Abstract
The exocrine pancreas is the organ with the highest level of protein synthesis in the adult—each day the pancreas produces litres of fluid filled with enzymes that are capable of breaking down nearly all organic substances. For optimal health, the pancreas must produce sufficient enzymes of the right character to match the dietary intake. Disruption of normal pancreatic function occurs primarily as a result of dysfunction of the acinar cells that produce these digestive enzymes, and can lead to acute or chronic diseases. For many years, the prevailing dogma has been that inappropriate intracellular activation of the digestive enzymes produced by acinar cells was the key to pancreatic inflammatory diseases, as digestive enzymes themselves are potentially harmful to the cells that secrete them. However, we now know that many stressors can affect pancreatic acinar cells, and that these stressors can independently trigger pancreatic pathology through various mechanisms. This Review focuses on protein synthesis and active digestive enzymes—two key stressors faced by the acinar cell that are likely to be the major drivers of pathology encountered in the pancreas.
Introduction
A well-functioning pancreas is easy to ignore. Situated deep within the recesses of the retroperitoneal cavity, the pancreas is out of the range of touch and its silent exocrine and endocrine functions occur below the level of consciousness. The pancreas acts as both an endocrine gland secreting hormones such as insulin directly into the blood, and as an exocrine gland secreting digestive enzymes into the duodenum. Stress and responses to stress in the pancreas are the triggers for disease, as they are in other organs. However, to understand pancreatic diseases, we must consider the function of the healthy organ and the stresses to which it is exposed.
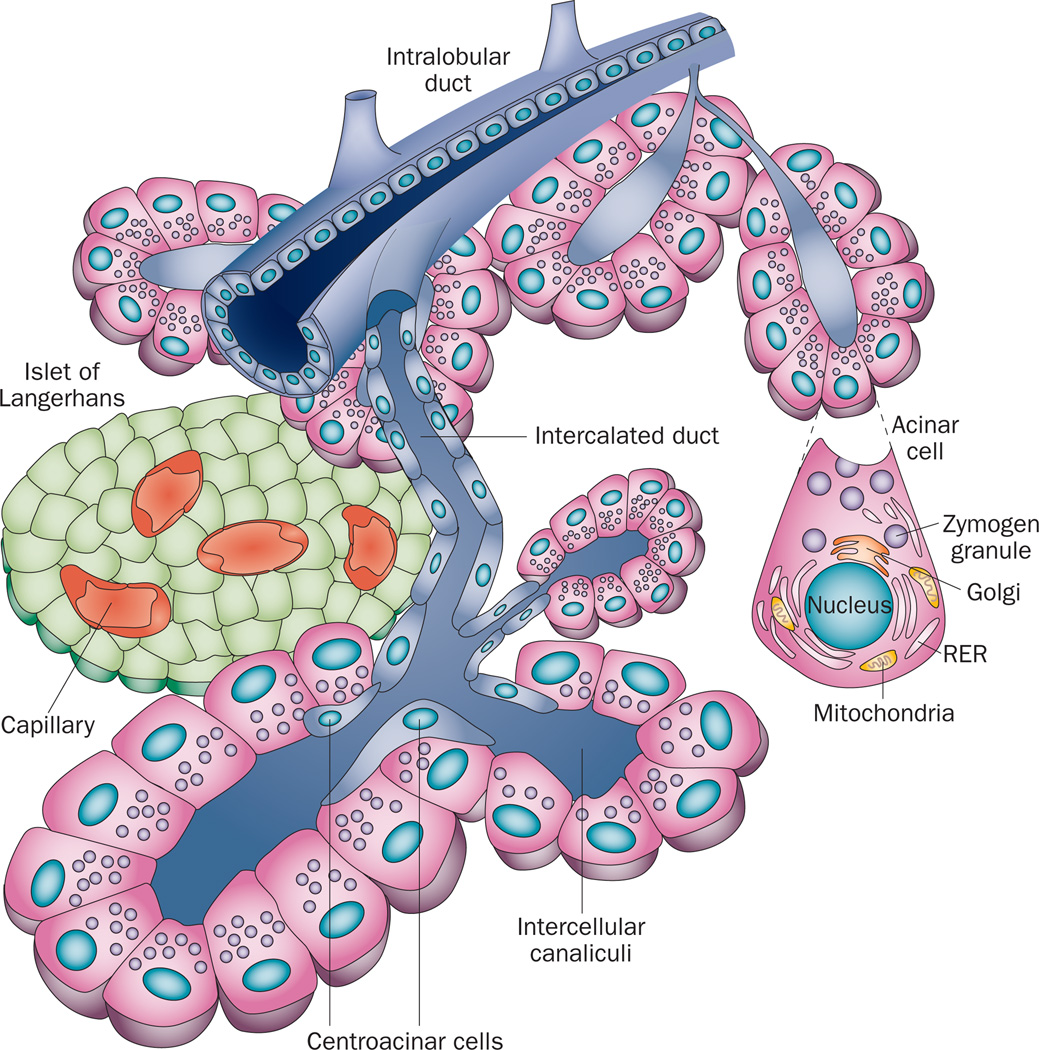
The exocrine pancreas is composed of acinar cells and ductal structures (Figure 1). The acinar cell is the workhorse of the exocrine pancreas—its primary responsibility is the production, storage and regulated secretion of the large amounts of enzymes necessary for the proper digestion and absorption of food. To this end, pancreatic acinar cells produce and secrete more protein than any other adult cell type.1 The processes of digestive enzyme gene expression, protein synthesis, storage and secretion are influenced by external inputs from nerves and hormones, as well as mechanisms within the acinar cells. Each of these processes is finely tuned, such that the production and delivery of digestive enzymes closely matches the dietary need.
Figure 1.
The components of the pancreas. The pancreas consists of endocrine cells localized within structures named the Islets of Langerhans, which contain multiple endocrine cell types including the β cells that secrete isulin, and the exocrine pancreas, which is composed of acinar calls and ductal structures. Pancreatic acinar cells form a basic structure called an acinus that surrounds a central lumen open to the duct system. Pancreatic acinar cells produce, store and secrete enzymes necessary for the digestion and absorption of food in the small intestine. Digestive enzymes are secreted through the apical membrane of the acinar cell into small intercalated ducts that are directly connected to increasingly larger intralobular ducts that join the main pancreatic duct. The main pancreatic duct joins the common bile duct just prior to the ampulla of Vater, where both pancreatic and liver products enter the small intestine. Blockage of the passage of materials through the ampulla of Vater, for example by the lodging of a bile stone or by the growth of a tumor, leads to increased pressure in the duct system and gives rise to acute pancreatitis.
