tone I obese hypertensives. Obes Res. 2005;3(Suppl):A47. [Google Scholar]
- 17.Marcelo Lima De Gusmao Correia. PhD diss. University of Iowa; 2007. Sympathetic Vascular Tone in Human Obesity. http://ir.uiowa.edu/etd/134. [Google Scholar]
- 18.Tappy L, Girardet K, Schwaller N, Vollenweider L, Jequier E, Nodod P, Scherrer U. Metabolic effects of an increase of sympathetic activity in healthy humans. Int J Obesity Metab Dis. 1995;19:419–422. [PubMed] [Google Scholar]
- 19.Young JB, Weiss J, Boufath N. Effects of dietary monosaccharides on sympathetic nervous system activity in adipose tissues of male rats. Diabetes. 2004;53:1271–1278. doi: 10.2337/diabetes.53.5.1271. [DOI] [PubMed] [Google Scholar]
- 20.Serazin V, Dos Santos E, Morot M, Giudicelli Y. Human adipose angiotensinogen gene expression and secretion are stimulated by cyclic AMP via increased DNA cyclic AMP responsive element binding activity. Endocrine. 2004;25:97–104. doi: 10.1385/endo:25:2:097. [DOI] [PubMed] [Google Scholar]
- 21.Licata G, Volpe M, Scaglione R, Rubattu S. Salt-regulating hormones in young normotensive obese subjects. Effects of saline load. Hypertension. 1994;23:1020–1024. doi: 10.1161/01.hyp.23.1_suppl.i20. [DOI] [PubMed] [Google Scholar]
- 22.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 23.Cooper R, Forrester T, Ogunbiyi O, Muffinda J. Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J Hypertens. 1998;16:571–575. doi: 10.1097/00004872-199816050-00003. [DOI] [PubMed] [Google Scholar]
- 24.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med. 2001;79:21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 25.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 26.Giacchetti G, Faloia E, Sardu C, Camilloni MA, Mariniello B, Gatti C, Garrapa GG, Guerrieri M, Mantero F. Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S142–S143. doi: 10.1038/sj.ijo.0801305. [DOI] [PubMed] [Google Scholar]
- 27.Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res. 2000;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 28.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilatation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension. 1992;19:621–627. doi: 10.1161/01.hyp.19.6.621. [DOI] [PubMed] [Google Scholar]
- 30.Hausberg M, Hoffman RP, Somers VK, Sinkey CA, Mark AL, Anderson EA. Contrasting autonomic and hemodynamic effects of insulin in healthy elderly versus young subjects. Hypertension. 1997;29:700–705. doi: 10.1161/01.hyp.29.3.700. [DOI] [PubMed] [Google Scholar]
- 31.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:625–628. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallman MF, Strack AM, Akana SF, Bradburry MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin JE, Zhang J, Geller DS. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J Am Soc Nephrol. 2008;19:1291–1299. doi: 10.1681/ASN.2007080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato A, Funder JW, Okubo M, Kubota E, Saruta T. Glucocorticoid-induced hypertension in the elderly relation to serum calcium and family history of essential hypertension. Am J Hypertens. 1995;8:823–828. doi: 10.1016/0895-7061(95)00149-J. [DOI] [PubMed] [Google Scholar]
- 35.Grassi G, Seravalle G, Dell Oro R, Turri C, Pasqualinotto L, Colombo M, Mancia G. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension. 2001;38:1316–1320. doi: 10.1161/hy1201.096117. [DOI] [PubMed] [Google Scholar]
- 36.Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metabol. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvery-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacher P, Batkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–1138. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brozoski DT, Dean C, Hopp FA, Hillard CJ, Seagard JL. Differential endocannabinoid regulation of baroreflex-evoked sympathoactivation in normotensive versus hypertensive rats. Auton Neurosci. 2009;150:82–93. doi: 10.1016/j.autneu.2009.05.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruilope LM, Despres JP, Scheen A, Pi-Sunyer X, Mancia G, Zanchetti A, Van Gaal L. Effect of rimonabant on blood pressure in overweight/obese patients with/without co-morbidities: analysis of pooled RIO study results. J Hypertens. 2008;26:357–367. doi: 10.1097/HJH.0b013e3282f2d625. [DOI] [PubMed] [Google Scholar]
