Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a significant public health problem caused by repeated episodes of upper airway closure that occur only during sleep. Attempts to treat OSA pharmacologically have been unsuccessful because there has not been identification of a target operating at cranial motor nuclei, blockade of which can reactivate pharyngeal muscle activity throughout sleep. Increasing potassium conductance is a common mechanism by which state-dependent neuromodulators reduce motoneuron excitability. Therefore, we aimed to determine if potassium channel blockade is an effective strategy to reactivate the pharyngeal musculature throughout sleep.
Design, Participants, and Interventions:
In rats chronically instrumented for recording sleep-wake states and respiratory motor activities, we locally microperfused pharmacological agents into the hypoglossal motor pool to modulate potassium channels of three major classes: inwardly rectifying, two-pore domain, and voltage-gated.
Measurements and Results:
Microperfusion of the inwardly rectifying potassium channel blocker, barium, as well as the voltage-gated potassium channel blockers, tetraethylammonium and 4-aminopyridine, increased tonic and respiratory-related genioglossus activities throughout nonrapid eye movement (non-REM) and rapid eye movement (REM) sleep to 133-300% of levels present during baseline wakefulness. In contrast, microperfusion of methanandamide (TWIK-related acid-sensitive potassium [TASK] channel blocker/cannabinoid receptor agonist) activated genioglossus in wakefulness but not in sleep.
Conclusions:
These findings establish proof-of-principle that targeted blockade of certain potassium channels at the hypoglossal motor pool is an effective strategy for reversing upper airway hypotonia and causing sustained reactivation of genioglossus throughout nonrapid eye movement and rapid eye movement sleep. These findings identify an important new direction for translational approaches to the pharmacological treatment of obstructive sleep apnea.
Citation:
Grace KP; Hughes SW; Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. SLEEP 2014;37(1):41-50.
Keywords: Animal models, genioglossus, obstructive sleep apnea, sleep, upper airway
INTRODUCTION
Obstructive sleep apnea (OSA) is a serious clinical problem due to its high prevalence and association with adverse cardiovascular, metabolic, and cognitive outcomes.1–4 OSA is characterized by repeated episodes of upper airway narrowing and closure during sleep.2 Although the causative factors for OSA are variable and complex within individuals,5 that the periods of upper airway closure occur only during sleep highlights the necessary role of sleep-induced changes in pharyngeal motor control in the pathogenesis of OSA.6
Because pharyngeal motor activity in wakefulness is sufficient to maintain airway patency even in patients with severe OSA, an effective pharmacotherapy should aim to restore pharyngeal motor activity in sleep to at least a physiological pattern of activity present when awake.5 Pharmacological attempts7–11 to reverse pharyngeal hypotonia in sleep have essentially used two major strategies: increasing central respiratory drive and attempting to potentiate excitatory neurotransmission onto the pharyngeal motoneurons that drive the upper airway musculature. These strategies have been largely unsuccessful, likely because they fail to address the root mechanisms of pharyngeal hypotonia in sleep and the varying contributions of different neural mechanisms across sleep-wake states.
The root cause of pharyngeal hypotonia in sleep is suppression of cranial motoneuron activity by state-dependent changes in synaptic input.10,12 In nonrapid eye movement (non-REM) sleep, hypoglossal motoneurons are disfacilitated by withdrawal of excitatory inputs, with noradrenergic, serotonergic, and glutamatergic influences being a particular focus of previous studies.13–16 In periods of rapid eye movement (REM) sleep, motoneuron activity can be abolished by additional recruitment of muscarinic receptor-mediated cholinergic inhibition.17 This REM sleep-specific mechanism is powerful, such that concomitant local stimulation of the hypoglossal motor pool with supraphysiological concentrations of excitatory neurotransmitters, or increased central respiratory inputs produced by hypercapnic respiratory stimulation, are insufficient to restore pharyngeal motor activity.16,18,19 In summary, the changing neurochemical environment at the hypoglossal motor pool from wakefulness to sleep is such that motoneuron activity and responsiveness to excitatory inputs can be reduced or virtually abolished, especially in REM sleep. Based on these basic observations, it should not be expected that pharmacological agents that simply increase respiratory drive or increase excitatory neuro-transmission onto hypoglossal motor neurons would be effective in the treatment of OSA.
To effectively reactivate pharyngeal motor activity in sleep, pharmacological strategies are needed that counteract the state-dependent suppression of hypoglossal motor excitability. The multiple state-dependent neurotransmitter systems identified previously13–17 potentially produce such suppression via a convergent ionic mechanism: increased potassium conductance. The inhibitory effect of monoaminergic disfacilitation of the hypoglossal motor pool may be mediated by an increase in potassium conductance, for example via TWIK-related acid-sensitive potassium (TASK) channels.20,21 Furthermore, the periods of atonia of the pharyngeal musculature during REM sleep are produced by cholinergic activation of G-protein coupled inwardly rectifying potassium channels.17 Considering the pivotal role of potassium conductance in the state-dependent modulation of hypoglossal motoneuron excitability, we tested the hypothesis that potassium channel blockade would be an effective pharmacological strategy capable of reactivating the pharyngeal musculature throughout sleep.
To this end, we locally microperfused pharmacological agents into the hypoglossal motor pool to modulate potassium channels of three major classes: inwardly rectifying (Kir), two-pore domain, and voltage-gated (Kv). In this respect, the inwardly rectifying family of potassium channels are important mediators of neuronal excitability.22 Significantly, the Kir2.4 channel subunit, a member of the constitutively active Kir2 channel subfamily, is the most restricted of all Kir subunits in the brain, being expressed mainly on cranial motoneurons such as the hypoglossal.23 Given their highly restricted expression, Kir2.4 is potentially a significant pharmacological target for the manipulation of upper airway muscle activity and OSA. There are currently no available blockers for this class of Kir channels. However, they would be of potential high priority for development and subsequent testing if their possible efficacy in reactivating the pharyngeal musculature can be identified or implicated. Kir channels are blocked with relative specificity by micromolar concentrations of barium,22 whereas millimolar concentrations block many other potassium channel subtypes including notable regulators of neuronal excitability such as two-pore domain TASK channels24,25 and voltage sensitive Kv7.2 (KCNQ2) channels.26,27 We aimed to achieve tissue concentrations of barium at the hypoglossal motor pool that would predominately affect Kir channels, and likewise for blockers of the other channels of interest.
