Abstract
Objective:
To evaluate the efficacy of a brief 4-w group-administered treatment program of cognitive behavior therapy for insomnia (CBT-I) for older adults with sleep maintenance insomnia.
Design:
Randomized controlled trial of CBT-I compared to waitlist control with comparisons at pretreatment, posttreatment, and 3-mo follow-up.
Setting:
Flinders University Sleep and Circadian Rhythm Research Laboratory, Adelaide, South Australia.
Participants:
One-hundred eighteen adults with sleep maintenance insomnia (mean age = 63.76 y, standard deviation = 6.45 y, male = 55).
Interventions:
A 4-w, group-based treatment program of CBT-I including bedtime restriction therapy, sleep education, and cognitive restructuring.
Measurements:
Seven-day sleep diaries, actigraphy, and several self-report measures to assess perceived insomnia severity, daytime functioning, and confidence in and beliefs about sleep.
Results:
The brief group-administered CBT-I program produced improvements in the timing and quality of sleep including later bedtimes, earlier out-of-bed times, reduced wake after sleep onset, and improved sleep efficiency. Participants also reported a reduction of the Insomnia Severity Index, Flinders Fatigue Scale, Epworth Sleepiness Scale, Daytime Feeling and Functioning Scale, Sleep Anticipatory Anxiety Questionnaire, the Dysfunctional Beliefs and Attitudes Scale, and increased Sleep Self-Efficacy Scale.
Conclusions:
The treatment program used in the current study has demonstrated potential for a brief, inexpensive, and effective treatment of sleep maintenance insomnia in the older adult population.
Citation:
Lovato N; Lack L; Wright H; Kennaway DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. SLEEP 2014;37(1):117-126.
Keywords: Insomnia, older adults, CBT-I
INTRODUCTION
Chronic insomnia is a highly prevalent and persistent health concern particularly within the older population.1 It has a substantial effect on the well-being of affected individuals, with many experiencing impairments to daytime functioning,2–4 depressed mood,5 anxiety, and difficulty maintaining social relationships.3,6 In recent years, surveys have indicated that approximately 20-40% of adults older than 55 y report waking a lot during the night, waking too early and not being able to get back to sleep, and waking feeling unrefreshed.7,8 The current nonpharmacological treatment of choice is cognitive behavior therapy for insomnia (CBT-I)3,9; however, individualized administration is costly and research indicates the absolute changes in sleep variables following the use of CBT-I are mild, particularly for older adults.10–12
Group-based administration of CBT-I conducted over six to eight weekly sessions has been suggested to provide a brief and inexpensive answer to the effective treatment of insomnia in the adult population.13–16 However, the efficacy of group-based CBT-I for older adults remains relatively uninvestigated. To the authors' knowledge, only one study has assessed the efficacy of group-administered CBT-I in older adults (> 60 y).
Morin et al.17 assessed the efficacy of a 10-w group-based CBT-I program in a group of 24 older adults (mean age = 61.4 y) with chronic insomnia who had used hypnotic agents for at least 3 mo. Treatment sessions occurred weekly, were approximately 90 min in duration, and administered to small groups of four to six individuals. Following treatment, participants reported a modest reduction in subjective total time awake, which was maintained at 3-mo follow-up. Although not significant immediately following treatment, improvements in sleep efficiency and reductions in sleep onset latency were observed at 3-mo follow-up. Participants also reported reductions in insomnia severity (measured by the Insomnia Severity Index3) both immediately following treatment and at follow-up. As acknowledged by Morin et al.,3 although this study demonstrated impressive potential for the use of group-based CBT-I in older adults, the generalizability of these findings is limited to those who have chronic insomnia and use hypnotic agents. Additionally, despite the group-based nature of this program, further cost savings could be obtained if the number of weekly therapy sessions could be reduced without loss of effectiveness.
Espie and colleagues18 assessed the efficacy of a five-session group-based, manualized, CBT-I intervention. Although this study was not restricted to older adults, the mean age of participants was 54 y. Treatment sessions were conducted weekly, were 60 min in duration, and administered by nurses to groups of four to six participants. Significant reductions in sleep onset latency, wake after sleep onset, and sleep efficiency were reported by participants immediately following treatment. Global self-reported sleep quality (assessed by the Pittsburgh Sleep Quality Index19) also improved following treatment. These improvements, however, were not maintained at 6-mo follow-up. This study demonstrates impressive potential for the use of a brief group-administered CBT-I treatment program; however, similar to the study by Morin et al.,3 the results of this study are largely limited to individuals with chronic insomnia and comorbid mental or physical health problems (70% of the sample), the majority of whom were using hypnotic agents (50% of the sample).