Critical secretions from the pancreas are delivered to the small intestine through a series of duct structures. Small intercalated ducts directly connect the acinar lumen to intralobular ducts residing within the subdivisions of the pancreas and progress through increasingly larger interlobular ducts and finally into the main pancreatic duct, which joins with the bile duct to form the common bile duct (Figure 1). Interference with the free passage of pancreatic secretions through this duct system is highly problematic. For example, gallstones lodged in the common bile duct are one of the most common aetiologies of acute pancreatitis. Blockage of bile secretions, owing to occlusion of the duct caused by the growth of a tumor mass, is one of the primary symptoms of pancreatic cancer. Thus, both the acinar and ductal components of the exocrine pancreas are required to function properly to avoid injury.
Under physiological conditions, the pancreatic acinar cells are subjected to high levels of stress, which is primarily due to their high level of protein synthetic and secretory activity, and because the proteins they produce are digestive enzymes that, if prematurely activated, are capable of damaging vital cellular components. Lifestyle choices such as the consumption of alcohol, diets high in fat, or smoking further increase the stress on acinar cells. Fortunately, the acinar cell has well-developed coping mechanisms that compensate for most of these stressors, and generally the pancreas functions without any apparent problems. However, increased severity of one type of stress increases the risk of injury to the pancreas when additional stressors are encountered. When the pancreatic coping mechanisms reach their limits and are overwhelmed, or if the actions of the coping mechanisms themselves become excessive, acinar cell damage results.
Pancreatic damage can be acute or chronic. Acute damage is associated with the generation of an inflammatory response, which can be mild and localized to the pancreas (acute pancreatitis, which accounts for a large volume of hospital admissions), or severe and systemic. The systemic inflammation resulting from pancreatic disease can lead to multiple organ failure and mortality. Chronic damage to the pancreas results in chronic pancreatitis with acinar cell destruction and replacement with fibrosis or fat, which if extensive, leads to exocrine insufficiency. Chronic damage also increases the probability of genetic instability and thus the risk of developing pancreatic cancer, especially pancreatic ductal adenocarcinoma (PDAC). Although this disease is fortunately fairly rare, PDAC ranks the fourth leading cause of cancer-related death in the USA.2 Currently, no effective treatments beyond surgery exist for either PDAC or pancreatitis, two diseases associated with severe pain.
This Review provides an overview of the current understanding of the sources of, and responses to, typical stressors encountered by pancreatic acinar cells. We focus on the two major stressors that affect pancreatic acinar cells: endoplasmic reticulum (ER) stress and digestive enzyme stress, since excessive levels of these two stresses are likely to be responsible for many pancreatic pathologies. We also outline the pancreatic acinar cell coping mechanisms that have evolved to compensate for these two stressors. This Review does not, however, describe other stresses, such as infection or duct blockage, that are directly pathological.
Endoplasmic reticulum stress
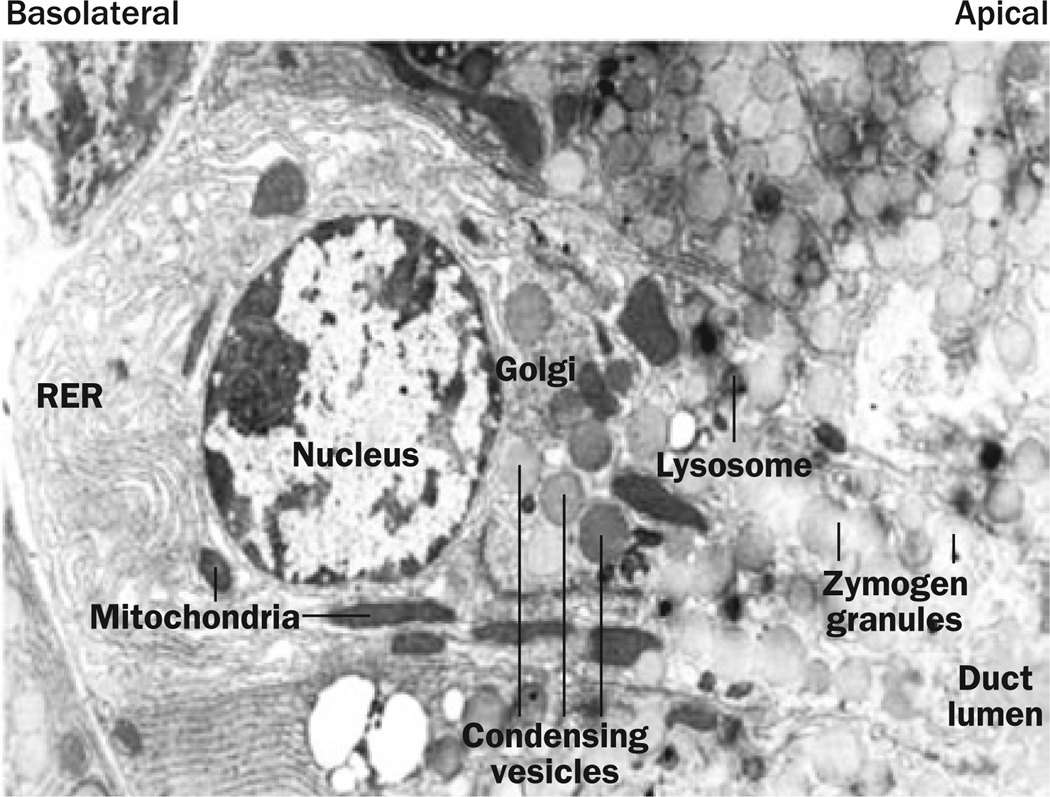
The primary function of the pancreatic acinar cell is the synthesis and secretion of digestive enzymes, which are destined for food digestion within the small intestine. In acinar cells, secretory protein transcripts pass across the ER membrane and are then processed within the ER, where folding, glycosylation and disulphide bond formation occurs.3 Thus, the ER forms the first compartment of the secretory pathway, which is then followed by the Golgi apparatus, condensing vacuoles and secretory granules (Figure 2).
Figure 2.