METHODS
Experiments were performed on 33 male Wistar rats (Charles River, Senneville, QC, Canada). Procedures conformed to the recommendations of the Canadian Council on Animal Care, and the University of Toronto Animal Care Committee approved the protocols.
Sterile surgery was performed to implant electrodes for chronic recording of the electroencephalogram and electromyo-grams of the trapezius (neck), diaphragm, and genioglossus muscles as previously described.17,28 Microdialysis guides were placed stereotaxically 3 mm above the hypoglossal motor pool (anterior-posterior, -14.0 mm; medio-lateral, -0.3 mm; dorsoventral, -6.7 mm with respect to bregma). The day before the experiments a microdialysis probe (240-μm-wide by 1-mm-long membrane, 6000 Dalton cutoff; CMA/11-14/01, Chromatography Sciences Company, Montreal, Canada) was inserted into the guide. The probes projected 3 mm from the tip of the guide and targeted the hypoglossal motor pool. Habituation of rats to the recording environment and processing of electrical signals were also performed as described.17,28
The probes were perfused with artificial cerebrospinal fluid (ACSF) for 2 h (control) followed by 2 h of drug. The first h following the switch to drug was excluded from data analysis to allow for diffusion and stabilization of responses. In separate experiments we applied 4-aminopyridine (4-AP, 500 μM, Tocris Bioscience, Minneapolis, MN, USA) in nine rats, tetraethylammonium chloride (TEA, 10 mM, Tocris) in nine rats, barium chloride (2.5 mM, Sigma- Aldrich, St. Louis, MO, USA) in six rats, and the cannabinoid receptor agonist/TASK channel blocker, (R)-(+)-methanandamide (Tocris) in nine rats. When adding TEA or barium chloride to the ACSF, the concentration of sodium chloride was reduced by 10 or 2.5 mM, respectively, to maintain the osmolarity of the perfusate. Stock solutions of methanandamide dissolved in a water-soluble emulsion (1:4 ratio of soya oil/water and the block copolymer Pluronic F68 - Tocri-solve™100, Tocris Bioscience) were diluted ∼140-fold in ACSF to a final concentration of 100 μM.
When microperfusing agents via reverse microdialysis, the tissue concentrations of drugs are only a fraction of the prepared concentrations. Tissue concentration and drug distribution was estimated ex situ by imaging the microperfusion of dye (2.5 mM potassium permanganate) into 0.6% agarose tissue phantoms, which mimics the diffusion characteristics of brain tissue.29 Permangate was chosen because its molecular weight (119 g/ mol) is similar to the lightest (i.e., most diffusible) compounds microperfused in vivo, i.e., barium, TEA, and 4-AP (range, 94-137 g/mol). For these studies, the color intensity of microperfused potassium permanganate dye surrounding the probe tip in agarose tissue phantoms was captured and analyzed using light microscopy (Olympus BX41, Olympus Canada Inc., Markham, Canada) and MATLAB software (Mathworks, Natick, MA, USA). Dye concentration was calculated by comparing color intensity values in the perfused tissue phantoms against intensity values from a standard agarose phantom prepared with the same concentration of dye used in the microperfusion (2.5 mM). Agarose tissue phantoms were incubated at 37°C prior to use.
Probe placements in the hypoglossal motor pool were confirmed by histology17,28 and referenced to a stereotaxic atlas of the rat brain.30 The electrophysiological signals were analyzed as previously described.17,28 For these analyses, sleep-wake states were identified by visual inspection of the neck electromyogram and the electroencephalogram and classified into wakefulness, non-REM, and REM sleep according to standard criteria.17,28 Specifically, each 5- sec epoch was scored as wake, non-REM sleep, REM sleep without transient muscle activations, i.e., “twitches” (REM sleep [-]), or REM sleep with transient muscle activations (REM sleep [+]).
As previously described,17,28 because changes between behavioral states are sometimes associated with difficulty in determining sleep-wake states and accompanied by unstable breathing, the following exclusion criteria were adopted to help ensure that all data included in the analysis of sleep-wake state phenomenology were obtained from temporally enduring periods within unequivocally defined sleep-wake states. Periods of wakefulness or sleep lasting < 30 sec were excluded from analysis. If a state transition occurred during a scoring epoch, then that epoch was also excluded from the analysis. All periods of wakefulness in which rats were eating, drinking, or grooming were excluded from the analysis. During such waking periods, the diaphragm electromyogram recordings can become contaminated by movement-related artefact, and so periods of active wakefulness were excluded from analysis because an inability to identify the peak and trough of each diaphragm breath prevents a meaningful analysis of the principal variables being measured (i.e., diaphragm amplitude, respiratory rate, and respiratory-related genioglossus activity).
Also as previously described17,28 and mentioned earlier, when reporting levels of genioglossus muscle activity in REM sleep we differentiated between periods of REM sleep without and with muscle twitching (REM sleep [-] and REM sleep [+], respectively). In order to distinguish genioglossus muscle twitching from respiratory modulation (i.e., in the event that atonia is prevented and respiratory activity emerges) we defined thresholds to differentiate between these two manifestations of motor activity. Muscle twitching occurs with a shorter inter-burst interval (i.e., a faster frequency) than respiratory modulation, and therefore an epoch of REM sleep was scored as having muscle twitching if the frequency of phasic muscle activation exceeded 125% of the respiratory rate for the same epoch.17,28 Twitch events were analyzed as follows. Muscle twitching activity was analyzed from the rectified raw genioglossus and neck muscle signals followed by derivation of the moving time average signal using a shorter time constant of 30 msec. Using an impulse function, the background tonic activity was filtered out, thereby isolating muscle twitches from the other components of the signal. Upon isolating the muscle twitches, their frequency and peak amplitude were calculated. In all cases, epochs of REM sleep identified as having twitching activity were confirmed via visual inspection of the sleep record.17,28
Even in the absence of motor activity during REM sleep without twitching, low levels of genioglossus muscle activity can always be measured from high-gain electromyogram recordings. Therefore, in each animal we zeroed baseline tonic and respiratory-related genioglossus activity in REM sleep without muscle twitching (i.e., biological zero) and equally adjusted levels in all other states/conditions.17,28
For each breath, the analysis of the genioglossus electro-myogram was time-locked to breathing as defined by the peak and trough of the diaphragm signal. Genioglossus activity was quantified as mean tonic activity (i.e., basal activity in expiration) and respiratory-related activity (i.e. peak inspiratory activity – tonic activity).17,28 As with previous studies,17,28 it is noted that measures of tonic genioglossus activity will inevitably include both basal tone and any background, spontaneous, continuous activity that may occur during certain behaviors, e.g., the sporadic twitches of REM sleep. Likewise, although clear sporadic genioglossus muscle twitches can occasionally occur in REM sleep, whether such sporadic activity that occurs in synchrony with the diaphragm is truly “respiratory” or random activation due to behavioral or REM sleep processes cannot be determined with this or other methods. However, such activations do not affect the measurements in quiet wakefulness, non-REM sleep, and REM sleep without such sporadic genioglossus muscle twitches, i.e., REM(-), that are a major focus of this study.
