Abstract
Selective iodide uptake and prolonged iodine retention in the thyroid is the basis for targeted radioiodine therapy for thyroid cancer patients; however, salivary gland dysfunction is the most frequent nonthyroidal complications. In this study, we have used noninvasive single photon emission computed tomography functional imaging to quantify the temporal dynamics of thyroidal and salivary radioiodine accumulation in mice. At 60 min post radionuclide injection, radionuclide accumulation in the salivary gland was generally higher than that in thyroid due to much larger volume of the salivary gland. However, radionuclide accumulation per anatomic unit in the salivary gland was lower than that in thyroid and was comparable among mice of different age and gender. Differently, radionuclide accumulation per anatomic unit in thyroid varied greatly among mice. The extent of thyroidal radioiodine accumulation stimulated by a single dose of exogenous bovine TSH (bTSH) in triiodothyronine (T3)-supplemented mice was much less than that in mice received neither bTSH nor T3 (nontreated mice), suggesting that the duration of elevated serum TSH level is important to maximize thyroidal radioiodine accumulation. Furthermore, the extent and duration of radioiodine accumulation stimulated by bTSH was less in the thyroids of the thyroid-targeted RET/PTC1 (thyroglobulin (Tg)-PTC1) mice bearing thyroid tumors compared with the thyroids in wild-type (WT) mice. Finally, the effect of 17-allyamino-17-demothoxygeldanamycin on increasing thyroidal, but not salivary, radioiodine accumulation was validated in both WT mice and Tg-PTC1 preclinical thyroid cancer mouse model.
Introduction
The primary function of the thyroid gland is to synthesize thyroid hormones, triiodothyronine (T3) and thyroxine (T4), which play important roles in systemic metabolism. Iodine is an essential component of thyroid hormone yet it is frequently not abundant in the diet; therefore, the thyroid gland has evolved an effective way to sequester iodine 20 to 40 times against its concentration gradient from the systemic circulation. Iodide is transported into thyroid follicular cells by the sodium iodide symporter (NIS), an intrinsic membrane glycoprotein expressed on the basolateral membrane of the thyroid follicular cells. Once in the thyroid cells, iodine is incorporated into thyroglobulin (Tg) through a process called iodine organification and stored in the follicular lumen of the thyroid. Effective radioiodine accumulation in thyroid tissues, mediated by both active uptake and organification, has allowed the clinical use of radioiodine to detect and/or ablate residual thyroid cancer tissue in patients who have undergone thyroidectomy (see review in Shen et al. (2001)).
Both iodide uptake and organification are mainly stimulated by TSH. To selectively induce radioiodine accumulation in thyroid tissues for effective radioiodine therapy, TSH is elevated either by T4 withdrawal to induce endogenous hypothyroidism or by exogenous administration of recombinant human TSH (rhTSH) prior to radioiodine administration (see guidelines published by Luster et al. (2008) and Cooper et al. (2009)). However, about 20–30% patients with metastatic thyroid cancer do not benefit from radioiodine therapy due to reduced or absent NIS expression/function (Caillou et al. 1998, Castro et al. 2001, Patel et al. 2002, Ward et al. 2003, Oler & Cerutti 2009). Thus, it is of clinical importance to identify/develop pharmacological reagents that could increase and/or restore radioiodine accumulation in these tumors. As radioiodine accumulation is the net outcome of iodide influx and efflux, strategies to increase radioiodine accumulation include selectively increase thyroidal iodide influx and/or decrease iodide efflux.
Much effort has been made to identify reagents that increase NIS mRNA levels and/or radioiodine accumulation in cultured thyroid cells. Among all agents tested, LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, has been reproducibly shown to increase thyroidal NIS expression (Garcia & Santisteban 2002, Zaballos et al. 2008, Liu et al. unpublished observations 2010) as well as NIS-mediated radioiodide uptake activity (Kogai et al. 2008, Liu et al. unpublished observations 2010). Although thyroidal NIS expression was increased by mitogen-activated protein kinase (MAPK) inhibitors (Knauf et al. 2003, Vadysirisack et al. 2007), it was not accompanied by comparable degree of increase in NIS-mediated radioiodide uptake activity (Vadysirisack et al. 2007, Liu et al. unpublished observations 2010). Histone deacetylase (H-DAC) inhibitor depsipeptide appeared to modestly increase NIS mRNA level as well as radioiodine accumulation in both thyroid and nonthyroid cancer cells (Kitazono et al. 2001). A recent study showed that combination treatment of inhibitors for H-DAC, MAPK, and PI3K increased radioiodine accumulation in several human thyroid cancer cell lines (Hou et al. 2010), but this finding is yet to be confirmed by others. Most importantly, to our knowledge, none of these findings have been validated using immune-competent genetically engineered mice carrying thyroid tumors surrounded by physiologically/pathologically relevant microenvironment.
Our laboratory has previously reported that 17-allyamino-17-demothoxygeldanamycin (17-AAG) increases NIS-mediated radioiodine accumulation by decreasing iodide efflux in NIS-expressing PC Cl3 rat thyroid cells and RET/PTC1-transformed PC Cl3 cells (Marsee et al. 2004). This finding was subsequently confirmed by another study showing that 17-AAG increases iodide retention in cultured human anaplastic thyroid cancer cells stably expressing human NIS (Elisei et al. 2006). In this study, we have used noninvasive radionuclide imaging with micro-single photon emission computed tomography (micro-SPECT) to validate the effect of 17-AAG on increasing thyroidal, but not salivary, radioiodine accumulation in wild-type (WT) mice and in our thyroid-targeted RET/PTC1 (Tg-PTC1) thyroid cancer mouse model. Our findings encourage the investigation of 17-AAG derivative pharmacological agents to increase the efficacy of radioiodine therapy in thyroid cancer patients, in addition to their potential roles as novel chemotherapeutic agents.
Materials and methods
Animals and treatment
The study protocol (2009A0118) was approved by our Institutional Animal Care and Use Committee that oversees the responsible use of animals in university research and instructional activities. All research activities were conformed to the statutes of the Animal Welfare Act and the guidelines of the Public Health Service as issued in the Guide for the Care and Use of Laboratory Animals (revised 1996). Mice received Harlan Teklad LM-485 Mouse/Rat sterilizable diet (Harlan Teklad Diets, Madison, WI, USA) containing 2.61 mg iodine/kg diet. Tg-PTC1 mice were generated in the FVB/N background, and all Tg-PTC1 mice developed thyroid tumors by 1 month of age (Jhiang et al. 1996, Cho et al. 1999). For T3-bovine TSH (bTSH) experiments, mice were supplemented with 12 μg/ml T3 in their drinking water daily. On day 5, mice received a single i.p. injection of bTSH in 200 μl 1% BSA/PBS and after 16 h the mice were subjected to SPECT imaging or killed for ex vivo γ counting, as described below. The bTSH dosages utilized are reported in the section ‘Results’.