The structure of the pancreatic acinar cell. Pancreatic digestive enzymes are processed within the RER located primarily in the basal domain of these cells. The RER is where folding, glycosylation and disulphide bond formation occurs during protein synthesis. From here, the digestive enzymes are transported to the Golgi where they are sorted and packaged into condensing vesicles. The condensing vesicles are compacted to form mature zymogen granules and are transported to the apical end of the cell in position for regulated secretion into the duct lumen. Abbreviation: RER, rough endoplasmic reticulum. Permission obtained from Nature Publishing Group © Adapted from Whitcomb, D. C.
The proper functioning of proteins depends upon their assuming the correct 3D shape. Protein folding in the ER is affected by many parameters, including pH, calcium levels, oxidative stress and availability of chaperones, foldases and protein disulphide isomerases. Many variables can, therefore, influence the functioning of the ER and can cause stress in this organelle in suboptimal conditions. Furthermore, alterations in protein sorting (conducted in the Golgi apparatus) and trafficking to various cellular compartments and organelles influences cell stress,4 suggesting that the intracellular distribution of these proteins influences a variety of cellular functions, including secretion and autophagy.
Proteins that do not fold into their appropriate 3D configuration are a well-known cause of ER stress. The ER has, however, evolved a set of sensitive mechanisms that can detect the presence of such misfolded proteins.5 A major component of this system is the chaperone protein GRP78 (78 kDa glucose-regulated protein), which detects exposed hydrophobic residues on the surface of nascent proteins. A properly folded protein has few such exposed residues, whereas a misfolded protein has many. Similarly, calreticulin and calnexin are sensors of the glycosylation status of proteins that also influence protein 3D folding and stability. Incorrectly folded or glycosylated proteins are initially retained in the ER, bound to chaperones, to enable completion of the folding process. However, if correct folding cannot be achieved, the misfolded proteins are translocated into the cytosol, where they are targeted for proteasomal breakdown in a process referred to as ER-associated protein degradation (ERAD).6,7 Additionally, in an autophagy-related, proteasome-independent mechanism of protein degradation, misfolded proteins are transported from the ER to lysosomes.8 This pathway might be of particular importance in acinar cells, as lysosomes contain cathepsins that can activate inactive digestive enzyme precursors (zymogens) to their active forms. Moreover, lysosomes are thought to be the cellular compartment where digestive enzymes are prematurely activated during acute pancreatitis.9
ER stress coping mechanisms
The unfolded protein response
A major component of the ER stress response is the unfolded protein response (UPR), which maintains equilibrium between the capacity and demand for protein folding within the ER.10 At low-to-moderate levels of ER stress, the UPR increases the folding capacity of the ER by enlargement of the ER11 and increased synthesis of molecular chaperones and foldases.12 Among the chaperones induced by ER stress are several members of the heat shock family of proteins, which have protective effects attributed to decreased trypsinogen activation,13 and preservation of the acinar cytoskeleton14 in the context of pancreatitis.13–16 Thus, heat shock proteins are able to reduce and prevent ER stress as well as ER-stress-generated apoptosis.17,18 Persistent pressure on the folding capacity of the ER leads to induction of additional compensatory measures. For example, as might be expected, increased demand for digestive enzymes initiated by diets high in protein leads to hypertrophy of pancreatic acinar cells.19
The ER overload response
The ER overload response (EOR) is initiated when there is an excessive intracellular build-up of proteins, as occurs during a viral infection. The EOR activates the NF-κB signalling cascade,20 probably via IRE1.21 Activation of NF-κB signalling occurs by phosphorylation and subsequent proteolytic degradation of NF-κB inhibitor IκBα.22 Another connection between the ER and the NF-κB pathway is likely to be intracellular calcium levels. Studies using intracellular calcium chelators suggest that release of calcium from the ER is required for EOR-mediated activation of NF-κB signalling.23 Furthermore, raising the cytosolic calcium concentration (by inhibition of the ER calcium ATPase, using thapsigargin or cyclopiazonic acid) also potently induces the NF-κB pathway.24 How the accumulation of proteins in the ER might increase its calcium permeability is currently unclear, although emerging evidence points to a role for HERPUD1, a protein with a ubiquitin- like domain that facilitates proteasome-mediated degradation of ER-resident calcium release channels.25
Reactive oxygen species (ROS) are likely to be among the inducers of ER stress that lead to NF-κB pathway activation, as antioxidants reduce NF-κB pathway activation. Thus, ROS might behave as a second messenger connecting calcium release from the ER to NF-κB activation.24,26 ROS are generated as a result of the intrinsic peroxidase activity of several ER-related enzymes, such as glutathione peroxidase, as well as intermediates formed as a result of cyclo-oxygenase and lipoxygenase activity. Treatment with the peroxidase inhibitor tepoxalin abrogated the increased production of reactive oxygen intermediates in response to Ca2+ release by thapsigargin.24 ROS generated by ER peroxidases are, therefore, likely to be involved in the EOR-mediated induction of NF-κB activity.