The current study used a randomized controlled trial to investigate the effects of a brief, group-based CBT-I treatment program in a large sample of hypnotic free older adults with chronic primary insomnia.
METHODS
Participants
Three hundred and seventy-eight potential participants were recruited into the study between June 2008 and February 2011. Participants were recruited from a variety of sources, including advertisements in the local newspapers of metropolitan Adelaide, announcements to social groups, such as senior citizens, and broadcasts in electronic media. Participants were assessed for eligibility using a semistructured telephone interview, 7-day sleep diary and actigraphy, several questionnaires, and a single night of home-based polysomnography.
Potential participants were screened for sleep maintenance insomnia using the Pittsburgh Sleep Quality Index19 and a brief semistructured telephone interview that asked about typical sleep timing, daytime functioning, medication use, and diagnoses of any sleep disorders. An overnight, home polysomnography recording (Compumedics enhanced Somte portable recorders, Melbourne, Victoria) was conducted to screen participants for sleep disordered breathing and periodic limb movements. A trained technician prepared all participants for the overnight recording and scored the recorded polysomnography in accordance with the current standard sleep scoring criteria.20 The Geriatric Depression Scale Short Form21 was used to screen potential participants for depression, and the Depression, Anxiety and Stress Short Form22 was used to screen potential participants for severe anxiety. Potential participants were also screened for mild cognitive impairment using two subtasks of the Weschler Abbreviated Scale of Intelligence.23
Individuals with an apnea-hypopnea index (AHI) greater than 15 (indicative of moderate/severe sleep apnea) were excluded from participation.24 Individuals with a score of 10 or greater on the Geriatric Depression Scale or those with a full-scale Weschler Abbreviated Scale of Intelligence quotient below 70, suggestive of mild cognitive impairment, were also excluded from participation.
Participants were included in this study if they experienced (1) wake after sleep onset of greater than 30 min, at least three nights per week for a reported duration of at least 6 mo, and (2) had impaired daytime functioning such as daytime fatigue or memory problems. All participants were required to remain free of sedatives/hypnotic medication for at least 1 mo prior to and throughout their involvement in the study. Participants with clear clinical symptoms of other sleep disorders or severe medical/psychiatric disorders were excluded. Participants were excluded if they (1) indicated the presence of sleep apnea (AHI > 15) or restless legs syndrome, (2) indicated the presence of major depression, anxiety, or serious cognitive impairment, or (3) consumed excessive amounts of caffeine (> 300 mg/day) or alcohol (> two standard drinks/day).
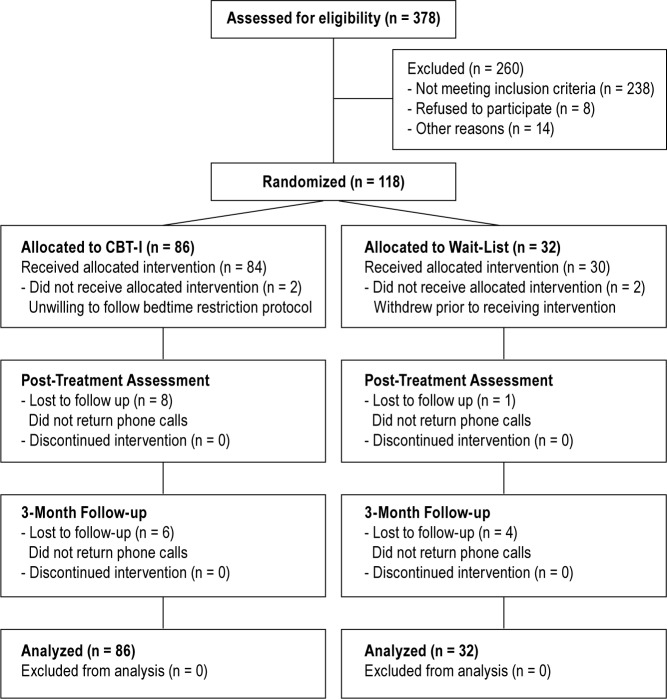
Of these potential participants, 238 did not meet inclusion criteria, eight declined to participate once understanding the requirements of their participation, and 14 gave other idiosyncratic reasons not to participate. The remaining 118 participants (mean age = 63.76 y, standard deviation = 6.45; male = 55) were randomized to either the treatment condition (N = 86) or to the waitlist control condition (N = 32). Figure 1 indicates the flow of participants through each stage of the study.