- 42.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 43.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 44.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 45.Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E. Ghrelin and the metabolic syndrome in older adults. J Clin Endocrinol Metab. 2005;90:6448–6453. doi: 10.1210/jc.2005-1358. [DOI] [PubMed] [Google Scholar]
- 46.Lambert E, Lambert G, Ika-Sari C, Dawood T, Lee K, Chopra R, Straznicky N, Eikelis N, Drew S, Tilbrook A, Dixon J, Esler M, Schlaich MP. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension. 2011;58:43–50. doi: 10.1161/HYPERTENSIONAHA.111.171025. [DOI] [PubMed] [Google Scholar]
- 47.Li ZF, Guo ZF, Yang SG, Zheng X, Cao J, Qin YW. Circulating ghrelin and ghrelin to obestatin ration are low in patients with untreated mild-to-moderate hypertension. Regul Pept. 2010;165:206–209. doi: 10.1016/j.regpep.2010.07.168. [DOI] [PubMed] [Google Scholar]
- 48.Berthold HK, Giannakidou E, Krone W, Tregouet DA, Gouni-Berthold I. Influence of ghrelin gene polymorphisms on hypertension and atherosclerotic disease. Hypertens Res. 2010;33:155–160. doi: 10.1038/hr.2009.194. [DOI] [PubMed] [Google Scholar]
- 49.Haqq AM, Stadler DD, Rosenfeld RG, Pratt KL, Weigle DS, Frayo RS, LaFranchi SH, Cummings DE, Purnell JQ. Circulating ghrelin levels are suppressed by meals and octreotide therapy in Prader-Willi Syndrome. J Clin Endocrinol Metab. 2003;88:3573–3576. doi: 10.1210/jc.2003-030205. [DOI] [PubMed] [Google Scholar]
- 50.Vogels A, Fryns JP. Age at diagnosis, body mass index and physical morbidity in children and adults with the Prader-Willi syndrome. Genet Couns. 2004;15:397–404. [PubMed] [Google Scholar]
- 51.Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med. 2007;232:390–397. [PubMed] [Google Scholar]
- 52.Vasunta RL, Kesaniemi YA, Ukkola O. Plasma adiponectin concentration is associated with ambulatory daytime systolic blood pressure but not with the dipping status. J Hum Hypertens. 2010;24:545–551. doi: 10.1038/jhh.2009.98. [DOI] [PubMed] [Google Scholar]
- 53.Celoria BMJ, Genelhu VA, Duarte SFP, Delfraro PAS, Francischetti EA. Hypoadiponectinemia is associated with prehypertension in obese individuals of multiethnic origin. Clin Cardiol. 2010;33:E61–E65. doi: 10.1002/clc.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim SY, Davidson SM, Paramanathan AJ, Smith CC, Yellon DM, Hausenloy DJ. The novel adipocytokine visfatin exerts direct cardioprotective effects. J Cell Mol Med. 2008;12:1395–1403. doi: 10.1111/j.1582-4934.2008.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, Abe M, Katoh A, Ostsuka N. Relationships between lipid profiles and metabolic syndrome, insulin resistance and serum high molecular adiponectin in Japanese community-dwelling adults. Lipids Health Dis. 2011;10:79. doi: 10.1186/1476-511X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamoto R, Tabara Y, Kohara K, Abe M, Kusunoki T, Miki T. Association of serum high molecular weight adiponectin and blood pressure among non-diabetic community dwelling men. Clin Exp Hypertens. 2011;33:336–344. doi: 10.3109/10641963.2010.531847. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi N, Anan F, Nakagawa M, Yufu K, Shinohara T, Tsubone T, Goto K, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Hara M, Saikawa T, Yoshimatsu H. Hypoadiponectinemia in type 2 diabetes mellitus in men is associated with sympathetic overactivity as evaluated by cardiac 123I–metaiodobenzylguanidine scintigraphy. Metabolism. 2007;56:919–924. doi: 10.1016/j.metabol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Leu H-B, Chung C-M, Lin S-J, Jong Y-S, Pan W-H, Chen JW. Adiponectin gene polymorphism is selectively associated with the concomitant presence of metabolic syndrome and essential hypertension. PLoS One. 2011;6:e19999. doi: 10.1371/journal.pone.0019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ai M, Otokozawa S, Asztalos BF, White CC, Cupples LA, Nakajima K, Lamon-Fava S, Wilson PW, Matsuzawa Y, Shaefer EJ. Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217:543–548. doi: 10.1016/j.atherosclerosis.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomopoulos C, Daskalaki M, Papazachou O, Rodolakis N, Bratsas A, Papadopoulos DP, Papavasileiou MV, Perrea D, Makris T. Association of resistin and adiponectin with different clinical blood pressure phenotypes. J Hum Hypertens. 2011;25:38–46. doi: 10.1038/jhh.2010.22. [DOI] [PubMed] [Google Scholar]