Two different experimental plans were used for 17-AAG (National Cancer Institute: NCI 330507) experiments. We started with the protocol of using T3–bTSH-treated mice i.p. injected with a single dose of 40 mg/kg 17-AAG (the highest dose used in mice reported in NCI Investigator Brochure, 2004) and performed SPECT imaging at 2 h post 17-AAG injection. We later modified the protocol using mice neither treated with T3 nor bTSH (nontreated mice) i.p. injected with a single dose of 30 mg/kg 17-AAG and performed SPECT imaging at 3 h post 17-AAG injection.
In vivo multi-pinhole SPECT imaging
Small animal SPECT imaging was performed by the X-SPECT preclinical platform (Gamma Medica, Northridge, CA, USA) equipped with two gamma cameras, each mounted with a multiple pinhole collimator with 1 mm aperture. After i.p. injection of 100–150 μCi Na123I (t1/2=13.3 h) or Na99mTcO4 (t1/2=6.01 h) in saline, mice were anesthetized using isofluorane inhalation and immobilized on the animal bed. For subsequent calibration of radioactivity in target tissues at various times, a 0.25 ml Eppendorf tube containing a known amount of the same radionuclide was included as a decay control (DC). SPECT imaging was acquired with 32 projections, each for 50 s on a 30 mm radius of rotation. SPECT images were acquired at 1, 6, and 24 h post Na123I injection or 1 h post Na99m TcO4 injection.
Ex vivo γ counting
Following T3–bTSH treatment, animals were i.p. injected with 20–40 μCi Na123I in saline. Animals were killed at 6 h post Na123I injection, and thyroid gland, salivary gland, and liver were harvested and their radioactivity was measured with a γ counter (PerkinElmer, Waltham, MA, USA) along with a known activity of Na123I as a standard for quantification.
Calculation of thyroidal and salivary radioiodine accumulation
The total injected dose was calculated by subtracting the postinjected syringe counts from the preinjected syringes counts. Using the Amira 3.1 software (Gamma Medica), region of interest (ROI) was manually drawn over the thyroid gland, salivary gland, or DC on xy, yx, and xz planes, and the total radioactivity in the 3D region defined by the three selected planes was automatically determined. Thyroidal and salivary radioiodine accumulation were calibrated by DC counts at each time point and reported as the percentage of injected dose (%ID).
Statistical analysis
As many of the experiments measured thyroidal radioiodine accumulation in mice over time, linear mixed models were used to take account of the dependency of observations within the same animals. To ensure unbiased hypothesis tests, we used a covariance structure estimation method for small samples, which avoids underestimation of experimental error. Two sample t-tests were used for simple two group comparisons and Holm’s step down procedure (Holm 1979) was used to adjust for multiple comparisons. P values <0.05 for single comparisons or after adjustment for multiple comparisons were considered as significant.
Results
Temporal dynamics of radioiodine accumulation in thyroid and salivary glands evaluated by 3D SPECT functional imaging
Thyroidal iodide accumulation is largely the sum of NIS-mediated iodide uptake along with iodide retention by iodide organification. In comparison, salivary glands accumulate iodide through NIS-mediated iodide uptake but they lack iodide organification. The temporal dynamics of 123I accumulation in thyroid and salivary glands in WT mice was examined by noninvasive SPECT nuclear imaging (Fig. 1). At 1 h post Na123I injection (t1), 123I accumulation was evident in both thyroid and salivary glands. At 6 h post Na123I injection (t6), 123I accumulated in the thyroid gland was further increased due to continued iodide uptake and organification. However, 123I accumulated in the salivary gland was decreased due to lack of iodide organification and decreased 123I in the blood circulation. At 24 h post Na123I injection (t24), 123I accumulation in the thyroid gland continued to increase when normalized by physical decay. Conversely, no 123I accumulation was detected in the salivary gland at t24, suggesting that 123I was completely cleared from the blood circulation by excretion or tissue uptake in other tissues such as the thyroid. Consequently, thyroidal radioiodine accumulation at t24 was mainly contributed by iodide retention.
Figure 1.
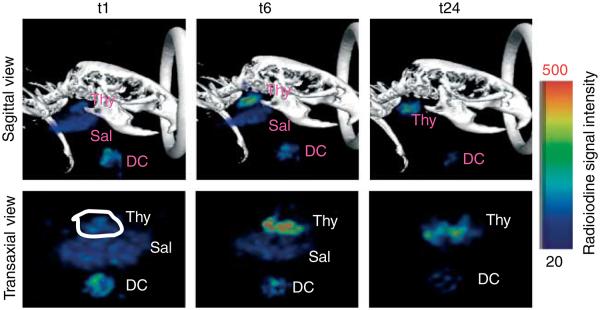
Temporal dynamics of thyroidal and salivary radioiodine accumulation in wild-type (WT) mouse. A 7-month-old male WT mouse was injected with ~150 μCi Na123I and subjected to SPECT imaging at 1(t1), 6(t6), and 24 h (t24) post Na123I injection. At t1, thyroidal radioiodine accumulation was mainly contributed by NIS-mediated 123I influx from blood circulation. At t6, thyroidal radioiodine accumulation was contributed by continuous 123I influx as well as 123I organification/retention. At t24, thyroidal radioiodine accumulation was mainly contributed by 123I organification/retention as NIS-mediated 123I uptake from blood circulation in the salivary gland was observed at t1 and t6, but not at t24. Thy, thyroidal functional image; Sal, salivary functional image; DC, decay control functional image. DC serves to normalize the counts of thyroidal radioiodine accumulation at each time point for quantification purposes. The color bar indicates radioiodine signal intensity increases from 20 (black) to 500 (red) in arbitrary units.