Overlap and crosstalk clearly occurs between the two main ER stress response pathways, UPR and EOR. Both are sensitive to ROS and changes in ER calcium levels. For example, calcium release during activation of the EOR can alter conditions inside the ER, leading to increased amounts of misfolded proteins and activation of the UPR. The UPR is also able to activate NF-κB by a unique mechanisms involving translational repression of the inhibitory subunit IKBα.27
Consequences of chronic ER stress
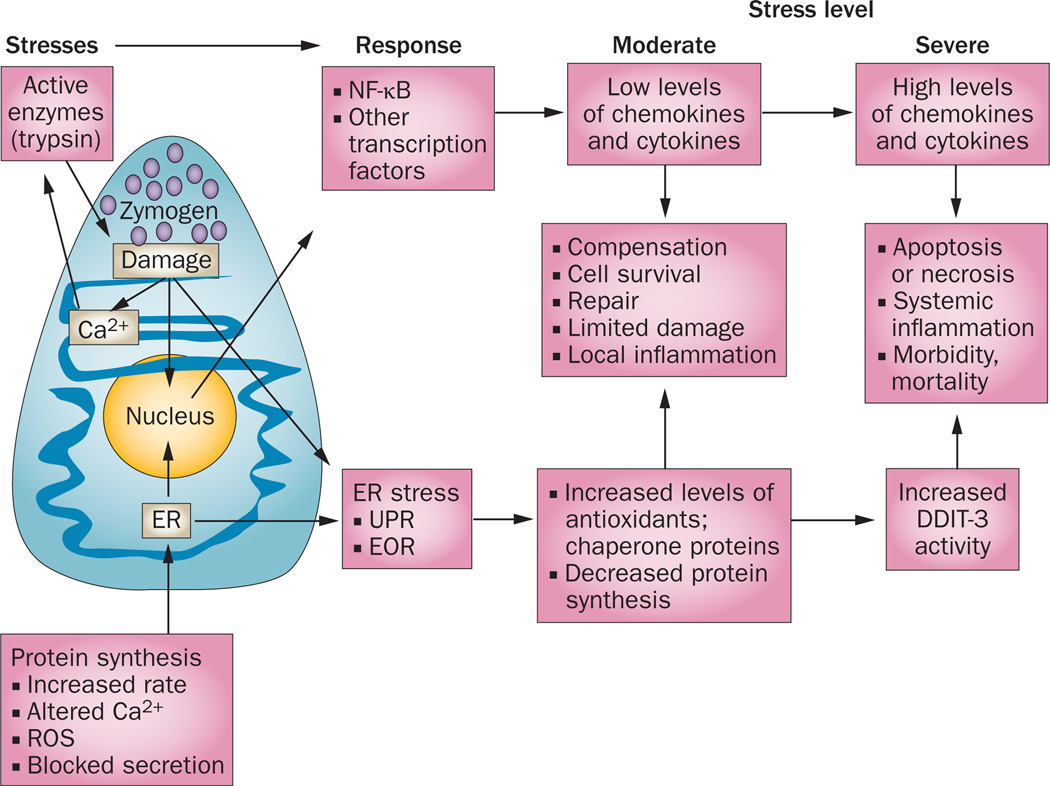
The accumulation of misfolded proteins is unhealthy for living cells. ER stress response mechanisms such as the UPR protect against the immediate consequences of ER stress by limiting the accumulation of misfolded proteins through translational attenuation, improving protein folding, increasing the levels of ER-resident chaperones, and inducing ERAD to clear misfolded molecules. Collectively, these processes are adaptive and protective mechanisms for cells to survive various stressors (Figure 3).
Figure 3.
ER stress responses that occur in pancreatic acinar cells. The very high rate of protein synthesis in acinar cells generates a constant low level of ER stress. Under normal conditions, this ER stress is readily compensated for by the UPR and EOR, which reduce the rate of protein synthesis while increasing production of molecules that facilitate cell recovery and repair. However, under conditions of increased ER stress, EOR and UPR lead to apoptosis. Excessive acinar cell apoptosis can lead to necrosis, a severe systemic inflammatory response, acute respiratory distress, and life-threatening multiorgan failure. Altered Ca2+ release from the ER, and ROS, are thought to activate NF-κB, a key regulator of inflammatory gene expression. Abbreviations: DDIT-3, DNA damage-inducible transcript 3 protein; EOR, ER overload response; ER, endoplasmic reticulum; ROS, reactive oxygen species; UPR, unfolded protein response.
Excessive exposure to chronically elevated ER stress will cause cell damage and these cells are eliminated by the induction of apoptosis, a mechanism that is protective from necrosis and inflammation at low levels. However, high levels of apoptosis overwhelm the capacity of the immune system to clear dead cells, resulting in necrosis. The link between ER stress and apoptosis involves several molecules, including the transcription factor DDIT-3, caspase 12, apoptosis regulator BAX, IRE1 and MAP kinases.28 DDIT-3 promotes cell-cycle arrest and/or apoptosis by regulating the expression of a variety of genes, including apoptosis regulator Bcl-2 family members, and by inducing ROS through the depletion of cellular glutathione.29 ER stress can also trigger mitochondrial mechanisms involved in apoptosis. The ER and mitochondria are interconnected and cooperate to control intracellular calcium levels.30 Movement of calcium from the ER into the mitochondria induces stress signals and can activate opening of the mitochondrial permeability transition pore, causing mitochondrial dysfunction and leading to the release of cytochrome c and downstream caspases 9 and 3. Increased mitochondrial sequestration of calcium also stimulates the generation of ROS, which contribute to opening of the mitochondrial permeability transition pore.31 ROS also promote and magnify calcium flux from the ER into mitochondria. Decreased calcium concentrations in the ER lumen result in increased misfolding of proteins and activation of the ER stress signalling pathways;31 collectively, these actions can result in a vicious cycle of increased ER stress and ROS formation that leads to irreversible damage to the cells. Increased ROS can also activate NF-κB and contribute directly to the development of local and systematic inflammation. NF-κB alters the transcription of a wide variety of genes, many of which promote inflammation or cell survival. Thus, like most coping mechanisms, the NF-κB pathway is a double-edged sword—although it helps pancreatic acinar cells to survive the stress associated with protein synthesis rates that exceed the capacity of the folding system, when NF-κB activity is too high, it leads to inflammation and other changes that ultimately damage these cells.32
Evidence of ER stress in pancreatic disease
Evidence of ER stress is present in all models of experimentally induced pancreatitis. Changes in the structure of the ER have been observed early in the development of acute pancreatitis. For example, vesiculation of the ER occurs within minutes of retrograde intraductal injection of sodium taurocholate into the pancreatic duct.33 In another model of pancreatitis induced by intraperitoneal injection of arginine, the ER cisternae showed partial dilatation or vacuolation,34 whereas pancreatic duct ligation in the American opossum causes acute necrotizing pancreatitis associated with extensive dilatation of the rough ER.35 Treatment with tauroursodeoxycholic acid, an ER chaperone, also reduced ER stress, acinar cell damage and systemic inflammation in rat models of acute pancreatitis.36 Although it is difficult to confirm that these early events also occur in human pancreatitis, changes in pancreatic ER have been noted in human pancreata under acute shock situations.37 These observations strongly suggest that ER stress is an early feature in pancreatitis, and is a reaction of acinar cells to a variety of different stressors.
Another indication that ER stress is prevalent in acute pancreatitis is that multiple ER stress response genes show altered expression during acute pancreatitis. Gene expression profiling studies in several rat models of acute pancreatitis have indicated that a complex pattern of alterations in gene expression occurs very early on during pancreatic acinar cell stress, both in vivo and in vitro.38 In particular, several ER-resident chaperones were upregulated in caerulein-induced and taurocholate-induced pancreatitis, including uracil diphosphate (UDP)-glycosyltransferase, UDP-glucose dehydrogenase, several proteins of the DnaJ homologue family, heme oxygenase 1, and reticulocalbin 2.