Figure 1.
Flowchart of participants throughout each stage of the study.
Four participants (3.4%) withdrew prior to completing treatment (N = 2 CBT-I condition, N = 2 waitlist condition). The two participants who were randomized to the treatment group ceased treatment after the first session. The two participants who were randomized to the waitlist group ceased participation after completing pretreatment assessment. Therefore, of the 118 participants, 114 (97%) were classified as treatment completers. These participants completed all four sessions of the treatment intervention. A total of 29 participants (25%) were lost to posttreatment or 3-mo follow-up assessments. Analyses revealed no significant differences between the two conditions in the rates of participants lost to follow-up (P > 0.05).
This study was granted ethics approval from the Flinders University Social and Behavioral Ethics Committee.
Design
This study used a 2 (treatment condition: cognitive behavior therapy and waitlist control) × 3 (time: pretreatment, posttreatment, 3-mo follow-up) mixed factorial design to evaluate the efficacy of a brief treatment of cognitive behavior therapy for the treatment of insomnia in older adults.
Interventions
Cognitive Behavior Therapy
CBT-I comprised four weekly 60-min sessions administered to small groups of four to five participants. The treatment was a structured, multicomponent intervention including bedtime restriction (based on sleep restriction but with modifications suggested by Morin3), cognitive therapy, and an educational component.
During the first treatment week, the behavioral components of CBT-I were administered. Sleep education and hygiene as well as the cognitive component of CBT-I were the focus of the second and third treatment weeks, whereas the fourth treatment week comprised a summary of all the information presented in the previous weeks and relapse prevention. All treatment sessions were conducted at Flinders University.
Behavioral Component
The behavioral component used in this study was bedtime restriction. Bedtime restriction involves restricting the amount of time the participant's spends in bed (TIB) as close as possible to their estimated total sleep time (TST) as calculated from their pretreatment sleep diary.3 For example, an individual's average pretreatment bedtime may be 22:00 and out-of-bed time 06:00 (TIB ≈ 8 h) but average reported sleep time may only be 5.5 h. Therefore, the new TIB would be 5.5 h. The participant would be asked to go to bed later and get out of bed earlier in the morning. This participant would, in this example, be asked to go to bed at 23:15 (1.25 h later) and get out of bed at 04:45 (1.25 h earlier), so their new TIB is equivalent to their reported TST. At each therapy session, participants' sleep diaries were individually reviewed prior to the start of the group session and in cases where sleep efficiency (TST/TIB *100) was greater than 90%, TIB was increased by 30 min for the following week. No participant was prescribed less than 5 h TIB, no matter how little his or her reported duration. Restriction of less than 5 h is likely to result in strong resistance from participants, making compliance difficult and may result in potentially dangerous impairments to daytime alertness and performance.3 TIB was titrated by the therapist in sessions two and three, and for the final time during the fourth treatment session.
Cognitive Component
The cognitive component is aimed at reevaluating the accuracy of participants' cognitions regarding their insomnia, the causes of their insomnia, and presumed consequences of sleeplessness.3 Specifically, the therapy is aimed at identifying dysfunctional cognitions related to sleep, challenging the validity of these cognitions, and providing more adaptive and rational substitute cognitions.3,25
The therapist used a variety of examples to demonstrate the interrelationship between cognitions, affect, and behavior.3,25,26 The therapist initially demonstrated this interrelationship using examples that are unrelated to sleep disturbances and then proceeded to demonstrate how the same principles apply in the context of sleep disturbances.3,25,26 The therapist worked through a range of examples regarding common dysfunctional beliefs and attitudes about sleep, identifying the dysfunctional sleep cognitions, challenging the validity of these cognitions and replacing them with more rational substitute cognitions.27 The therapist also spent time explaining that frequent feelings of anxiety and frustration can cause arousal of the autonomic nervous system, which can in turn contribute to feelings of daytime fatigue.
Education Component
The education component focused on providing participants with basic information about sleep and sleep hygiene practices.3 The therapist discussed information such as the 90-min cyclical nature of sleep, circadian rhythms, sleep needs, and the effects some sleep disorders, such as insomnia, can have on the sleep pattern.3,25,26 Sleep hygiene practices were also discussed with particular reference to the effect common substances (such as caffeine, alcohol, and nicotine) and diet, exercise, and the bedroom environment can have on sleep.