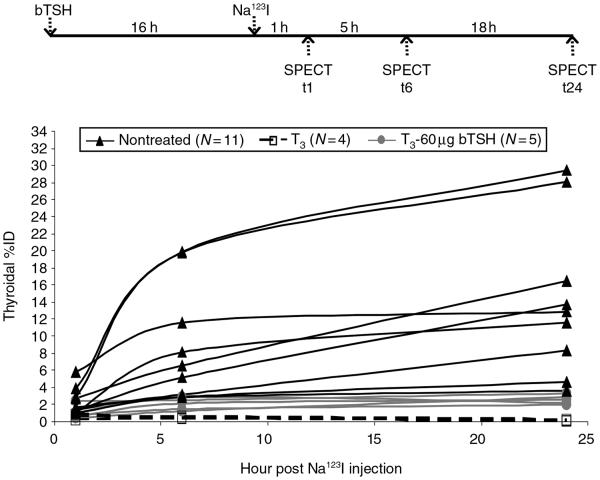
Several mice were subjected to SPECT imaging in the same experimental settings as stated above. The amount of 123I accumulation in the thyroid (%ID) varied extensively among mice and the degree of 123I accumulation at t6 was not always near maximum, i.e. to the similar extent seen at t24 (Fig. 2). This variability may be contributed by various sizes of follicular lumen, as radioactivity equilibrium is reached faster in follicular lumen with smaller radius (Cantraine & Dewandre 1975). Thyroidal %ID at t1, t6, and t24 for each mice studied is shown in Table 1 (t1: 0.63–6.45%, mean=2.07%; t6: 1.59–23.21%, mean=8.88%; and t24: 1.48–24.66%, mean=11.55%). Albeit our small sample number, mice of 14–15 months of age tended to have lower iodide influx as indicated by thyroidal %ID at t1. Mice of 2–3-months old appeared to have lower iodide retention ability as indicated by lower ratios of t6 or t24 over t1 thyroidal %ID. This may be at least in part contributed by their faster renal clearance rate as shown by a sharper decrease in 123I accumulation in salivary gland at t6 versus t1 (t6/t1 %ID: 2–3 months: 0.1–0.3, mean=0.22, N=3; >6 months: 0.73–1.01, mean=0.86, N=4). These findings warrant further study using a larger cohort.
Figure 2.
The extent of induced thyroidal radioiodine accumulation by one dose of 60 μg bTSH in T3 mice is much less than that induced by continuous endogenous mTSH in nontreated mice. WT mice were given 12 μg/ml T3 in drinking water daily. On day 5, mice were injected with one dose of 60 μg bTSH (T3-60 μg bTSH; N=5). Na123I (100–150 μCi) was administered into mice at 16 h post bTSH injection. SPECT images were taken at 1(t1), 6(t6), and 24 h (t24) post Na123I injection. For comparison, T3 mice (N=4) and nontreated mice (N=11) were subjected to SPECT imaging as well. In T3 mice, thyroidal radioiodine accumulation was almost undetected (P value=0.0017), confirming that T3 supplementation for 5 consecutive days is sufficient to suppress endogenous TSH-induced thyroidal radioiodine accumulation. While one dose of 60 μg bTSH was sufficient to induce some extent of thyroidal radioiodine accumulation, the extent of 60 μg bTSH-induced radioiodine accumulation was far less (P value=0.027) than that of nontreated mice.
Table 1.
Temporal dynamics of thyroidal and salivary radioiodine accumulation in nontreated wild-type mice
| Thyroidal %ID |
Thyroidal iodide retention ability |
Salivary %ID |
Salivary iodide retention ability |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Age (months) |
t1 | t6 | t24 | t6/t1 | t24/t1 | t1 | t6 | t6/t1 |
| M | 2 | 1.49 | 2.88 | 3.62 | 1.94 | 2.43 | 2.74 | 0.83 | 0.30 |
| M | 3 | 1.64 | 3.15 | 8.30 | 1.93 | 5.07 | 6.47 | 1.7 | 0.26 |
| M | 7 | 2.94 | 18.45 | 21.26 | 6.27 | 7.22 | 13.62 | 9.92 | 0.73 |
| M | 14 | 0.90 | 5.29 | 13.06 | 5.88 | 14.52 | 4.59 | 3.94 | 0.86 |
| M | 15 | 1.12 | 8.14 | 11.60 | 7.25 | 10.33 | 6.46 | 6.54 | 1.01 |
| F | 3 | 6.45 | 12.74 | 10.46 | 1.98 | 1.62 | 6.57 | 0.69 | 0.10 |
| F | 4 | 0.63 | 1.59 | 1.48 | 2.51 | 2.34 | 0.93 | 1.37 | 1.48 |
| F | 6 | 2.14 | 23.21 | 24.66 | 10.85 | 11.52 | 4.89 | 4.13 | 0.84 |
The extent of single dose bTSH-induced thyroidal radioiodine accumulation in T3-supplemented mice is less than that induced by continuous stimulation of endogenous mouse TSH in nontreated mice
To examine the effect of exogenous bTSH on temporal dynamics of thyroidal radioiodine accumulation in the absence of endogenous mouse TSH (mTSH), endogenous mTSH was suppressed by T3 supplementation in WT mice. As shown in Fig. 2, thyroidal radioiodine accumulation was diminished in all mice that underwent T3 supplementation (t1: 0.18–1.06%, mean=0.49%; t6: 0.26–0.59%, mean=0.41%; t24: 0–0.2%, mean=0.06%, N=4), despite extensive variability of thyroidal radioiodine accumulation seen in nontreated mice (N=11). These data confirm that our T3 supplementation protocol is sufficient to suppress endogenous mTSH-induced thyroidal radioiodine accumulation, regardless of age and gender.
In mice treated with 6, 30, or 60 μg of bTSH at day 5 of T3 supplementation, thyroidal radioiodine accumulation was increased in a dose-dependent manner (data not shown). Further increase of bTSH to 90 μg did not further increase (P value=0.71) thyroidal radioiodine accumulation as determined by ex vivo γ counting of thyroids at 6 h post Na123I injection (Fig. 3A). As shown in Fig. 2, thyroidal radioiodine accumulation average across time was significantly increased (P value=0.0007) by a single dose of 60 μg bTSH in T3-supplemented mice (t1: 0.34–1.99%, mean=0.93%; t6: 1.22–2.84%, mean=2.01%; t24: 1.97–3.36%, mean=2.46%, N=5). However, thyroidal %ID in T3–bTSH-treated mice was much lower (P value=0.027) than that in nontreated mice. This is most likely due to the short half-life of bTSH (<3 h, Guyot et al. 2007), such that a single dose of 60 μg bTSH in T3-supplemented mice is not sufficient to induce thyroidal radioiodine accumulation comparable to nontreated mice, which had continuous low-level stimulation of endogenous mTSH.
Figure 3.