- 61.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 62.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–942. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 64.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 65.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlyle M, Jones OK, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 67.Farooqi SI, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 68.Ozata M, Ozdemir IC, Licino J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 69.Shintani M, Ikegami H, Fujisawa T, Kamaguchi Y, Ohishi M, Katsuya T, Higaki J, Shimmoto K, Ogihara T. Leptin gene polymorphism is associated with hypertension independent of obesity. J Clin Endocrinol Metab. 2002;87:2909–2912. doi: 10.1210/jcem.87.6.8595. [DOI] [PubMed] [Google Scholar]
- 70.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56:623–628. doi: 10.1161/HYPERTENSIONAHA.109.148213. [DOI] [PubMed] [Google Scholar]
- 72.Grøntved A, Steene-Johannessen J, Kynde I, Franks PW, Helge JW, Froberg K, Anderssen SA, Andersen LB. Association between plasma leptin and blood pressure in two population-based samples of children and adolescents. J Hypertens. 2011;29:1093–1100. doi: 10.1097/HJH.0b013e328346d787. [DOI] [PubMed] [Google Scholar]
- 73.Abramson JL, Lewis C, Murrah NV. Body mass index, leptin, and ambulatory blood pressure variability in healthy adults. Atherosclerosis. 2011;214:456–461. doi: 10.1016/j.atherosclerosis.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 75.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Mitogen activated protein kinase: a newly discovered mediator of selective leptin actions. Hypertension. 2005;46:867. [Google Scholar]
- 76.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 77.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 78.Fruhbeck G. Intracellular signaling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 80.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39:486–490. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 81.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 82.Haynes WG, Morgan DA, Djalali A, Sivitz W, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 83.Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens. 2002;20:1633–1641. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 84.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 85.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 86.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 87.Rhamouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lund IK, Hansen JA, Andersen HS, Moller NP, Billestrup N. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signaling. J Mol Endocrinol. 2005;34:339–351. doi: 10.1677/jme.1.01694. [DOI] [PubMed] [Google Scholar]
- 89.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–118. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 90.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;4:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 91.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefaly WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1 contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Meyers MG, Jr, Ozan U. Endoplasmic reticulum plays a central role in development of leptin resistance. Cell Metabolism. 2009;9:31–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85 alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;4:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agata J, Masuda A, Takada M, Hiashura K, Murakami H, Miyazaki Y, Shimamoto K. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997;10:1171–1174. doi: 10.1016/s0895-7061(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 95.Matsubara M, Chiba H, Maruoka S, Katayose S. Elevated serum leptin concentrations in women with components of multiple risk factor clustering syndrome. J Atheroscler Thromb. 2000;7:231–237. doi: 10.5551/jat1994.7.231. [DOI] [PubMed] [Google Scholar]
- 96.Barba G, Russo O, Siani A, Iacone R, Farinaro E, Gerardi MC, Russo P, Della Valle E, Strazzullo P. Plasma leptin and blood pressure in men: graded association independent of body mass and fat pattern. Obes Res. 2003;11:160–166. doi: 10.1038/oby.2003.25. [DOI] [PubMed] [Google Scholar]