Waitlist Control
Participants who were allocated to the waitlist control group followed the same general procedure as the CBT-I group through to the 3-mo follow-up but without any of the CBT-I component. They were treated with the CBT-I program immediately after completing the 3-mo follow-up. No posttreatment assessment data were collected on waitlist control participants after completing the intervention.
Therapists
The group sessions were administered by five trainee psychologists (four female, one male) with experience in CBT-I. Participants received the same therapist for each therapy session. Each therapist was provided with a treatment manual to ensure participants in each group received identical information. All therapists took at least one waitlist control group. For all therapists, a clinical psychologist specialized and highly experienced in treating insomnia (HW) was consulted weekly to discuss clinical issues, ensure proper provision of treatment, and maintain fidelity of treatment. Analyses revealed the credibility and participant satisfaction with treatment, assessed using the Treatment Credibility and Satisfaction Scale36 did not differ across the therapists (P = 0.28).
Treatment Fidelity
A randomly chosen subset of eight therapy sessions were reviewed to assess treatment fidelity. These therapy sessions were recorded using a small, tripod-mounted video camera. The subset of sessions used to assess treatment fidelity included two recordings of each weekly session. Two assessors independently reviewed each recording to determine whether the therapist adhered to the guidelines provided in the treatment manual. The assessors were asked to establish whether the therapy session included all information presented within the treatment manual for that particular weekly session. Additionally, assessors were asked to determine whether the therapy session included any other treatment recommendations beyond those provided in the treatment manual. Both assessors concluded the eight therapy sessions reviewed did not differ from the treatment manual and that therapists did not provide any additional therapeutic recommendations to participants.
Treatment Adherence
Although there were no formal compliance measures used in the current study, TIB was used as an indicator of compliance to CBT-I. Compliance to CBT-I was assessed by examining nightly TIB as reported on sleep diaries and recorded using actigraphy. At each weekly therapy session, participants' sleep diary reports and actigraphy recordings of nightly TIB were compared to those prescribed as per the bedtime restriction procedure. TIB as reported on sleep diaries or recorded using actigraphy did not differ significantly from allocated TIB (P < 0.05).
Measures
Sleep Diaries and Wrist Actigraphy
Participants completed 7-day sleep diaries at screening, pretreatment, during treatment, post-treatment, and at 3-mo follow-up. Participants were required to record their bedtime, lights out time, and out-of-bed time on a daily basis. Participants also provided subjective estimates of their sleep onset latency, number and duration of awakenings, final wakeup time, TST across the night, and the amount of TIB. From these estimates, sleep onset time and sleep efficiency were calculated.
Actiwatch physical activity monitors (AW64 Mini Mitter, Oregon, USA) were used to provide an objective measure of wake after sleep onset, TST, and sleep efficiency at pretreatment, during treatment, posttreatment, and at follow-up. Each monitor contains a piezoelectric sensor that registers and digitally records body movements (via an accelerometer) at a high frequency sampling rate (40 times/sec). This signal is then converted into data counts for sequential 30-sec time epochs. Following each 7-day interval of recording, the Actiwatch data were downloaded and examined using ActiTrac 5.59 software. The Actiware-Sleep scoring algorithm relies on the use of a threshold sensitivity level in determining whether a participant is awake or asleep for any given 30-sec epoch. The algorithm scores epochs as either wake or sleep by comparing activity counts for that epoch, and the surrounding epochs, to a threshold value. In this study, the threshold sensitivity was set at the recommended default medium sensitivity value of 40. An epoch is scored as sleep when the activity counts fall below or are equal to this threshold sensitivity.
Questionnaires Assessing Perceived Severity of Insomnia, Daytime Functioning, Confidence In and Beliefs About Sleep
The Insomnia Severity Index3 was used to assess changes in participants subjective impressions of the severity and effect of their insomnia.
Daytime functioning was assessed using the Flinders Fatigue Scale,29 the Epworth Sleepiness Scale,30 and the Daytime Feeling and Functioning Scale,31 which asked a series of questions on a four-point frequency scale (never or seldom = 0, to frequently or almost all the time = 3) about daytime impairments over the past 2 w such as “felt irritable,” “had trouble with memory,” and “had a reduced quality of life.”