The extent of thyroidal radioiodine accumulation and temporal duration of NIS-mediated thyroidal Na99mTcO4 influx activity induced by bTSH is less in Tg-PTC1 mice than that in wild-type mice. (A) Seven-month-old WT and 4-month-old Tg-PTC1 mice were given 12 μg/ml T3 in drinking water daily. On day 5, mice were injected with either one dose of 60 or 90 μg bTSH. Na123I (20–40 μCi) was injected into the mice at 16 h post bTSH injection. At 6 h (t6) post Na123I injection, mice were euthanized and thyroids were harvested and subjected for counting of activity by a γ counter. Statistical analysis showed that WT mice had significantly higher (P value=0.001) thyroidal radioiodine accumulation at t6 than Tg-PTC1 mice, while there was no significant difference (P value=0.71) between 60 and 90 μg bTSH-induced thyroidal radioiodine accumulation in both WT and Tg-PTC1 mice. (B) T3-supplemented WT and Tg-PTC1 mice were administered with one dose of 60 μg bTSH. Na99mTcO4 (100 μCi), a substrate of NIS yet could not be organified by thyroid, was first injected into mice 16 h post bTSH injection and SPECT images were acquired at 1 h post Na99mTcO4 injection, i.e. at 17 h post bTSH injection. A second dose of Na99mTcO4 (100 μCi) was injected into mice 40 h post bTSH injection and SPECT images were acquired at 1 h post the second Na99mTcO4 injection, i.e. at 41 h post bTSH injection. Two out of three WT mice had comparable NIS-mediated 99mTcO4 influx activity at the two different time points, whereas all three Tg-PTC1 mice had decreased NIS-mediated 99mTcO4 influx activity at later time point. The difference in duration of NIS-mediated thyroidal 99mTcO4 influx activity induced by single dose of bTSH between Tg-PTC1 mice and WT mice was statistically significant (P value=0.014).
The extent and duration of thyroidal radioiodine accumulation induced by bTSH is less in Tg-PTC1 mice than that in WT mice
Tg-PTC1 mice can serve as a preclinical animal model to examine factors that modulate radioiodine accumulation in thyroid tumors. We previously reported that thyroidal radioiodine accumulation in Tg-PTC1 mice is decreased compared with WT mice at 6 h post Na123I injection when normalized with tissue weight despite of elevated serum TSH level in Tg-PTC1 mice (Cho et al. 1999). This is consistent with the clinical findings that malignant thyroid tumors are typically ‘cold’ nodules. Our pilot study showed that thyroidal radioiodine accumulation was also suppressed by T3 supplementation in Tg-PTC1 mice (data not shown). To compare the responsiveness of WT and Tg-PTC1 mice to exogenous bTSH toward induction of thyroidal radioiodine accumulation, 123I accumulation at t6 was evaluated ex vivo in T3-supplemented mice injected with a single dose of bTSH. As shown in Fig. 3A, the extent of bTSH-induced thyroidal radioiodine accumulation at t6 in Tg-PTC1 mice (1.6–2.5%, mean=2.04%, N=4) was significantly lower (P value=0.001) than that in WT mice (8.9–16.3%, mean=12.44%, N=4) without normalization for thyroid gland volume or weight. However, the size of thyroid glands in Tg-PTC1 mice was consistently larger than that in WT mice.
To compare the duration of bTSH-induced NIS activity between WT mice and Tg-PTC1 mice, Na99mTcO4, which cannot be organified, was used to evaluate NIS-mediated radionuclide influx at 17 or 41 h post bTSH administration. As shown in Fig. 3B, thyroidal %ID of Na99mTcO4 was modestly decreased (P value=0.28) at 41 h compared to 17 h post bTSH administration in WT mice (17 h: 1.27–1.54%, mean=1.41%; 41 h: 0.23–1.50%, mean=0.93%, N=3). In comparison, thyroidal %ID of Na99mTcO4 was significantly decreased (P value=0.0076) at 41 h in all three Tg-PTC1 mice examined (17 h: 1.7–2.60%, mean=2.01%; 41 h: 0.00–0.57%, mean=0.32%, N=3). Indeed, statistical analysis by linear mixed model showed that the difference of NIS activity between 17 and 41 h post bTSH administration for Tg-PTC1 mice was much more evident (P value=0.014) than that for WT mice. Taken together, thyroid tumor-bearing thyroid glands of Tg-PTC1 mice not only were less responsive to bTSH in the induction of radioiodine accumulation but the duration of NIS induction was also much shorter than normal thyroids in WT mice. It is interesting to note that thyroidal %ID of Na99mTcO4 at 17 h post bTSH injection in Tg-PTC1 mice was higher compared to WT mice, suggesting that the number of thyroid follicular cells responsive to bTSH at an early time point in Tg-PTC1 mice is much more than that in WT mice. This phenomenon can be explained by increased proliferation of thyroid follicular cells (Cho et al. 1999) and the much larger size of thyroids observed in Tg-PTC1 mice.
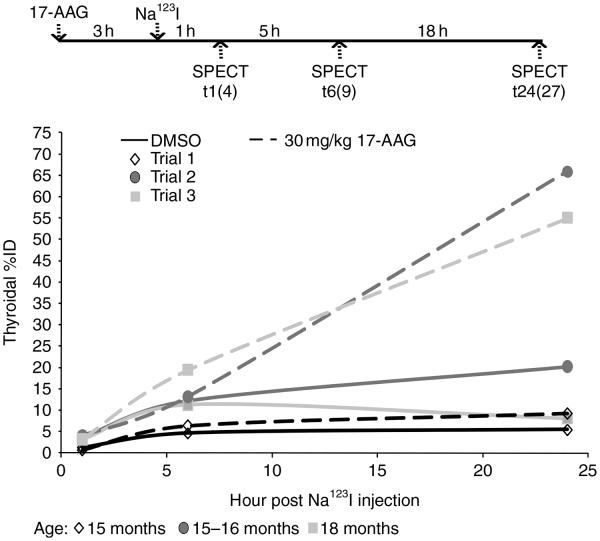
The area under time–activity curve of thyroid in WT mice is markedly increased by 30 mg/kg 17-AAG
None of the T3–bTSH mice injected with a single dose of 40 mg/kg 17-AAG survived during SPECT imaging acquisition (N=3, data not shown), indicating the experimental condition was not tolerable by the mice. We then examined the effect of 30 mg/kg 17-AAG in nontreated mice that had Na123I injection at 3 h post 17-AAG injection (see the top panel of Fig. 4). Three experimental trials with identical design were performed to compare temporal radioiodine accumulation between dimethyl sulfoxide (DMSO)- and 17-AAG-treated mice. As shown in Fig. 4, thyroidal radioiodine accumulation at t24 in 17-AAG-treated mice was much higher (P value=0.15) than DMSO-treated mice in each experimental trial (DMSO versus 17-AAG: 5.42 vs 9.22%; 20.66 vs 65.88%; 8.07 vs 55.05%), despite extensive variations of thyroidal %ID among different experimental trials. Conversely, thyroidal radioiodine accumulation at t1 was not increased (P value=0.98) by 17-AAG treatment (DMSO versus 17-AAG: 3.58 vs 4.02%; 1.18 vs 0.63%; 3.24 vs 3.44%). These results indicate that 17-AAG markedly increases iodide retention although it has little effect on NIS-mediated iodide influx.