- 97.Alvarez-Aguilar C, Mondragon-Jimenez LI, Ramirez-Enriquez J, Gomez-Garcia A, Paniagua-Sierra R, Amato D. Hyperleptinemia as a risk factor in obesity-related hypertension. Med Clin. 2004;123:766–769. doi: 10.1157/13069809. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura Y, Ueshima H, Okuda N, Murakami Y, Miura K, Kita Y, Okamura T, Turin TC, Rodriguez B, Curb JD, Stamler J. Relation of serum leptin to blood pressure of Japanese in Japan and Japanese-Americans in Hawaii. Hypertension. 2009;54:1416–1422. doi: 10.1161/HYPERTENSIONAHA.109.133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kramer CK, von Muhlen D, Barrett-Connor E. Does leptin predict incident hypertension in older adults? Clin Endocrinol. 2010;73:201–205. doi: 10.1111/j.1365-2265.2010.03781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 101.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes Res. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 102.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banglore S, Parkar S, Grossman E, Messerli F. A meta-analysis of 94492 patients with hypertension treated with beta-blockers to determine the risk of new-onset diabetes mellitus. J Am Coll Cardiol. 2007;100:1254–1262. doi: 10.1016/j.amjcard.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 104.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Ledeballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 105.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Östergren J for the ASCOT investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required, in the Anglo-Scandinavian Cardiac Outcomes Trial Blood-Pressure Lowering Arm (ASCOT-BPLA): a multicenter randomized controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 106.Simone G, Wachtell K, Palmieri V, Hille DA, Beevers G, Dhlof B, Faire U, Fyhrquist F, Ibsen H, Julius S, Kjeldsen SE, Laderballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Devereux RB. Body build and risk of cardiovascular events in hypertension and left ventricular hypertrophy: the LIFE (Losartan Intervention for Endpoint reduction in hypertension) Study. Circulation. 2005;111:1924–1931. doi: 10.1161/01.CIR.0000161799.91577.0A. [DOI] [PubMed] [Google Scholar]
- 107.UKPDS. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications of type 2 diabetes: UKPDS 39. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 108.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 109.Grassi G, Trevano FQ, Facchini A, Toutouzas T, Chanu B, Mancia G. Efficacy and tolerability profile of nebivolol vs atenolol in mid-to-moderate essential hypertension: results of a double-blind randomized multicentre trial. Blood Press Suppl. 2003;2:35–40. [PubMed] [Google Scholar]
- 110.Correia ML, Haynes WG. Emerging drugs for obesity: linking novel biological mechanisms to pharmaceutical pipelines. Expert Opin Emerg Drugs. 2005;10:643–660. doi: 10.1517/14728214.10.3.643. [DOI] [PubMed] [Google Scholar]
- 111.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretics versus beta-blocker as first-step antihypertensive therapy: the final results from the antihypertensive therapy. The final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 112.Cushman WC, Ford CE, Einhorn PT, Wright JT, Preston RA, Davis BR, Basile JN, Whelton PK, Weiss RJ, Bastien A, Courtney DL, Hamilton BP, Kirchner K, Louis GT, Retta TM, Vidt DG for the ALLHAT Collaborative Research Group. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2008;10:751–760. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright JT, Harris-Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, Basile JN, Black HR, Dart R, Gupta AK, Hamilton BP, Einhorn PT, Haywood J, Jafri SZA, Lois GT, Whelton PK, Scott CL, Simmons DL, Stanford C, Davis BR. Clinical outcomes by race in patients with or without the metabolic syndrome ALLHAT. Arch Intern Med. 2008;168:207–217. doi: 10.1001/archinternmed.2007.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barzilay JI, Davis BR, Bettencourt J, Margolis KL, Goff DC, Black H, Habib G, Ellsworth A, Force RW, Wiegman T, Ciocon JO, Basile JN. Cardiovascular outcomes using doxazosin vs chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT Study. J Clin Hypertens. 2004;6:116–125. doi: 10.1111/j.1524-6175.2004.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Topal E, Cikim AS, Cikim K, Temel I, Ozdemir R. The effect of moxonidine on endothelial dysfunction in metabolic syndrome. Am J Cardiovasc Drugs. 2006;6:343–348. doi: 10.2165/00129784-200606050-00007. [DOI] [PubMed] [Google Scholar]
- 116.Reisin E, Weir MR, Falkner B. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo-controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension. 1997;30(1 Part 1):140–145. doi: 10.1161/01.hyp.30.1.140. [DOI] [PubMed] [Google Scholar]
- 117.Jordan J, Engeli S, Boye SW, Le Breton S, Keefe DL. Direct renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension. 2007;49:1047–1055. doi: 10.1161/HYPERTENSIONAHA.106.084301. [DOI] [PubMed] [Google Scholar]