In some cases, changes in genioglossus muscle activity were also analyzed as a function of brain arousal state. We used a ratio of electroencephalographic power in two frequency bands (power, 0-20Hz/power, 0-55Hz) to define the spectrum of arousal according to the method of “state-space analysis.”31
Data are presented as means ± standard error of the mean. For the two-way analyses of variance with repeated measures (ANOVA-RM), the two factors were Treatment (i.e., ACSF versus drug applied to the hypoglossal motor pool) and State (i.e., wakefulness, non-REM sleep, and REM sleep). For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using a two-tailed test. Where post hoc comparisons were performed after ANOVARM, the Holm-Sidak test was used to test statistical significance. All analyses were performed using Sigmastat (SPSS, Chicago, IL, USA).
RESULTS
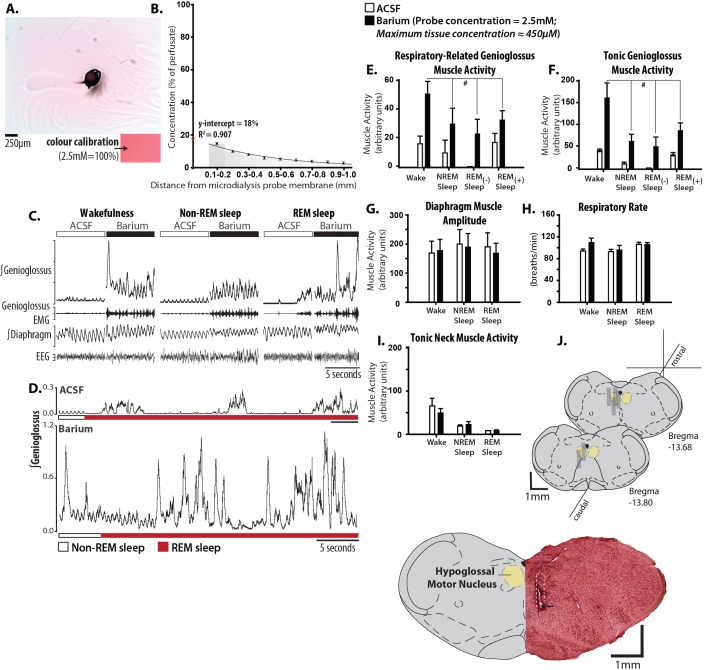
Figure 1A and B shows that after 2 h of microperfusion, the maximum concentration of drug in the phantom tissue surrounding the microdialysis probe tip was ≤ 18% of the concentration in the perfusion medium. We therefore micro-perfused 2.5 mM barium into the hypoglossal motor pool, yielding an estimated tissue concentration of ≤ 450 μM. This concentration is reasonable for the barium sensitivity of Kir2.4 (IC50 = 390 μM).23 Under these conditions, microperfusion of barium into the hypoglossal motor pool reactivated genioglossus throughout sleep (Figure 1C and D). Figure 1D also shows that microperfusion of barium into the hypoglossal motor pool completely prevented the periods of motor atonia that normally accompany REM sleep, such that tonic and respiratory genioglossus muscle activities were preserved throughout the REM sleep episodes. Given that waking pharyngeal motor activity is sufficient to prevent airway collapse even in patients with severe OSA, this targeted manipulation of the hypoglossal motor pool reveals a mechanism that is capable of increasing genioglossus activity throughout both non-REM and REM sleep to levels that even exceed those during baseline wakefulness.
Figure 1.
Microperfusion of barium into the hypoglossal motor pool restores genioglossus muscle activity throughout sleep to waking levels. Ex situ determination of tissue drug concentration relative to internal probe concentration is shown in A and B. (A) An aerial view of a 0.6% agarose slab microperfused with 2.5 mM permanganate dye (i), with the bottom inset (ii) showing an agarose standard prepared with the same dye. The black distortion in the middle of the agarose is where the microdialysis probe was placed. (B) Histogram showing the average decay profile of drug concentration along multiple radii stemming from the origin of drug diffusion: two agarose slabs with eight radii per experiment. (C) Example in one rat showing genioglossus muscle activation with microperfusion of 2.5 mM barium into the hypoglossal motor pool across sleep-wake states, with longer rapid eye movement (REM) episodes shown in (D). Note that in the presence of barium at the hypoglossal motor pool the motor suppression during REM sleep is completely reversed. Group data (n = 6) showing the effects of barium on respiratory-related (E) and tonic (F) genioglossus muscle activities during wakefulness, non-REM, and REM sleep with ([-]) and without ([+]) muscle twitching. The lack of effects of barium in sleep and wakefulness on other control variables are shown in G-I: diaphragm muscle amplitude, respiratory rate, and tonic neck muscle activity. (J) Example and group data showing the location of microdialysis probes. The example shows a coronal section of tissue with the site of microdialysis within the hypoglossal motor pool. The location of the ventral tip of the probe site is indicated by the black arrow, and the approximate position of the entire microdialysis probe membrane is denoted by the white dotted section. The coronal diagrams of the rat medulla show the locations of all the sites of microdialysis. Gray rectangles represent the space occupied by the membrane portion of the microdialysis probes. Values are means ± standard error of the mean. # indicates significant effect of barium relative to artificial cerebrospinal fluid (ACSF) controls independent of sleep-wake state (P < 0.05, from analysis of variance).