Figure 4.
The area under time–activity curve (AUC) of thyroid in wild-type (WT) mice is markedly increased by 30 mg/kg 17-AAG. WT mice (15–18 months) were weighed and injected with 30 mg/kg 17-AAG in 50 μl DMSO. Na123I (100–150 μCi) was injected into mice at 3 h post 17-AAG injection. SPECT images were acquired at t1(4), t6(9), and t24(27) post Na123I injection (time post 17-AAG injection). At t1, the extent of thyroidal radioiodine accumulation was quite comparable between DMSO versus 17-AAG-treated mice, suggesting that 17-AAG had minimal effect on thyroidal iodide influx. However, at t24, the extent of thyroidal radioiodine accumulation was markedly increased (P value=0.15) in 17-AAG-treated mice versus that in DMSO-treated mice, suggesting that 17-AAG increased iodide retention ability. Consequently, the exposure of thyroid with radioiodine over time was markedly increased by 17-AAG as defined by the AUC.
The average of thyroidal %ID at t1, t6, and t24 among three experimental trials as well as the average ratio of thyroidal %ID at t6 or t24 versus t1 in each experimental trial are shown in Table 2. Iodide retention ability in WT mice was moderately increased by 17-AAG at t6 (~2-fold), and further increased at t24 (~4-fold) as compared with DMSO-treated mice. Consequently, the exposure of the thyroid to radioiodine over time was markedly increased by 17-AAG as defined by the area under time–activity curve (AUC). Indeed, while the value of AUC varied greatly among experimental trials (DMSO: 224.4±133.2, N=3; 17-AAG: 583.1±362.1, N=3), the fold increase in AUC by 17-AAG calculated in each experimental trial ranged from 1.55 to 3.96. Taken together, the effect of 17-AAG on increasing radioiodine accumulation in cultured thyroid cells is validated in WT mice using temporal SPECT imaging. Furthermore, 17-AAG did not appear to have an effect on salivary %ID (data not shown).
Table 2.
Effect of 17-allyamino-17-demothoxygeldanamycin (17-AAG) on thyroidal radioiodine accumulation in wild-type (WT) and thyroglobulin (Tg)-PTC1 mice
| Thyroidal %ID |
Iodide retention ability |
||||||
|---|---|---|---|---|---|---|---|
| Mice | Treatment | t1 | t6 | t24 | 6 h/1 h | 24 h/1 h | AUCa |
| WT | DMSO | 2.6±1.3 | 9.3±4.1 | 11.2±7.9 | 3.6±0.2 | 4.3±1.6 | 224.4±133.2 |
| 17-AAG | 2.7±1.8 | 13.0±6.6 | 43.4±30.1 | 6.3±3.4 | 15.7±0.9 | 583.1±362.1 | |
| Tg-PTC1 | DMSO | 7.4±5.6 | 8.8±5.1 | 3.0±0.7 | 1.3±0.3 | 0.6±0.6 | 148.8±44.3 |
| 17-AAG | 9.2±1.0 | 16.7±2.9 | 22.6±4.7 | 1.9±0.5 | 2.6±0.6 | 442.6±62.7 | |
AUC, area under time–activity curve; it is calculated by ; f(t), thyroidal %ID; t, time (h).
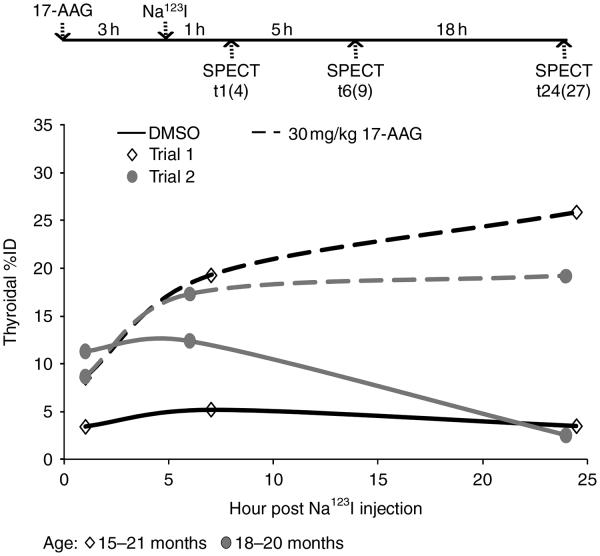
The AUC of thyroid tumor in Tg-PTC1 mice is markedly increased by 30 mg/kg 17-AAG
We next examined the effect of 30 mg/kg 17-AAG on thyroidal radioiodine accumulation in thyroid tumor-bearing thyroid glands of Tg-PTC1 mice. While WT mice were tolerant of 30 mg/kg 17-AAG treatment, two out of four Tg-PTC1 mice did not survive through SPECT imaging acquisition. However, as shown in Fig. 5, both of the remaining Tg-PTC1 mice treated with 17-AAG had evident increase in thyroidal radioiodine accumulation compared with mice treated with DMSO at t24 (DMSO versus 17-AAG: 3.50 vs 25.89%; 2.53 vs 19.23%). Similar to WT mice, 17-AAG appeared to increase radioiodine retention rather than radioiodide influx in Tg-PTC1 mice. As shown in Table 2, iodide retention was moderately increased by 17-AAG at t6 (~1.5-fold), yet it was further increased at t24 (~4-fold) as calculated by the average ratios of thyroidal %ID at t6 or t24 versus t1 in each experimental trial. The value of AUC was less variable between the two experimental trials in Tg-PTC1 mice (DMSO: 148.8±44.3, N=2; 17-AAG: 442.6±62.7, N=2), and the fold increase in AUC by 17-AAG in the two experimental trials was 2.21 and 4.14. Thyroidal iodide retention ability in 17-AAG-treated Tg-PTC1 mice (t24/t1 %ID=2.6±0.6) was much lower than that in WT mice (t24/t1 %ID=15.7±0.9) at t24, yet the fold increase of iodide retention ability by 17-AAG was comparable between Tg-PTC1 mice and WT mice at t24. Taken together, the effect of 17-AAG on increasing thyroidal radioiodine accumulation is confirmed in a preclinical thyroid cancer animal model.