Evidence also suggests that ER stress might even, in some patients, be linked to hereditary pancreatitis,39 a disease that is generally caused by hyperactivation of, or mutations in, trypsin-1. A 346C>T mutation (Arg116Cys), in the human PRSS1 gene (which encodes trypsin-1, also known as cationic trypsinogen), generates an unpaired Cys residue, causing incorrect disulphide bond formation and misfolding of the protein. The misfolded protein showed no important differences in activation or degradation compared with wild-type trypsinogen; however, secretion of the Arg116Cys mutant protein was dramatically reduced, with much of the misfolded protein present in an insoluble form in cell extracts.39 As expected, expression of this misfolded protein was associated with elevated levels of ER stress markers, suggesting that ER stress (rather than aberrant trypsin activity) might be involved in the initiation of some mutant-trypsinogen-induced forms of hereditary pancreatitis.
ER stress might also be involved in alcohol-induced pancreatic disease. High alcohol consumption is the major aetiological factor in many patients with pancreatitis, both acute and chronic. However, most individuals who abuse alcohol do not develop pancreatitis. Thus, although alcohol consumption increases the risk of pancreatitis, it is not sufficient to cause development of the disease. One possibility is that alcohol consumption increases acinar cell stress, but that coping mechanisms are generally able to compensate for it. Evidence that alcohol consumption induces ER stress came to light from studies that demonstrated that rats placed on an alcohol-containing diet have altered expression levels of several ER stress response genes.40 A subsequent study in mice, which showed that ethanol activated the UPR—as indicated by increased levels of the UPR sensor Xbp1 (X-box binding protein 1)—confirmed that alcohol intake could influence the expression of ER stress response genes.41 Furthermore, Xbp1 levels were substantially lower in ethanol-fed Xbp1+/− mice than in ethanol-fed wild-type mice, and ER stress was associated with a histologically altered ER, decreased numbers of zymogen granules, increased numbers of autophagic vacuoles and increased acinar cell death. These data support a model in which ethanol stresses the acinar cell by perturbing ER function, which increases the activity of an ER stress coping mechanism (the UPR). Disruption or hyperactivation of this coping mechanism is likely to result in acinar cell damage and pancreatitis.
It is worth emphasizing that combinations of several stressors, each of which alone does not cause obvious damage, can lead to the development of severe pancreatitis. Support for this concept comes from experiments that demonstrate that an alcohol diet that is insufficient to cause pancreatitis in wild-type mice can cause pancreatitis in mice that express mutant digestive enzymes (C. D. Logsdon, unpublished work). These findings provide an explanation for observations that neither alcohol consumption nor inherited trypsinogen mutations lead to 100% penetrance of pancreatitis. In most people, therefore, the physiological coping mechanisms are able to deal with the presence of one major stressor.
Digestive enzyme activity stress
The products produced by healthy pancreatic acinar cells are essential for proper digestion and absorption of ingested foodstuffs. However, the digestive enzymes produced by these cells are capable of damaging the pancreas if prematurely activated inside the cells, and multiple protective mechanisms have consequently evolved to reduce the danger. First, proteases, the digestive enzymes most capable of harming the acinar cell, are produced as precursor molecules (zymogens) including inhibitory domains that keep them inactive. Physiologically, these inhibitory domains are removed in the small intestine by a cascade of enzymatic cleavage steps, starting with enteropeptidase-mediated cleavage of trypsinogen into the active enzyme, trypsin. Trypsin is then able to cleave and activate the other zymogens, as well as trypsinogen itself. However, the zymogens (including trypsinogen) are capable of some level of autoactivation, depending upon local environmental conditions, such as enzyme concentration, pH and calcium levels.42 Second, zymogens are packaged within acinar cells into a membrane-enclosed compartment, the zymogen granule. This packaging prevents access of these molecules to sensitive cytoplasmic proteins, even if some zymogens do become activated. The acinar cell produces various inhibitory molecules (including pancreatic secretory trypsin inhibitor [PSTI] protein, also known as SPINK1, which can block trypsin activity) that are packaged into zymogen granules along with the digestive enzymes. In addition, the digestive enzymes themselves are subject to protease degradation, which terminates their activity. Specialized enzymes are critical mediators of the destruction of digestive enzymes. In particular, chymotrypsin-C has been identified as a major regulator of trypsin degradation within acinar cells in humans.43,44
Digestive enzyme activity coping mechanisms
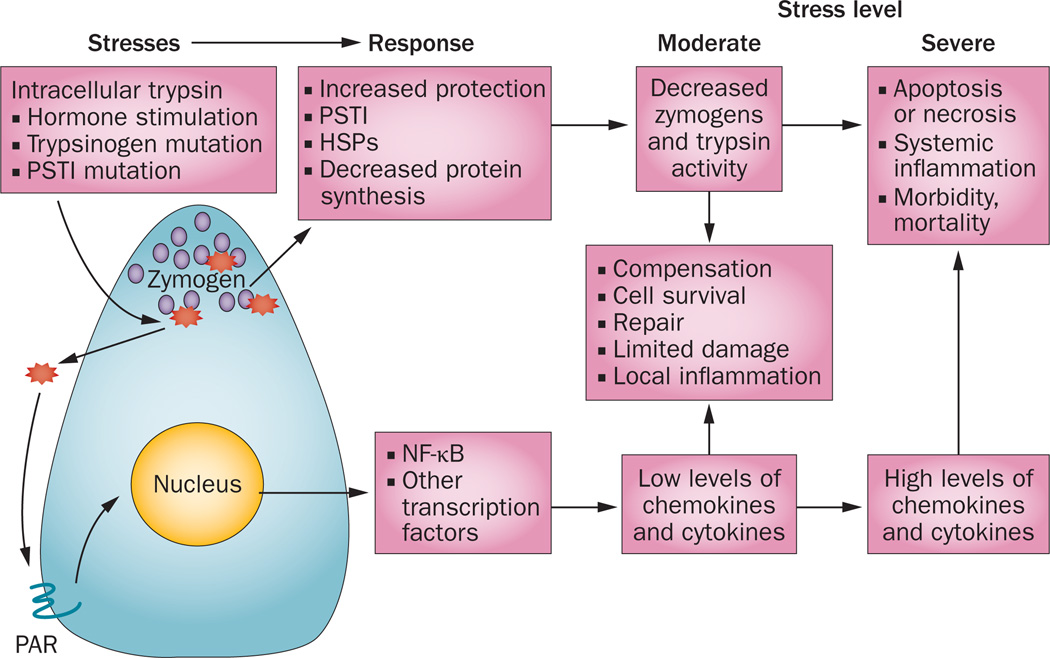
Several mechanisms have evolved to mitigate the dangers posed by inappropriate activation of digestive enzymes within the pancreas (Figure 4). Animals engineered to express a mutant trypsinogen that becomes activated within the trans-Golgi network of the secretory pathway showed a transient loss of zymogen granules, which was followed by complete recovery of granule production despite continued expression of this mutant.45 This observation suggests that pancreatic acinar cells can upregulate protective mechanisms. However, as yet, these mechanisms have not been identified. Reasonable candidates include the upregulation of trypsin inhibitors such as pancreatic secretory trypsin, or an increase in trypsin-degrading enzymes, such as chymotrypsin-C. Trypsin-stimulated induction of apoptosis might be another potential coping mechanism; high levels of intracellular trypsin activity can cause acinar cells to undergo apoptosis.45,46 Apoptosis is a means of both eliminating unhealthy cells and limiting the inflammatory cascade.