Group data from six rats (Figure 1E and F) identified that microperfusion of barium into the hypoglossal motor pool significantly increased tonic and respiratory-related genioglossus muscle activities, relative to ACSF controls, independent of the prevailing sleep-wake state (range of t5 = 4.44 to 4.03, range of P = 0.010 to 0.008, post hoc paired t-tests following identification of: (1) a significant main effect of treatment, range of F1,5 = 16.22 to 18.13, range of P = 0.008 to 0.010, and (2) a nonsignificant interaction between treatment and state, F3,15 = 0.84 to 2.58, P = 0.517 to 0.069, two-way ANOVA-RM). To our knowledge, such a robust effect in activating pharyngeal motor activity throughout sleep has not been achieved with any other blocking agent that targets a mechanism that appears largely restricted to the pharyngeal motor nuclei.23 This observation identifies proof-of-principle and opens up the possibility of targeting a small molecule to Kir2.4 channels to reactivate respiratory and tonic pharyngeal muscle activity throughout sleep.
The effects of microperfusion of barium into the hypoglossal motor pool were specific to genioglossus muscle as other variables were not significantly affected. The lack of effects on other variables included the amplitude of diaphragm activity (Figure 1G, F1,5 = 0.11, P = 0.750, two-way ANOVA-RM), respiratory rate (Figure 1H, F1,5 = 0.64, P = 0.461), neck muscle activity (Figure 1I, F = 0.40, P = 0.461), and electroencephalographic power within any frequency band (F1,5 = 1.29 to 0.042, P = 0.308 to 0.812). Figure 1J shows that the sites of microperfusion of barium were within the hypoglossal motor pool in all rats.
Two-pore domain TASK channels produce ‘background’ or ‘leak’ potassium currents that maintain membrane potential at hyperpolarized levels.32 TASK channels are responsible for most of the resting potassium conductance of hypoglossal moto-neurons in vitro, and can be inhibited by arousal-related excitatory neurotransmitters such as serotonin, noradrenaline, and glutamate (via group I metabotropic glutamate receptors).20,21,33 Accordingly, modulation of TASK channel activity may be a potentially significant pharmacological target for the manipulation of upper airway muscle activity. As for Kir channels, however, there are few available blockers for TASK channels.
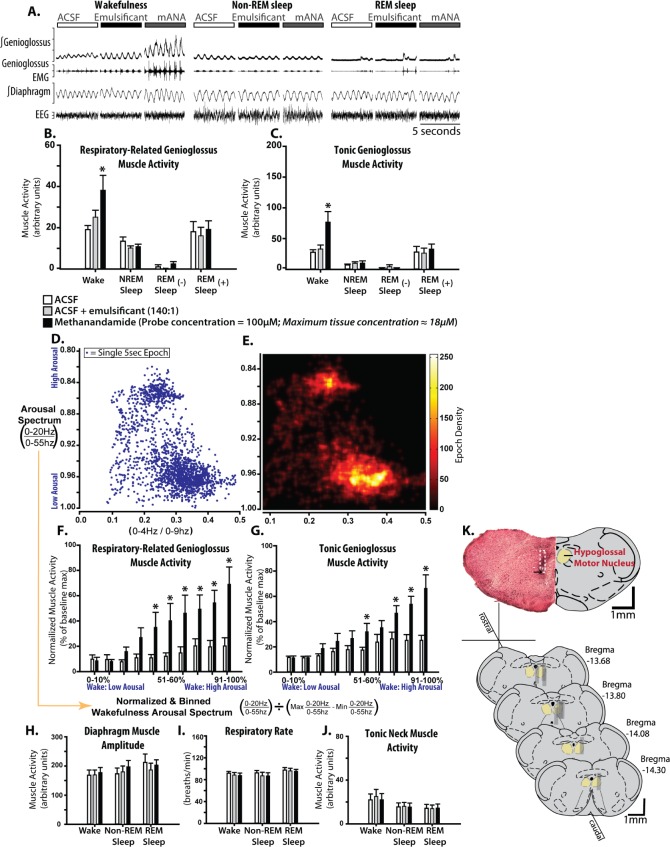
Based on the estimates of the maximum concentration of drug in the phantom tissue surrounding the microdialysis probe tip being ≤ 18% of the concentration in the perfusion medium (Figure 1A-C), we microperfused 100 μM methanandamide for an estimated tissue concentration of ≤ 18 μM. TASK-1 currents are nearly abolished by 10 μM methanandamide in vitro.34 The example shown in Figure 2A shows that microperfusion of methanandamide into the hypoglossal motor pool did not reactivate pharyngeal motor activity in sleep, but did produce a motor activation that was present only in wakefulness.
Figure 2.
Microperfusion of methanandamide into the hypoglossal motor pool activates genioglossus muscle activity exclusively in wakefulness. (A) Example in one rat showing genioglossus muscle activity during microperfusion of: artificial cerebrospinal fluid (ACSF) (control), a water soluble emulsificant (control) and 100 μM emulsified methanandamide into the hypoglossal motor pool across sleep-wake states. Note the selective genioglossus muscle activation by methanandamide compared to controls. Group data (n = 9) showing the effects of methanandamide on respiratory-related (B) and tonic (C) genioglossus muscle activities during wakefulness, non-REM, and REM sleep with ([-]) and without ([+]) muscle twitching, compared with ACSF and emulsificant controls. (D) A scatterplot bounded by two electroencephalographic ratios showing the position of 5-sec segments of wakefulness and non-REM sleep in a two-dimensional state-space. (E) A density plot of the points in D. Note that in D and E, separate wake and non-REM sleep clusters are visible along the y-axis, meaning that the spectral power ratio (0-20Hz/0-55Hz) is an effective indicator of the arousal spectrum (high in wake to low in non-REM sleep). (F and G) Group data showing the effects of methanandamide on respiratory and tonic genioglossus muscle activities during waking epochs as a function of waking arousal level identified previously. Note the relationship between increased level of arousal and increased methanandamide-mediated genioglossus muscle activation. The lack of effect of methanandamide across sleep-wake states on the control variables are shown in H-J for diaphragm amplitude, respiratory rate and tonic neck muscle activity. (K) Example and group data showing the location of microdialysis probes from coronal sections. The example from a single animal indicates the site of microdialysis within the hypoglossal motor pool. The location of the ventral tip of the probe site is indicated by the black arrow and the approximate position of the entire microdialysis probe membrane is denoted by the white dotted section. The locations of all the sites of microdialysis are also shown. Gray rectangles represent the space occupied by the semi-permeable membrane portion of the microdialysis probes. Values are means ± standard error of the mean. Asterisk indicates significant effect of methanandamide compared with controls (P < 0.05, from analysis of variance). mANA, methanandamide.