Figure 5.
The area under time–activity curve (AUC) of thyroid tumor in Tg-PTC1 mice is markedly increased by 30 mg/kg 17-AAG. Tg-PTC1 mice (15–21 months) were weighed and injected with 30 mg/kg 17-AAG in 50 μl DMSO. Na123I (100–150 μCi) was injected into mice at 3 h post 17-AAG injection. SPECT images were acquired at t1(4), t6(9), and t24(27) post Na123I injection (time post 17-AAG injection). At t24, the difference in the extent of increased thyroidal radioiodine accumulation was evidently enhanced in 17-AAG-treated mice compared with that in DMSO-treated mice at least in part due to the decreased thyroidal %ID by DMSO at t24 in Tg-PTC1 mice. As a result, AUC was evidently increased by 17-AAG in Tg-PTC1 mice.
Discussion
In this study, we quantified temporal radioiodine accumulation in both thyroid and salivary glands modulated by T3, bTSH, and/or 17-AAG in mice using SPECT functional imaging. By comparing temporal dynamics of radioiodine accumulation between salivary and thyroid glands, we found that administered radioiodine was almost completely cleared from blood circulation after 24 h, and that iodide organification contributes greatly to thyroidal radioiodine accumulation over time. The extent of thyroidal radioiodine accumulation stimulated by a single dose of exogenous bTSH in T3 mice was much less than that in nontreated mice, justifying the clinical use of multiple doses of rhTSH or the merit of T4 withdrawal in thyroid cancer patients prior to radioiodine therapy. Furthermore, the extent and duration of radioiodine accumulation stimulated by bTSH was reduced in thyroid tumor-bearing thyroid glands of Tg-PTC1 mice compared with the thyroids in WT mice. Finally, 17-AAG significantly increased thyroidal exposure of radioiodine over time in our Tg-PTC1 preclinical thyroid cancer mouse model, which encourages the study of 17-AAG derivatives not only as novel chemotherapeutic agents to impede tumor progression but also to improve radioiodine therapy in patients with thyroid cancer. Optimally, 17-AAG derivatives could permit those tumors with low radioiodine accumulation to become amenable to radioiodine treatment and allow for those tumors with normal radioiodine accumulation to be affectively treated with lower radioiodine activities.
Salivary gland dysfunction is the most frequent nonthyroidal complications of radioiodine therapy for patients with thyroid cancer (Kloos 2009). A better understanding of the temporal dynamics of radioiodine accumulation in the thyroid and salivary glands may lead to strategies to mitigate radioiodine-induced salivary gland damage without compromising the efficacy of thyroid cancer radioiodine therapy. A recent study showed that the time required to reach maximal accumulation of 99mTcO4 uptake in thyroid glands is shorter than that in salivary glands of C57 BL6/6J mice (Franken et al. 2010). This difference could be explained by higher NIS transport activity and/or smaller NIS-expressing epithelial-cell-delimiting compartments of the thyroid, i.e. thyroid colloid versus salivary acini and duct lumens. Similarly, the rate of decrease in 99mTcO4 accumulation is faster in the thyroid than in the salivary gland after the administration of competitive inhibitors (Franken et al. 2010). In this study, we showed that salivary gland %ID was 1.02–5.75-fold higher than thyroid %ID at t1 (see Table 1), which has been reported by others (Zuckier et al. 2004, Franken et al. 2010) and is anticipated by the larger size of the salivary gland. Indeed, voxel counts in the selected ROI of salivary were 1.93–9.38-fold higher than those of the thyroid at t1 (data not shown), although functional voxel counts in selected ROI may not exactly reflect anatomic volume of the target tissue. Interestingly, the normalized means (total signal intensity counts divided by voxel counts) of salivary glands at t1 were lower than those of the thyroid (salivary/thyroid=0.47–0.94 with a mean of 0.68), indicating that NIS expression/activity per anatomic unit is lower in salivary glands compared to the thyroid gland. Salivary normalized means at t1 were quite comparable among mice (62–80 with a mean of 72), yet thyroid normalized means at t1 varied greatly among mice (66–163 with a mean of 113). Thus, NIS activity per anatomic unit in the salivary gland is not changed by gender or age among different mice.
Although rhTSH administration has been shown to be equally effective compared to thyroid hormone withdrawal in preparation of low-risk patients for postoperative thyroid remnant ablation (Pacini et al. 2006, Chianelli et al. 2009, Elisei et al. 2009, Tuttle et al. 2010), it remains uncertain whether rhTSH is equally effective in treating high-risk patients with known residual cancer. The level and duration of serum TSH levels required for effective ablation of thyroid remnant could be very different from that for residual cancer and may vary depending on various somatic tumor mutations that affect radioiodine accumulation. Indeed, Pötzi et al. (2006) reported that a higher radioactivity of 131I is required to achieve comparable radioiodine accumulation in thyroid cancer metastases in patients administered radioiodine after rhTSH versus thyroid hormone withdrawal preparation. This study showed that thyroidal radioiodine accumulation in T3–bTSH mice was much lower than that in nontreated mice suggesting that the duration of exposure to TSH is important to promote desirable thyroidal radioiodine accumulation. We also showed that thyroid tumor-bearing thyroid glands in Tg-PTC1 mice were less responsive to bTSH-induced thyroidal radioiodine accumulation than normal thyroids in WT mice. Taken together, thyroid cancer foci may require higher 131I activity for treatment as opposed to normal thyroid remnants and longer duration of elevated TSH level may be required to achieve the desirable 131I activity in thyroid cancer foci. Accordingly, various thyroid cancer mouse models (see review in Knostman et al. (2007) and Burniat et al. (2008)) can be invaluable to examine the concepts of magnitude and duration of serum TSH elevation required to confer sufficient radioiodine accumulation in thyroid tumors of different histologies that are initiated by various genetic events.