Figure 4.
Digestive enzyme stress responses in pancreatic acinar cells. Digestive enzymes produced by acinar cells are normally inactive until exported, but their inappropriate intracellular activation can result in cellular damage. Trypsinogen is the key digestive enzyme as, once activated, it can activate other enzymes—initiating a cycle of intracellular activation of trypsin, intracellular and extracellular digestive enzyme activity, and further cell damage. Whereas intracellular trypsin activity does not seem to activate NF-κB, extracellular trypsin does, probably by activation of PAR proteins, which subsequently activate transcription factors, including NF-κB. Low levels of these transcription factors have a local inflammatory effect and induce expression of various protective and repair genes. However, high levels of inflammatory transcription factors generate a systemic inflammatory response. Abbreviations: HSP, heat shock protein; PAR, proteinase-activated receptor; PSTI, pancreatic secretory trypsin inhibitor.
Digestive enzyme stress in pancreatitis
For many years, intracellular premature activation of trypsinogen was widely thought to be the key initiator of both acute and chronic pancreatitis. This idea originated with observations made more than a century ago by the anatomist Chiari, who suggested that pancreatitis was the result of pancreatic autodigestion.47 After this original observation, improved understanding of the function and control of digestive enzymes led to the idea that trypsin is the key digestive enzyme involved in pancreatitis. This concept is sometimes referred to as the trypsin hypothesis of pancreatitis, and support for this hypothesis has come from a number of indirect observations. For example, increased trypsin activity was observed early in the course of experimentally induced pancreatitis, and inhibition of intracellular trypsinogen activation (using pharmacological inhibitors of cathepsins) seemed to ameliorate the disease.48 However, the strongest evidence in support of the trypsin hypothesis came from the observation that mutations in trypsinogen are associated with hereditary pancreatitis.49 Subsequently, researchers also observed that mutations in PSTI, the pancreatic secretory trypsin inhibitor gene, also predispose patients to pancreatitis.50 In animal models, genetic deletion of PSTI led to acinar cell death and impaired regeneration of acinar cell populations.51 Similarly, transgenic expression of PSTI ameliorated secretagogue-induced pancreatitis in mice.52 Further support for the trypsin hypothesis includes the observation that deletion of Ctsb, the cathepsin B gene, affects trypsinogen activation and reduces the severity of experimentally introduced pancreatitis.53
Although the trypsin hypothesis has prevailed as the central paradigm of pancreatic pathology, objective evaluation of the major observations thought to support a central role for trypsinogen activation as the key initiator of pancreatitis indicate that the evidence is indirect and inconclusive. A major problem is that the standard experimental animal models of acute pancreatitis involve generalized nonspecific damage to the pancreas, rather than specific trypsinogen activation. Pharmacological protease inhibitors lack specificity,54 and the results of some studies using these inhibitors suggest that trypsin is not involved in the activation of its zymogen, nor that of other digestive enzymes in injured acinar cells.55 The data from genetic studies are also inconclusive. Mice engineered to express the mutations associated with human hereditary pancreatitis do not reproducibly develop chronic pancreatitis. Furthermore, some mutations that are associated with hereditary pancreatitis have little effect on the enzymatic properties of proteases, but rather lead to protein misfolding and consequently to ER stress.39 Importantly, no strong evidence exists that protease inhibitors can alter the clinical course of acute pancreatitis in more than 70 clinical trials performed over the past 40 years.56 The most reasonable explanation for the inconsistency between the popular paradigm and clinical reality is that the animal models utilized to develop the paradigm are not suitable for determining the specific role of intracellular trypsinogen activation in pancreatitis.
A mutant trypsinogen, which is activated intracellularly by endogenous cellular mechanisms, has been utilized to test the sufficiency of intracellular active trypsin to induce pancreatitis.45 This model enabled the direct determination of the effects of intracellular trypsinogen activation on acinar cell function. The level of intracellular activation of trypsin observed in heterozygous animals had only a transient disruptive effect on acinar cell histology; these cells adjusted to the presence of active trypsin and went back to normal functioning within a few days. Further studies in this new animal model should aid our understanding of how acinar cells deal with stress caused by the presence of active intracellular trypsin, and the mechanisms involved in this coping strategy. Notably, however, animals that were homozygous for this same mutation developed severe acute pancreatitis. High levels of intracellular active trypsin are, therefore, sufficient to cause pancreatitis.
Novel studies in a different mutant mouse model, which lacks intracellular trypsin activity as a result of deletion of the gene encoding trypsinogen 7, indicate that pancreatitis can be initiated by the activation of inflammatory pathways, independent of trypsin activation.57 Trypsinogen 7 is the mouse equivalent of human cationic trypsinogen; deletion of the gene encoding trypsinogen 7 reduced the total trypsinogen content of pancreatic acinar cells by 60%, but did not affect their physiological function. Surprisingly, however, administration of caerulein did not cause pathological activation of trypsinogen in these mice, although they did maintain pathological activation of the NF-κB pathway.57 These observations support the findings of a previous study, which demonstrated that activation of the NF-κB pathway can cause pancreatitis in the absence of activation of trypsinogen.58 Thus, trypsin activity is not required for the development of pancreatitis. Perhaps this finding should not be surprising, as many organs that do not express trypsinogen can develop inflammation. Inappropriate trypsinogen activation does seem to impose extra stress on acinar cells, and we can safely assume that active digestive enzymes within the pancreas are harmful. However, active digestive enzymes seem unlikely to be the only, or even the dominant, component of acinar cell stress and damage.