Group data from nine rats (Figure 2B and C) shows a significant activating effect of methanandamide at the hypoglossal motor pool on both respiratory and tonic genioglossus motor activities compared to both the ACSF and emulsificant controls, with this activation effect being present only during wakefulness (range of t8 = 4.13 to 6.41, all P < 0.001, post hoc paired t-tests following identification of: (1) a significant main effect of treatment, F2,16 = 6.56 to 6.95, P = 0.007 to 0.008; and (2) a significant interaction between treatment and state, F3,16 = 4.57 to 6.03, P ≤ 0.001, two-way ANOVA-RM). The emulsificant used to solubilize methanandamide did not affect genioglossus activity relative to ACSF controls (t8 = 0.71 to 1.92, P = 0.479 to 0.059, post hoc paired t-tests), and therefore was not responsible for the wakefulness-dependent genioglossus activation.
To further characterize this wakefulness-dependent motor activation with methanandamide at the hypoglossal motor pool, we used an electroencephalographic frequency ratio that reflects moment-to-moment shifts along the spectrum of arousal from active wakefulness through to deep non-REM sleep; i.e., ‘state-space analysis’31 (Figure 2D and E). Here we show that the activating effects of methanandamide on respiratory and tonic genioglossus motor activities increased with increasing levels of waking electrocortical arousal but was absent at lower levels of waking arousal (Figure 2F and G; range of t8 = 2.06 to 5.36, P = 0.043 to < 0.001, post hoc paired t-tests following identification of: (1) a significant main effect of treatment, F1,8 = 9.07 to 21.32, P = 0.017 to 0.002, and, (2) a significant interaction between treatment and level of electroencephalographic arousal, F9,8 = 2.99 to 4.83, P = 0.005 to < 0.001, two-way ANOVA-RM). For these studies, the ACSF and emulsificant control groups were pooled due to the absence of significant differences between them in the previous analysis. Overall, these data show that the activating effect of methanandamide at the hypoglossal motor pool is restricted to periods of high-waking electrocortical arousal. This result suggests that other such agents with the pharmacological profile of methanandamide may not be an effective strategy for direct reactivation of the upper airway musculature during sleep if their primary targeted site of action was the hypoglossal motor pool. However, such a definitive result can only be confirmed with more specific TASK channel blockers but these are not currently commercially available. Overall, these findings are significant because modulating these channels is cited as a possible pharmacotherapeutic target for OSA.35–37
The effects of microperfusion of methanandamide into the hypoglossal motor pool were also specific to the genioglossus because other variables were not significantly affected. The lack of effects on other variables included diaphragm activation (Figure 2H; F2,16 = 9.83, P = 0.002, two-way ANOVA-RM), respiratory rate (Figure 2I; F2,16 = 2.31, P = 0.131), and neck muscle activity (Figure 2J; F2,16 = 1.16, P = 0.339). Micro-perfusion of methanandamide also did not affect electroencephalographic power within any frequency band (range of F2,16 = 0.32 to 0.88, P = 0.718 to 0.131, two-way ANOVA-RM). The absence of changes in these indices of brain electrocortical arousal state and postural (neck) motor activity indicate that the wakefulness-specific genioglossus activation produced by microperfusion of methanandamide into the hypoglossal motor pool was not the indirect result of increased motor drive related to a change in behavioural state per se; i.e., they were the direct result of the intervention. Figure 2K shows that the sites of microperfusion of methanandamide were within the hypoglossal motor pool in all rats.
Control of neuronal activity by Kv channels is complex. High-threshold Kv channels act to reduce action potential duration thus permitting high rates of repetitive firing, whereas low-threshold Kv channels oppose neuronal depolarization to action potential threshold, thereby restraining presynaptic and postsynaptic hyperexcitability.38,39 Consistent with a predominant effect on the latter group, microperfusion of the Kv channel blockers TEA and 4-AP into the hypoglossal motor pool strongly activated genioglossus muscle activity throughout sleep.
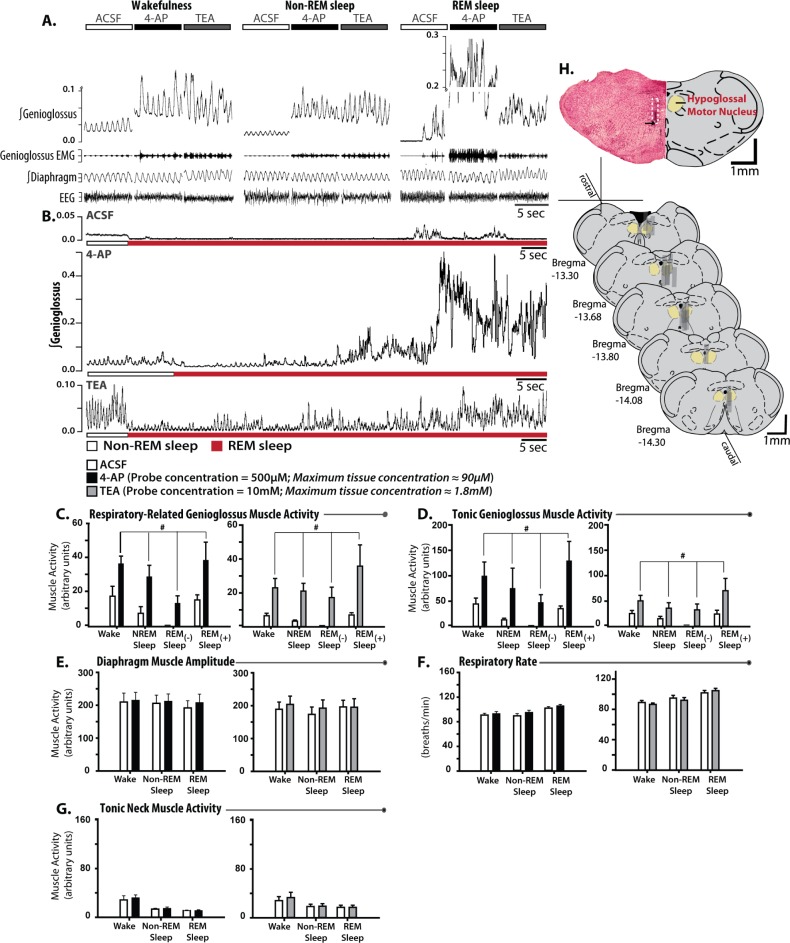
Figure 3A shows examples of increased genioglossus muscle activity across sleep-wake states during local microperfusion of 4-AP and TEA into the hypoglossal motor pool. Estimates based on the maximum concentration of drug in the phantom tissue surrounding the microdialysis probe tip being ≤ 18% of the concentration in the perfusion medium (Figure 1A-C) suggest tissue concentrations of ≤ 90 μM and 2.1 mM for 4-AP and 10 mM TEA, respectively. In Figure 3A, note that 4-AP and TEA increased genioglossus activity throughout both non-REM and REM sleep to levels exceeding those in normal wakefulness. Figure 3B shows that local microperfusion of 4-AP and TEA into the hypoglossal both completely prevented the periods of motor atonia that normally accompany REM sleep, such that tonic and respiratory genioglossus muscle activities were preserved throughout the REM sleep episodes.