Findings using cultured cells do not always translate into preclinical animal models or patients. For example, leptin decreases radioiodine uptake activity in FRTL-5 immortalized rat thyroid cells (Isozaki et al. 2004) and rat thyroid slices (de Oliveira et al. 2007), yet it increases thyroidal radioiodine uptake activity in live rats (de Oliveira et al. 2007). The discordance is most likely explained by tissue microenvironments and/or systemic effects that do not exist in isolated cultured cell system. In this study, we confirmed that 17-AAG increases thyroid exposure to radioiodine over time in WT mice as well as Tg-PTC1 transgenic mice carrying thyroid tumors. The fact that 17-AAG did not increase thyroidal radioiodine accumulation at t1, but did at t6 and t24, indicates that 17-AAG has little effect on NIS-mediated radioiodine influx but mainly acts on iodide retention/organification. This result is consistent with our previous finding that 17-AAG increases radioiodine accumulation by decreasing iodide efflux in cultured cells (Marsee et al. 2004) and that 17-AAG did not increase thyroidal 99mTcO4 accumulation at either 4 or 27 h post treatment (data not shown). The mechanism underlying the increase in thyroidal iodide retention by 17-AAG is currently unknown. Since heat shock protein 90 (Hsp90) is known for its role as molecule chaperone, it would be interesting to investigate whether 17-AAG acts to impair the secretion of iodinated Tg derivatives.
The following issues need to be addressed before our findings can be translated into clinical trials. Is 17-AAG’s effect on thyroidal radioiodine accumulation mainly mediated by Hsp90 inhibition? Do all Hsp90 inhibitors increase thyroidal radioiodine accumulation? Which Hsp90 inhibitor has the desired pharmacokinetic and pharmacodynamic profiles? What are the optimal dose, duration, and timing of Hsp90 inhibitor treatment to increase the efficacy of radioiodine therapy? Is the magnitude of increased radioiodine accumulation by Hsp90 inhibitors sufficient to meaningfully improve the efficacy of radioiodine therapy? Are there any differences in the effects of Hsp90 inhibitors on thyroid tumors derived from various genetic abnormalities, such as B-RAFV600E mutation? Finally, while not immediately needed for Hsp90 inhibitor clinical application, what are the molecular targets of this treatment in thyroid tumors, and can they be more optimally modulated by other approaches?
Acknowledgements
We thank Dr Mitch Phelps and Dr Jeffrey S Johnston for sharing their expertise and experiences in pharmacokinetics of 17-AAG.
Funding
This work was supported in part by the National Institutes of Health P01 CA124570 (Project 3 leader: S M Jhiang), R01 EB001876 (PI: S M Jhiang), and P30 CA16058 (PI: M A Caligiuri).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Burniat A, Jin L, Detours V, Driessens N, Goffard JC, Santoro M, Rothstein J, Dumont JE, Miot F, Corvilain B. Gene expression in RET/PTC3 and E7 transgenic mouse thyroids: RET/PTC3 but not E7 tumors are partial and transient models of human papillary thyroid cancers. Endocrinology. 2008;149:5107–5117. doi: 10.1210/en.2008-0531. (doi:10.1210/en.2008-0531) [DOI] [PubMed] [Google Scholar]
- Caillou B, Troalen F, Baudin E, Talbot M, Filetti S, Schlumberger M, Bidart JM. Na+/I− symporter distribution in human thyroid tissues: an immunohistochemical study. Journal of Clinical Endocrinology and Metabolism. 1998;83:4102–4106. doi: 10.1210/jcem.83.11.5262. (doi:10. 1210/jc.83.11.4102) [DOI] [PubMed] [Google Scholar]
- Cantraine FR, Dewandre B. Kinetics of the thyroid follicle lumen diffusion subsystem: application to iodide accumulation by the thyroid follicle in vitro. Bulletin of Mathematical Biology. 1975;37:301–321. doi: 10.1007/BF02461448. (doi:10.1007/BF02461448) [DOI] [PubMed] [Google Scholar]
- Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. Journal of Clinical Endocrinology and Metabolism. 2001;86:5627–5632. doi: 10.1210/jcem.86.11.8048. (doi:10.1210/jc.86.11.5627) [DOI] [PubMed] [Google Scholar]
- Chianelli M, Todino V, Graziano FM, Panunzi C, Pace D, Guglielmi R, Signore A, Papini E. Low-activity (2.0 GBq; 54 mCi) radioiodine post-surgical remnant ablation in thyroid cancer: comparison between hormone withdrawal and use of rhTSH in low-risk patients. European Journal of Endocrinology/European Federation of Endocrine Societies. 2009;160:431–436. doi: 10.1530/EJE-08-0669. (doi:10.1530/EJE-08-0669) [DOI] [PubMed] [Google Scholar]
- Cho JY, Sagartz JE, Capen CC, Mazzaferri EL, Jhiang SM. Early cellular abnormalities induced by RET/PTC1 oncogene in thyroid-targeted transgenic mice. Oncogene. 1999;18:3659–3665. doi: 10.1038/sj.onc.1202709. (doi:10.1038/sj.onc.1202709) [DOI] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. (doi:10.1089/thy.2009.0110) [DOI] [PubMed] [Google Scholar]
- Elisei R, Vivaldi A, Ciampi R, Faviana P, Basolo F, Santini F, Traino C, Pacini F, Pinchera A. Treatment with drugs able to reduce iodine efflux significantly increases the intracellular retention time in thyroid cancer cells stably transfected with sodium iodide symporter complementary deoxyribonucleic acid. Journal of Clinical Endocrinology and Metabolism. 2006;91:2389–2395. doi: 10.1210/jc.2005-2480. (doi:10. 1210/jc.2005-2480) [DOI] [PubMed] [Google Scholar]
- Elisei R, Schlumberger M, Driedger A, Reiners C, Kloos RT, Sherman SI, Haugen B, Corone C, Molinaro E, Grasso L, et al. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. Journal of Clinical Endocrinology and Metabolism. 2009;94:4171–4179. doi: 10.1210/jc.2009-0869. (doi:10.1210/jc.2009-0869) [DOI] [PubMed] [Google Scholar]
- Franken PR, Guglielmi J, Vanhove C, Koulibaly M, Defrise M, Darcourt J, Pourcher T. Distribution and dynamics of (99m)Tc-pertechnetate uptake in the thyroid and other organs assessed by single-photon emission computed tomography in living mice. Thyroid. 2010;20:519–526. doi: 10.1089/thy.2009.0213. (doi:10.1089/thy.2009.0213) [DOI] [PubMed] [Google Scholar]