- 118.Sowers JR, Raij L, Jiall I, Egan BM, Ofili EO, Zappe DH, Purkayastha D, Deedwanis PC. Angiotensin receptor blocker/diuretic combination preserves insulin responses in obese hypertensives. J Hypertens. 2010;28:1761–1799. doi: 10.1097/HJH.0b013e32833af380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jordan J, Engeli S, Boschmann M, Weidinger G, Luft FC, Sharma AM, Kreuzberg U. Hemodynamic and metabolic responses to valsartan and atenolol in obese hypertensive patients. J Hypertens. 2005;23:2313–2318. doi: 10.1097/01.hjh.0000188734.98463.82. [DOI] [PubMed] [Google Scholar]
- 120.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 121.Jansen PM, Danser JAH, Spiering W, Meiracket AH. Drug mechanisms to help in managing resistant hypertension in obesity. Curr Hypertens Rep. 2010;12:220–225. doi: 10.1007/s11906-010-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 123.Ernst ME, Carter BL, Goerdt C, Steffensmeir JJG, Philips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 124.Cooper-Dehoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, Gong Y, Hall K, Parekh V, Chapman AB, Boerwinkle E, Johnson JA. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2010;55:61–68. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gentille CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in non-obese humans. Am J Physiology Regal Integra Comp Physiology. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 126.Andersson B, Elam M, Wallin BG, Bjorntorp P, Andersson OK. Effect of energy-restricted diet on sympathetic muscle nerve activity in obese women. Hypertension. 1991;18:783–789. doi: 10.1161/01.hyp.18.6.783. [DOI] [PubMed] [Google Scholar]
- 127.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 128.Trombetta IC, Batalha LT, Rondon MUPB, Laterza MC, Kuniyoshi FHS, Gowdak MMG, Barretto ACP, Halpern A, Villares SMF, Negrao CE. Weight loss improves neurovascular muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–H982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- 129.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 130.Neter JE, Stam BE, Kok FJ, Robbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 131.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR and the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 132.Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett S, Kaplan RA, Comstock J, Lucas CP, Lodewick PA, Canovatchel W, Chung J, Hauptman J. Role of orlistat in the treatment of obese patients with type 2 diabetes. Diabetes Care. 1998;21:1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- 133.Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M. A randomized double-blind placebo-controlled study of the long term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Metab Disord. 2004;28:1399–1410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- 134.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 135.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomized, double-blind, placebo-controlled, phase 5 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 136.The Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sjostrom L, Narbro K, Sjostrom D, Karason K, Larrsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgreen S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Senlof K, Torgerson J, Agren G. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 138.James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal L, Maggioni AP, Torp-Pedrsen C, Sharma AM, Shepherd GM, Rode RA, Renz CL SCOUT Investigator Group. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 139.Agapitov AV, Correia ML, Sinkey CA, Phillips BG, Haynes WG. Weight loss does not alter sympathetically-mediated vascular tone in normotensive obese humans. Pharmacotherapy. 2002;22:1329–1330. [Google Scholar]
- 140.Correia ML, Agapitov A, Sinkey CA, Haynes WG. Vasodilatation induced by beta2 adrenergic receptor activation is impaired in obese hypertensives before and after weight loss. J Hypertens. 2010;28:e437. [Google Scholar]