Figure 3.
Blockade of voltage-gated potassium channels at the hypoglossal motor pool produces suprawaking levels of genioglossus muscle activity throughout sleep. (A) Examples from individual rats showing genioglossus muscle activation by microperfusion of 500 μM 4-AP (n = 9) and 10 mM TEA (n = 9) into the hypoglossal motor pool across sleep-wake states (ACSF sections taken from rat administered 4-AP), with longer REM episodes shown in (B; ACSF sections taken from rat-administered TEA). Group data showing the effects of 4-AP and TEA on respiratory-related (C) and tonic (D) genioglossus muscle activities during wakefulness, non-REM, and REM sleep with ([-]) and without ([+]) muscle twitching. E-H shows the effects of 4-AP/TEA across sleep-wake states on the control variables: diaphragm amplitude, respiratory rate, tonic neck muscle activity, and the amplitude of REM sleep-specific muscle twitching. (I) Example and group data showing the location of microdialysis probes. The example from a single animal indicates the site of microdialysis within the hypoglossal motor pool. The location of the ventral tip of the probe site is indicated by the black arrow and the approximate position of the entire microdialysis probe membrane is denoted by the white dotted section. The locations of all the sites of microdialysis are also shown. Gray rectangles represent the space occupied by the semipermeable membrane portion of the microdialysis probes. Values are means ± standard error of the mean. # indicates significant effect of either 4-AP or TEA relative to ACSF controls independent of sleep-wake state (P < 0.05, from analysis of variance). ACSF, artificial cerebrospinal fluid; REM, rapid eye movement;TEA, tetraethylammonium chloride; 4-AP, 4-aminopyridine.
Group data from nine rats with microperfusion of 4-AP into the hypoglossal motor pool, and the other nine rats with micro-perfusion of TEA, identified a significant increase in respiratory-related and tonic genioglossus muscle activities relative to the ACSF controls that occurred independently of the prevailing sleep-wake state (Figure 3C and D; range of t8 = 2.39 to 3.35, P = 0.044 to 0.010, post hoc paired t-tests following identification of: (1) a significant main effect of treatment, F1,8 = 7.97 to 15.27, P = 0.022 to < 0.001, and (2) an insignificant interaction between treatment and state, F3,24 = 0.44 to 2.21, P = 0.728 to 0.113, two-way ANOVA-RM).
The effects of microperfusion of TEA and 4-AP into the hypoglossal motor pool were specific to the genioglossus muscle as other variables were not significantly affected. The lack of effects on other variables included the amplitude of diaphragm activity (Figure 3E, range of F1,8 = 0.48 to 2.80, P = 0.508 to 0.133, two-way ANOVA-RM), respiratory rate (Figure 3F, F1,8 = 1.25 to 1.60, P = 0.296 to 0.240), neck muscle activity (Figure 3G, F1,8 = 3.88 to 1.59, P = 0.700 to 0.084) or electroencephalographic power within any frequency band (F1,8 = 0.008 to 4.99, P = 0.932 to 0.056). Figure 3H shows that the sites of microperfusion of TEA and 4-AP were within the hypoglossal motor pool in all rats.
DISCUSSION
Here we show that targeted modulation of potassium channel conductance at the hypoglossal motor pool is, in principle, an effective strategy to reactivate genioglossus muscle throughout sleep to at least normal waking levels. It is important to note that the restoration and sustained reactivation of both tonic and respiratory components of pharyngeal muscle activity, throughout both non-REM and REM sleep, has not been achieved by other blocking agents at this motor pool. These results, therefore, establish an important new direction for translational sleep science by identifying potassium channel modulation as an appropriate target for developing a small molecule pharmacotherapy for OSA. As discussed in the next paragraphs, manipulation of such a target is viable because certain potassium channel subunits show highly restricted expression in the brain, mainly on cranial motoneurons such as the hypoglossal.23 Given the highly restricted expression, such channels provide an appropriate and high-priority molecular target for drug development to selectively manipulate upper airway muscle activity for OSA.
Kir channels significantly influence the excitability of hypoglossal motoneurons. Barium-mediated blockade of hypoglossal Kir channels in vitro produces motoneuron depolarization, tonic spike firing, and increased spiking frequency in response to suprathreshold current injections.23 Here we show that putative barium-mediated blockade of Kir channels at the hypoglossal motor pool in vivo produces suprawaking levels of pharyngeal motor activity during both non-REM and REM sleep. We have previously shown that targeted blockade of G-protein coupled Kir3 channels restores respiratory-related genioglossus activity specifically in REM sleep.17 That study provided evidence of a powerful and REM sleep-specific motor inhibitory mechanism that can be viewed as a genuine and significant mediator of REM sleep pharyngeal motor inhibition because its blockade has its largest influence in REM sleep and trivial, or no, effect in other sleep-wake states.17 In contrast and of significance, the relatively nonspecific blockade of Kir channels in the current study produced activation of pharyngeal motor activity across all sleep-wake states, an effect consistent with a state-independent increase in motoneuronal gain stemming from the additional blockade of constitutively open Kir channels. Importantly, hypoglossal motoneurons together with other cranial motor pools express the Kir2.4 channel, whereas evidence of its expression is lacking elsewhere.23 Kir2.4 is a member of the constitutively open Kir2 channel subfamily, and appears to be the most abundantly expressed Kir2 family subunit at the hypoglossal motor pool.23 The near-exclusive expression of Kir2.4 to cranial motor pools together with the efficacy of Kir channel blockade in restoring waking levels of pharyngeal motor activity throughout sleep suggest that Kir2.4 is a rational and high-priority target for the future development of a pharmacological treatment for OSA. There are currently no available agents to specifically modulate Kir2.4 channel function.