- Garcia B, Santisteban P. PI3K is involved in the IGF-1 inhibition of TSH-induced sodium/iodide symporter gene expression. Molecular Endocrinology. 2002;16:342–352. doi: 10.1210/mend.16.2.0774. (doi:10.1210/me.16.2.342) [DOI] [PubMed] [Google Scholar]
- Guyot H, Sulon J, Beckers J-F, Closset J, Lebreton P, de Oliveira LA, Rollin F. Development and validation of a radioimmunoassay for thyrotropin in cattle. Journal of Veterinary Diagnostic Investigation. 2007;19:643–651. doi: 10.1177/104063870701900605. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. Journal of Clinical Endocrinology and Metabolism. 2010;95:820–828. doi: 10.1210/jc.2009-1888. (doi:10.1210/jc.2009-1888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki O, Tsushima T, Nozoe Y, Miyakawa M, Takano K. Leptin regulation of the thyroids: negative regulation on thyroid hormone levels in euthyroid subjects and inhibitory effects on iodide uptake and Na+/I− symporter mRNA expression in rat FRTL-5 cells. Endocrine Journal. 2004;51:415–423. doi: 10.1507/endocrj.51.415. (doi:10.1507/endocrj.51.415) [DOI] [PubMed] [Google Scholar]
- Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/endo.137.1.8536638. (doi:10. 1210/en.137.1.375) [DOI] [PubMed] [Google Scholar]
- Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na+/I− symporter and iodine accumulation on poorly differentiated thyroid carcinoma cells. Journal of Clinical Endocrinology and Metabolism. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. (doi:10.1210/jc.86.7.3430) [DOI] [PubMed] [Google Scholar]
- Kloos RT. Protecting thyroid cancer patients from untoward effects of radioactive iodine treatment. Thyroid. 2009;19:925–928. doi: 10.1089/thy.2009.0236. (doi:10.1089/thy.2009.0236) [DOI] [PubMed] [Google Scholar]
- Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22:4406–4412. doi: 10.1038/sj.onc.1206602. (doi:10.1038/sj.onc. 1206602) [DOI] [PubMed] [Google Scholar]
- Knostman KA, Jhiang SM, Capen CC. Genetic alterations in thyroid cancer: the role of mouse models. Veterinary Pathology. 2007;44:1–14. doi: 10.1354/vp.44-1-1. (doi:10.1354/vp.44-1-1) [DOI] [PubMed] [Google Scholar]
- Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. Journal of Endocrinology. 2008;199:243–252. doi: 10.1677/JOE-08-0333. (doi:10.1677/JOE-08-0333) [DOI] [PubMed] [Google Scholar]
- Luster M, Clarke SE, Ditelein M, Lassman M, Lind P, Oyen WJG, Tennvall J, Bombardieri E. Guidelines for radioiodine therapy of differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. (doi:10.1007/s00259-008-0883-1) [DOI] [PubMed] [Google Scholar]
- Marsee DK, Venkateswaran A, Tao H, Vadysirisack D, Zhang Z, Vandre DD, Jhiang SM. Inhibition of heat shock protein 90, a novel RET/PTC1-associated protein, increases radioiodide accumulation in thyroid cells. Journal of Biological Chemistry. 2004;279:43990–43997. doi: 10.1074/jbc.M407503200. (doi:10.1074/jbc.M407503200) [DOI] [PubMed] [Google Scholar]
- Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodidemetabolizing genes. Cancer. 2009;115:972–980. doi: 10.1002/cncr.24118. (doi:10.1002/cncr.24118) [DOI] [PubMed] [Google Scholar]
- de Oliveira E, Teixeira Silva Fagundes A, Teixeira Bonomo I, Curty FH, Fonseca Passos MC, de Moura EG, Lisboa PC. Acute and chronic leptin effect upon in vivo and in vitro rat thyroid iodide uptake. Life Sciences. 2007;81:1241–1246. doi: 10.1016/j.lfs.2007.08.030. (doi:10.1016/j.lfs.2007.08.030) [DOI] [PubMed] [Google Scholar]
- Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C, Molinaro E, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. Journal of Clinical Endocrinology and Metabolism. 2006;91:926–932. doi: 10.1210/jc.2005-1651. (doi:10.1210/jc.2005-1651) [DOI] [PubMed] [Google Scholar]
- Patel A, Jhiang S, Dogra S, Terrell R, Powers PA, Fenton C, Dinauer CA, Tuttle RM, Francis GL. Differentiated thyroid carcinoma that express sodium–iodide symporter have a lower risk of recurrence for children and adolescents. Pediatric Research. 2002;52:737–744. doi: 10.1203/00006450-200211000-00021. [DOI] [PubMed] [Google Scholar]
- Pötzi C, Moameni A, Karanikas G, Preitfellner J, Becherer A, Pirich C, Dudczak R. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clinical Endocrinology. 2006;65:519–523. doi: 10.1111/j.1365-2265.2006.02626.x. (doi:10.1111/j.1365-2265.2006.02626.x) [DOI] [PubMed] [Google Scholar]
- Shen DH, Kloos RT, Mazzaferri EL, Jhiang SM. Sodium iodide symporter in health and disease. Thyroid. 2001;11:415–425. doi: 10.1089/105072501300176372. (doi:10.1089/105072501300176372) [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Lopez N, Leboeuf R, Minkowitz SM, Grewal R, Brokhin M, Omry G, Larson S. Radioactive iodine administered for thyroid remnant ablation following recombinant human thyroid stimulating hormone preparation also has an important adjuvant therapy function. Thyroid. 2010;20:257–263. doi: 10.1089/thy.2009.0401. (doi:10.1089/thy. 2009.0401) [DOI] [PubMed] [Google Scholar]
- Vadysirisack DD, Venkateswaran A, Zhang Z, Jhiang SM. MEK signaling modulates sodium iodide symporter at multiple levels and in a paradoxical manner. Endocrine-Related Cancer. 2007;14:421–432. doi: 10.1677/erc.1.01263. (doi:10.1677/erc. 1.01263) [DOI] [PubMed] [Google Scholar]
- Ward LS, Santarosa PL, Granja F, da Assumpção LV, Savoldi M, Goldman GH. Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Letters. 2003;200:85–91. doi: 10.1016/s0304-3835(03)00392-6. (doi:10.1016/S0304-3835(03)00392-6) [DOI] [PubMed] [Google Scholar]
- Zaballos MA, Garcia B, Santisteban P. Gbg dimers released in response to thyrotropin activate phosphoinositide 3-kinase and regulate gene expression in thyroid cells. Molecular Endocrinology. 2008;22:1183–1199. doi: 10.1210/me.2007-0093. (doi:10. 1210/me.2007-0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckier LS, Dohan O, Li Y, Chang CJ, Carrasco N, Dadachova E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. Journal of Nuclear Medicine. 2004;45:500–507. [PubMed] [Google Scholar]