Consequences of digestive enzyme activity
High intracellular concentrations of active trypsin induce acinar cell death primarily through apoptosis.46 However, massive or chronic overproduction of active trypsin initiates apoptosis on a large scale, resulting in necrosis.45 Interestingly, in animal models, the same total amount of acinar cell death caused much higher mortality when apoptosis occurred over a short period of time than when it occurred over a long period.45 This discovery suggests that coping mechanisms are able to deal with a moderate loss of acinar cells, but if they are overwhelmed, the outcome can be serious. Furthermore, mice with dramatically reduced trypsin activity as a result of deletion of trypsinogen 7 manifest equivalent local and systemic inflammatory responses, resulting from intraacinar NF-κB activation, to those observed in wild-type mice upon treatment with caerulein.57 Yet, the absence of trypsinogen activation in these mice almost completely inhibited acinar cell death in vitro and led to a 50% reduction in acinar cell necrosis in vivo. Thus, trypsin seems to be a major regulator of acinar cell apoptosis and necrosis.
Another important early event during the development of pancreatitis is increased acinar cell expression of inflammatory regulators, including chemokines and cytokines.59 These acinar-cell-derived factors lead to infiltration and activation of inflammatory cells, and initiation of an inflammatory cascade. The expression of inflammatory regulators is controlled by a number of related transcription factors, including NF-κB and EGR-1.59,60 Administration of caerulein, for example, caused comparable levels of intra-acinar NF-κB activation in mice lacking trypsinogen 7.57 Other studies in which cathepsin B was either genetically deleted or inhibited also found substantial reductions in trypsin activity, although systemic inflammation was little affected.53 In concordance with these observations, direct activation of intracellular trypsin does not activate NF-κB.46 Intra-acinar trypsin activation is, therefore, independent of the initiation of other critical pathways of pancreatitis, as previously suggested.54 However, extracellular trypsin is able to activate NF-κB46 and could still be involved in the progression of pancreatitis.
Perhaps the most important message from studies involving genetically engineered mouse models is that multiple pathways are activated simultaneously by the various factors that cause pancreatitis. Trypsinogen activation is definitely not the only factor that determines the severity of pancreatitis. Indeed, acute pancreatitis can be initiated by genetic activation of the NF-κB pathway58 as well as by elevated Ras signaling, which is present in many people due to gain-of-function mutations.61 The notion that trypsin is sufficient, but not required, for the development of pancreatitis might be able to explain the negative results of the clinical trials of trypsin inhibitors.
Conclusions
The stresses experienced by acinar cells in modern humans are generally similar to those experienced by the acinar cells of our ancestors. The production of large amounts of proteins, management of dangerous digestive enzymes and restoration of pancreatic function after trauma—these processes have evolved such that the compensatory mechanisms to manage these stresses are highly effective. However, we are often exposed to additional stresses, such as alcohol, tobacco, chemicals and unhealthy diets. Furthermore, genetic alterations in any of several molecules can impair acinar cell function. To the extent that these new stressors evoke the same pathways and processes as are initiated during normal physiological conditions, coping mechanisms to manage these stresses are already in place. However, the acinar cell compensatory mechanisms do not have unlimited capacity and cannot, for example, handle the levels of stress that can be generated by nonphysiological environmental factors or the combined effects of multiple stressors.
Within the exocrine pancreas, elevated stress on the acinar cells, either directly or indirectly, is the source of most pancreatic diseases. Stress derived from environmental and intrinsic factors is part of the normal physiological state and homeostasis is maintained by induction of appropriate responses to these stresses that balance opposing reactions. Coping mechanisms, consisting of the UPR, EOR, ERAD and autophagy, are capable of dealing with all but the highest levels of ER stress. However, if ER stress becomes excessive, it can be associated with acinar cell death. Apoptotic acinar cell death is generally not associated with inflammation and is not life-threatening at low levels. However, if the extent of acinar cell apoptosis overwhelms the ability of the immune system to clear dead cells, necrosis occurs. Acinar cell necrosis is associated with very severe inflammation and can be fatal.
The digestive enzymes that are at the heart of physiological functioning of the pancreas can also be a source of stress if inappropriately activated. For many years, research into pancreatic disease focused on the stress induced by trypsinogen activation. Intracellular trypsin causes cell death, primarily through apoptosis. Trypsin is also able to activate the proinflammatory NF-κB signaling pathway once released from zymogen granules into the intracellular space. Studies from hereditary pancreatitis and a recent genetic model support the hypothesis that trypsin is able to initiate pancreatitis and is likely to have a role in the damage that occurs during the disease.45 However, although trypsin is sufficient to induce pancreatitis, it is not required in this process.57
Understanding the exact mechanisms leading to pancreatitis in an individual patient is challenging, and tools to manipulate the stress response system are, as yet, crude. Further knowledge regarding the complexity of the stress response system, and its inherent abilities to deal with stressors will be useful in the development of effective treatments for both acute and chronic pancreatitis. Future directions for the prevention and therapy of pancreatic disease should focus on a multifaceted model of initiation of pancreatic disease, rather than on a single pathway.
Key points.
-
▪
The pancreas is a highly active organ, primarily because it contains the acinar cells that produce digestive enzymes
-
▪
Physiological stresses on acinar cells include those associated with high levels of protein synthesis and with the production, storage and secretion of potentially damaging digestive enzymes
-
▪
Synthesis of secreted proteins occurs in the endoplasmic reticulum (ER) and can be affected by many factors that result in protein misfolding or ER stress
-
▪
Several compensatory mechanisms have evolved to protect the acinar cell from ER stress and inappropriate activation of digestive enzymes
-
▪
Under normal circumstances, the compensatory mechanisms for ER stress and digestive enzyme activation are sufficient to fully protect the cells without any decrease in function
-
▪
At high levels of stress, these same mechanisms result in acinar cell destruction and disease
Acknowledgements
C. D. Logsdon’s scientific research is supported by grant DK052067 and AA020822.
Footnotes
Competing interests
The authors declare no competing interests.