Inhibition of TASK channels is cited as a possible pharmacotherapeutic target for OSA.35–37 TASK channels are strongly expressed at motor nuclei including the cranial motor pools, are major determinants of hypoglossal motoneuron resting potassium conductance in vitro, and are inhibited by neuromodulators arising from neuronal groups that are active in wakefulness but less active in sleep.20,21,33 Together, these observations suggest that withdrawal of endogenous wake-active neuromodulators from wakefulness to sleep may lead to TASK channel opening and reduced motoneuronal excitability, whereas targeted inhibition of such channels would prevent this endogenous mechanism and reactivate pharyngeal motor activity in sleep. Here we show that local microperfusion of the TASK channel blocker/ cannabinoid receptor agonist methanandamide into the hypoglossal motor pool does activate pharyngeal motor activity, but this effect is apparent only in wakefulness and not in sleep. Further analysis confirmed that the magnitude of the methanandamide-mediated increase in waking genioglossus activity was related to the level of waking arousal as judged by frequency analysis of the electroencephalogram.
The wakefulness-only genioglossus activating effect of microperfusion of methanandamide into hypoglossal motor pool is consistent with exaggerated nocturnal motor activity in TASK-3 knockout mice.40 However, the lack of other observable motor or neurological differences in TASK channel knockout mice may be due to a compensatory increase in inhibitory γ-amino-butyric acid type-A (GABAA) receptor function that can occur independently of changes in GABAA receptor expression.41,42 It is possible, therefore, that in our study the physiological significance of TASK channel modulation is masked by a rapid homeostatic regulation of membrane potential mediated by GABAA receptors. Even if this scenario of compensation for the intervention is correct, however, TASK channel blockade at the hypoglossal motor pool may not be an effective strategy to reactivate pharyngeal motor activity in sleep (as the effects are confined to wakefulness) such that targeting these channels may not be effective for OSA. Such a definitive conclusion can only be confirmed, however, with more specific TASK channel blockers but none are currently commercially available.
In addition to blocking TASK channels, methanandamide is also an agonist of cannabinoid receptors. A function of cannabinoid receptors throughout the nervous system, including the hypoglossal motor pool,43 is presynaptic depression of inhibitory neurotransmitter release. Accordingly, the motor-activating effect of microperfusion of methanandamide into the hypoglossal motor pool could be explained by such a mechanism. If such a scenario is correct, however, we would expect a priori that depression of inhibitory neurotransmission at the hypoglossal motor pool would produce motor activation in sleep as well as wakefulness, and this did not occur. Methanandamide can also inhibit voltage-gated sodium channels,44 N-type/T-type calcium channels,45,46 and shaker-type voltage gated potassium channels.47 With the exception of the postassium channels, however, such non-TASK channel effects potentially mediated by methanandamide would be expected to inhibit hypoglossal motoneurons.
We also showed that blockade of either TEA or 4-AP sensitive potassium conductances at the hypoglossal motor pool were sufficient to activate pharyngeal muscle activity throughout sleep to waking levels. TEA and 4-AP sensitive Kv channels having high voltage thresholds mediate the fast component of the after-hyperpolarization in hypoglossal motoneurons; blockade of such channels increases action potential duration, reduces firing frequency, and would therefore tend to suppress downstream motor activity.48 As a consequence, the TEA and 4-AP-mediated activation of pharyngeal muscle activity is likely mediated by blockade of low threshold Kv channels, which normally act to suppress presynaptic and postsynaptic membrane excitability.39
Low-threshold Kv channels operate below the threshold for action potential generation, producing outward currents in response to depolarizations from resting membrane potential.38 The relatively low concentration of 4-AP in this study (estimated maximum tissue concentration of ∼90 μM) would be expected to broadly inactivate the low threshold Kv1 subfamily whereas TEA (estimated maximum tissue concentration of ∼1.8mM) would be expected to more specifically block Kv1.1 and 4-AP insensitive Kv7.2 (KCNQ2) channels.22,32,49–57 Blockade of these channels likely contributes to the increased pharyngeal motor activity observed during sleep in the presence of microperfusion of TEA or 4-AP into the hypoglossal motor pool. Inactivation of low threshold Kv channels would be expected to increase the probability of action potential firing via sensitization of the postsynaptic membrane to depolarizing inputs. Low threshold Kv channels, specifically Kv1.1, are also located presynaptically where they prevent hyperexcitability and spontaneous action potential generation. Presynaptic actions of TEA and 4-AP also likely contribute to the observed pharyngeal motor activation in sleep, particularly REM sleep, when significant facilitatory input is required to overcome motoneuron inhibition. Ultimately, the combination of presynaptic and postsynaptic effects of 4-AP and TEA may underlie their ability to activate motor activity in REM sleep.
In summary, these findings establish proof-of-principle that targeted blockade of certain potassium channels at the hypoglossal motor pool is an effective strategy to produce sustained activation of tonic and respiratory components of pharyngeal muscle activity throughout non-REM and REM sleep. Such responses have not been observed with other blocking agents at this motor pool. Certain potassium channels show highly restricted expression to the cranial motor pools, particularly the hypoglossal.23 Accordingly, development of specific agents (currently lacking) to manipulate such channels—aiming to restore physiological patterns of activity previously present in wakefulness—may provide a viable and tractable pharmacological target of benefit, for example, to patients with OSA. Further studies may also identify the broad applicability of the current findings to other cranial motor pools (as would be suggested by the regionalized distribution of channel expression23), and the type of motor units preferentially affected by the interventions and the mechanical consequences on airway stability (e.g., tonic and/or inspiratory-modulated units58,59).