- 141.Nogueiras R, Perez D, Christelle VD, Varela L, Haynes WG, Patterson JT, Disse E, Pfluger PT, Lopez M, Woods SC, DiMarchi R, Diegues C, Rahmouni K, Rohner-Jeanrenaud F, Tschop MH. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet induced obesity. J Neurosci. 2009;29:5916–5925. doi: 10.1523/JNEUROSCI.5977-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Astrup A, Bosser S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Efffects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled stud. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 143.Toplak H, Haman A, Moore A, Masson E, Gorska M, Vercryusse F, Sun X, Fitchet M. Efficacy and safety of topiramate in combination with metformin in the treatment of the obese subject with type 2 diabetes: a randomized, double-blind, placebo controlled study. Int J Obes. 2007;31:138–146. doi: 10.1038/sj.ijo.0803382. [DOI] [PubMed] [Google Scholar]
- 144.Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147:5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 145.Roth JD, Roland BL, Cole R, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amylin Pharmaceuticals USA. Pramlintide Acetate Prescribing Information. San Diego, CA: Amylin Pharmaceuticals USA; 2008. Revised July 2008. [Google Scholar]
- 147.Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity. 2010;18:1739–1746. doi: 10.1038/oby.2009.478. [DOI] [PubMed] [Google Scholar]
- 148.Wulffele´ MG, Kooy A, Zeeuw D, Stehouwer CDA, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med. 2004;256:1–14. doi: 10.1111/j.1365-2796.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 149.Holman RR, Paul SK, Bethel A, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in Type 2 diabetes. N Engl J Med. 2008;359:1–13. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 150.Diabetes Prevention Program Research Group. Intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.O’brien PE, Dixon JB, Laurie C, Skinner S, Rioretto J, Mcneil JM. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding. Ann Intern Med. 2006;144:625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 152.Hofso D, Nordtrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, Bollersev J, Godang K, Sandbu R, Roilsien J, Hjelmeseth J. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735–745. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Erondu N, Addy C, Kaifeng L, Mallick M, Musser B, Gantz I, Prioretto J, Astrup A, Toubro S, Rissannen A, Tonstad S, Haynes WG, Gottesdiener K, Kaufan KD, Amatruda JM, Heymsfield SB. NPY5R Antagonism does not augment the weight loss efficacy of orlistat vs sibutramine. Obesity. 2007;15:2027–2042. doi: 10.1038/oby.2007.242. [DOI] [PubMed] [Google Scholar]
- 154.Endocrinologic and Metabolic Drugs Advisory Committee Meeting. Gaithersburg, MD: 2010. United States Food and Drug Administration (FDA) NDA 22580 Briefing Documents. [Google Scholar]
- 155.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12:876–882. doi: 10.1111/j.1463-1326.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 156.Cercato C, Roizenblatt VA, Leanca CC, Segal A, Lopes Filho AP, Mancini MC, Halpern A. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes. 2009;33:857–865. doi: 10.1038/ijo.2009.124. [DOI] [PubMed] [Google Scholar]
- 157.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 158.Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Kempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 159.Torgerson JS, Boldrin MN, Hauptman J, Sjostrom L. Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study: a randomize study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 160.Miles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, Forey T, Aronne L, Klein S. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care. 2002;25:11123–11128. doi: 10.2337/diacare.25.7.1123. [DOI] [PubMed] [Google Scholar]
- 161.Buse JB, Rosenstock J, Sesti G, Schimidt WE, Montanya E, Brett JH, Zychma M. Blonde L, LaEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;10:633–641. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 162.Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, Perry BH. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 163.Tonstad S, Tykarski A, Weissgarten J, Ivleva A, Levy B, Fitchet M. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243–251. doi: 10.1016/j.amjcard.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 164.Stenlof K, Rossner S, Verruysse F, Kumar A, Fitchet M, Sjostrom L. Topiramate in the treatment of obese subjects with drug-naı¨ve type 2 diabetes. Diabetes Obes Metab. 2007;9:360–368. doi: 10.1111/j.1463-1326.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 165.Gadde KM, Franciscy DM, Wagner IIHR, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289:1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]