Review criteria
The references for this Review were selected from the authors’ personal reference libraries on the basis of the personal experience of the authors.
Author contributions
C. D. Logsdon researched the data for the article, and wrote the manuscript. C. D. Logsdon and B. Ji contributed to discussions of its content and undertook review and editing of the manuscript before submission.
References
- 1.Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol. Rev. Camb. Philos. Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jamieson JD, Palade GE. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J. Cell Biol. 1971;50:135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J. Biol. Chem. 2007;282:9635–9645. doi: 10.1074/jbc.M611108200. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino JS, Suzuki CK, Klausner RD. A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science. 1990;247:79–82. doi: 10.1126/science.2294595. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- 8.Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 9.Mareninova OA, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J. Clin. Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao SS, Kaufman RJ. Unfolded protein response. Curr. Biol. 2012;22:R622–R626. doi: 10.1016/j.cub.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J. Biol. Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- 12.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 13.Bhagat L, et al. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J. Clin. Invest. 2000;106:81–89. doi: 10.1172/JCI8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubisch C, et al. Overexpression of heat shock protein Hsp27 protects against cerulein-induced pancreatitis. Gastroenterology. 2004;127:275–286. doi: 10.1053/j.gastro.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Bhagat L, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 16.Hietaranta AJ, et al. Water immersion stress prevents caerulein-induced pancreatic acinar cell NF-κb activation by attenuating caerulein-induced intracellular Ca2+ changes. J. Biol. Chem. 2001;276:18742–18747. doi: 10.1074/jbc.M009721200. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, et al. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1α-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8:e1000410. doi: 10.1371/journal.pbio.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beere HM, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 19.Green GM, et al. Role of cholecystokinin in induction and maintenance of dietary protein-stimulated pancreatic growth. Am. J. Physiol. 1992;262:G740–G746. doi: 10.1152/ajpgi.1992.262.4.G740. [DOI] [PubMed] [Google Scholar]
- 20.Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-κB. Trends Biochem. Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel T, et al. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 23.Kuang E, et al. ER Ca2+ depletion triggers apoptotic signals for endoplasmic reticulum (ER) overload response induced by overexpressed reticulon 3 (RTN3/HAP) J. Cell Physiol. 2005;204:549–559. doi: 10.1002/jcp.20340. [DOI] [PubMed] [Google Scholar]
- 24.Pahl HL, Baeuerle PA. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 25.Belal C, et al. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum. Mol. Genet. 2012;21:963–977. doi: 10.1093/hmg/ddr502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorman AM, Healy SJ, Jäger R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm S. The ER-mitochondria interface: the social network of cell death. Biochim. Biophys. Acta. 2012;1823:327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, et al. Activation of Nuclear Factor-κB in Acinar Cells Increases the Severity of Pancreatitis in Mice. Gastroenterology. 2012;144:201–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aho HJ, Koskensalo SML, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand. J. Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 34.Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J. Nutr. 1984;114:467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- 35.Lerch MM, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum [see comments] Gastroenterology. 1993;104:853–861. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- 36.Seyhun E, et al. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G773–G782. doi: 10.1152/ajpgi.00483.2010. [DOI] [PubMed] [Google Scholar]
- 37.Hegewald G, Nikulin A, Gmaz-Nikulin E, Plamenac P, Bärenwald G. Ultrastructural changes of the human pancreas in acute shock. Pathol. Res. Pract. 1985;179:610–615. doi: 10.1016/S0344-0338(85)80203-X. [DOI] [PubMed] [Google Scholar]
- 38.Ji B, et al. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiol. Genomics. 2003;14:59–72. doi: 10.1152/physiolgenomics.00174.2002. [DOI] [PubMed] [Google Scholar]
- 39.Kereszturi E, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum. Mutat. 2009;30:575–582. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubisch CH, et al. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas. 2006;33:68–76. doi: 10.1097/01.mpa.0000226878.81377.94. [DOI] [PubMed] [Google Scholar]
- 41.Lugea A, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JM, et al. Evolution of trypsinogen activation peptides. Mol. Biol. Evol. 2003;20:1767–1777. doi: 10.1093/molbev/msg183. [DOI] [PubMed] [Google Scholar]
- 43.Beer S, et al. Comprehensive functional analysis of chymotrypsin C (CTRC) variants reveals distinct loss-of-function mechanisms associated with pancreatitis risk. Gut. doi: 10.1136/gutjnl-2012-303090. http://dx.doi.org/10.1136/gutjnl-2012-303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szabó A, Sahin-Tóth M. Increased activation of hereditary pancreatitis-associated human cationic trypsinogen mutants in presence of chymotrypsin C. J. Biol. Chem. 2012;287:20701–20710. doi: 10.1074/jbc.M112.360065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaiser S, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-κB activation. J. Biol. Chem. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiari H. Über die Selbstverdauung des menschlichen Pankreas [German] Zeitschrift für Heilkunde. 1896;17:69–96. [Google Scholar]
- 48.Van Acker GJ, et al. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G794–G800. doi: 10.1152/ajpgi.00363.2001. [DOI] [PubMed] [Google Scholar]
- 49.Whitcomb DC, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 50.Witt H, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat. Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 51.Ohmuraya M, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696–705. doi: 10.1016/j.gastro.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 52.Nathan JD, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 53.Halangk W, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-κB in rat pancreatic acinar cells. Am. J. Physiol. Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 55.Halangk W, et al. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G367–G374. doi: 10.1152/ajpgi.00315.2001. [DOI] [PubMed] [Google Scholar]
- 56.Singh VP, Chari ST. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology. 2005;128:2172–2174. doi: 10.1053/j.gastro.2005.03.087. [DOI] [PubMed] [Google Scholar]
- 57.Dawra R, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X, et al. NF-κB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 59.Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-κB activation. Am. J. Physiol. 1999;277:C74–C82. doi: 10.1152/ajpcell.1999.277.1.C74. [DOI] [PubMed] [Google Scholar]
- 60.Ji B, et al. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiol. Genomics. 2003;14:59–72. doi: 10.1152/physiolgenomics.00174.2002. [DOI] [PubMed] [Google Scholar]
- 61.Ji B, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. e1–e6. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]