DISCLOSURE STATEMENT
This work was cosupported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563), and a collaborative academic research discovery grant with full legal agreements between Lilly UK and University of Toronto. Dr. Horner is supported by a Tier I Canada Research Chair in Sleep and Respiratory Neurobiology. Mr. Grace was supported by Canadian Institutes of Health Research Team Research and Training Grant in Sleep and Biological Rhythms. Dr. Hughes is an employee of Eli Lilly and Company. Work was performed at the University of Toronto.
REFERENCES
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 5.Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15:587–95. doi: 10.1111/j.1440-1843.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 7.Lin CM, Huang YS, Guilleminault C. Pharmacotherapy of obstructive sleep apnea. Expert Opin Pharmacother. 2012;13:841–57. doi: 10.1517/14656566.2012.666525. [DOI] [PubMed] [Google Scholar]
- 8.Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(2):CD003002. doi: 10.1002/14651858.CD003002.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Smith IE, Quinnell TG. Pharmacotherapies for obstructive sleep apnoea: where are we now? Drugs. 2004;64:1385–99. doi: 10.2165/00003495-200464130-00001. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veasey SC. Will we ever have an effective pharmacotherapy for obstructive sleep apnea? Sleep. 2005;28:18–9. [PubMed] [Google Scholar]
- 12.Horner RL. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2553–64. doi: 10.1098/rstb.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–73. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 14.Steenland HW, Liu H, Horner RL. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci. 2008;28:6826–35. doi: 10.1523/JNEUROSCI.1019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–30. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess C, Lai D, Siegel J, Peever J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle. J Neurosci. 2008;28:4649–60. doi: 10.1523/JNEUROSCI.0334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–9. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 18.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164:179–96. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 20.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 21.Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997;110:139–50. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 22.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 23.Topert C, Doring F, Wischmeyer E, et al. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci. 1998;18:4096–105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayliss DA, Viana F, Berger AJ. Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992;68:1733–45. doi: 10.1152/jn.1992.68.5.1733. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ND, Nistri A. A study of the barium-sensitive and -insensitive components of the action of thyrotropin-releasing hormone on lumbar motoneurons of the rat isolated spinal cord. Eur J Neurosci. 1993;5:1360–9. doi: 10.1111/j.1460-9568.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 27.Constanti A, Galvan M. M-current in voltage-clamped olfactory cortex neurones. Neurosci Lett. 1983;39:65–70. doi: 10.1016/0304-3940(83)90166-0. [DOI] [PubMed] [Google Scholar]
- 28.Grace KP, Liu H, Horner RL. 5-HT1A receptor-responsive pedunculopontine tegmental neurons suppress REM sleep and respiratory motor activity. J Neurosci. 2012;32:1622–33. doi: 10.1523/JNEUROSCI.5700-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen ZJ, Gillies GT, Broaddus WC, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. J Neurosurg. 2004;101:314–22. doi: 10.3171/jns.2004.101.2.0314. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- 31.Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci. 2004;24:11137–47. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2013;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 33.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coburn C, Wang J, Santarelli V, et al. (Merck & Co., West Point, USA). TASK Channel Antagonists. Int. Pat. Appl. WO 2011/103715, 2011. [Google Scholar]
- 36.Brendel J, Goegelein H, Wirth K, Kamm W. (Sanofi-Aventis Deutschland GMBh, Frankfurt-Am-Main, Germany). Inhibitors of the TASK-1 and TASK-3 Ion Channel. Int. Pat. Appl. WO 2007/124849, 2007. [Google Scholar]
- 37.Coburn CA, Luo Y, Cui M, et al. Discovery of a pharmacologically active antagonist of the two-pore-domain potassium channel K2P9.1 (TASK-3) Chem Med Chem. 2011;7:123–33. doi: 10.1002/cmdc.201100351. [DOI] [PubMed] [Google Scholar]
- 38.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–7. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Linden AM, Sandu C, Aller MI, et al. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther. 2007;323:924–34. doi: 10.1124/jpet.107.129544. [DOI] [PubMed] [Google Scholar]
- 41.Linden AM, Aller MI, Leppa E, Rosenberg PH, Wisden W, Korpi ER. K+ channel TASK-1 knockout mice show enhanced sensitivities to ataxic and hypnotic effects of GABA(A) receptor ligands. J Pharmacol Exp Ther. 2008;327:277–86. doi: 10.1124/jpet.108.142083. [DOI] [PubMed] [Google Scholar]
- 42.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 43.Mukhtarov M, Ragozzino D, Bregestovski P. Dual Ca2+ modulation of glycinergic synaptic currents in rodent hypoglossal motoneurones. J Physiol. 2005;569:817–31. doi: 10.1113/jphysiol.2005.094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–9. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- 46.Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–40. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poling JS, Rogawski MA, Salem N, Jr, Vicini S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35:983–91. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 48.Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–63. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- 49.Grissmer S, Nguyen AN, Aiyar J, et al. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–34. [PubMed] [Google Scholar]
- 50.Stephens GJ, Garratt JC, Robertson B, Owen DG. On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. J Physiol. 1994;477:187–96. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavanaugh MP, Varnum MD, Osborne PB, et al. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991;266:7583–7. [PubMed] [Google Scholar]
- 52.Coetzee WA, Amarillo Y, Chiu J, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–85. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 53.Fedida D, Bouchard R, Chen FS. Slow gating charge immobilization in the human potassium channel Kv1.5 and its prevention by 4-aminopyridine. J Physiol. 1996;494:377–87. doi: 10.1113/jphysiol.1996.sp021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fedida D, Wible B, Wang Z, et al. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993;73:210–6. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- 55.Kalman K, Nguyen A, Tseng-Crank J, et al. Genomic organization, chromosomal localization, tissue distribution, and biophysical characterization of a novel mammalian Shaker-related voltage-gated potassium channel, Kv1.7. J Biol Chem. 1998;273:5851–7. doi: 10.1074/jbc.273.10.5851. [DOI] [PubMed] [Google Scholar]
- 56.Wang HS, Pan Z, Shi W, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 57.Yang WP, Levesque PC, Little WA, et al. Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy. J Biol Chem. 1998;273:19419–23. doi: 10.1074/jbc.273.31.19419. [DOI] [PubMed] [Google Scholar]
- 58.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 59.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]