Participants' confidence in their ability to sleep was assessed using the Sleep Self-Efficacy Scale,32 and the Sleep Anticipatory Anxiety Questionnaire.33 Participants' beliefs about sleep were assessed using the Dysfunctional Beliefs and Attitudes About Sleep Scale.34
Procedure
Following screening, eligible participants were instructed to begin keeping the 7-day sleep diary and wear the wrist actigraphy. The sleep diary and wrist actigraphy were returned prior to the commencement of therapy.
Each participant was then randomly allocated to one of two conditions (CBT-I or waitlist) on an approximately 3:1 basis. Participants were blocked into groups of approximately eight and allocated to the two conditions (six and two per condition, respectively) using a computer-generated random digit sequence.
Participants were required to keep sleep diaries and wear actigraphy each week during the treatment period. Upon arrival at each treatment session, the previous 7-day sleep diary was collected and actigraphy was downloaded. At the end of the session, the therapist provided each participant with a new sleep diary and titrated TIB as per the bedtime restriction protocol.
Posttreatment assessment occurred during the week following the final treatment session. Participants kept the sleep diary and wore the wrist actigraphy across the week and completed the questionnaires at the end of the week. This assessment was repeated 3 mo following completion of therapy. All outcome assessment data was collected in person.
Overview of Statistical Analyses
Linear mixed-model analyses (also referred to as mixed effects models or random effects models) were conducted to investigate between group differences in sleep timing and quality variables across pretreatment, posttreatment, and 3-mo follow-up for the CBT-I and waitlist groups. For each dependent variable, fixed effects included treatment group (CBT-I, waitlist), within-subjects factor was time (pretreatment, post-treatment, and 3-mo follow-up), and the two-way interaction of treatment group and time.
In each case, an unstructured covariance matrix was fitted to account for the correlation of participants' repeated measures over time. Any variables with significant differences between the groups at pretreatment were entered as covariates to facilitate direct comparisons of change in the dependent variable across the groups. Planned comparisons were conducted for significant interactions between treatment group and time to assess the extent to which changes are attributed specifically to the treatment.
The clinical significance of the change in dependent variables was assessed using two methods. For each dependent variable, a treatment effect size (ES) was calculated using the mean scores for each group with the formula d = (MT1 − MT2) / SDpooled. MT1 refers to the mean score at pretreatment, MT2 refers to the mean score at posttreatment, or MT3 for 3-mo follow-up, and SDpooled refers to the pooled pretreatment standard deviation across treatment type. To further examine the clinical significance of changes over time, chi-squared analyses were used to compare group differences in the proportion of individuals who continued to meet clinical cutoff scores for insomnia at post-treatment and 3-mo follow-up. Participants were considered to no longer meet the diagnostic criteria for insomnia if (1) their sleep diary reported nightly average wake after sleep onset was < 45 min, and their sleep efficiency was ≥ 80%,35–37 or (2) their reported insomnia severity was nonclinical (defined here as ISI score of < 7).3 Because the current sample comprised older adults who initially had a mean sleep efficiency of 66% and a mean wake after sleep onset of 2 h and 22 min, a more lenient cutoff for wake after sleep onset of 45 min or less was considered rather than the more traditional cutoff of 30 min or less.37
RESULTS
Treatment Sample Characteristics
The sample comprised equal numbers of male and females with an average age of 64 y. The majority of participants (72%) reported suffering from insomnia for more than 5 years. The mean intelligence quotient (IQ) of the participants was 107, within the normal IQ range. The mean Pittsburgh Sleep Quality Index score for the overall sample was 11.81. Analyses revealed no significant differences between the two groups on any variable at pretreatment, with the exception of perceived insomnia severity. At pretreatment, the CBT-I group reported significantly greater insomnia severity (mean = 16.34, SD = 3.83) when compared to the waitlist group (mean = 14.40, SD = 4.03), F(1,107) = 5.40, P = 0.022. Pretreatment insomnia severity scores were statistically controlled as a covariate in subsequent between group comparisons involving this variable.
Comparisons of Sleep Quality and Timing Following Treatment
Subjective Sleep Quality and Timing
Table 1 shows sleep diary reported sleep quality and timing, including the mean wake after sleep onset, sleep efficiency, number of awakenings, sleep onset latency, TST, bedtime, lights out time, sleep onset time, wake-up time, out-of-bed time, and TIB for both groups across time.
Table 1.
Predicted means, standard error and treatment effect sizes for sleep diary reported sleep quality and timing measures across time for each group
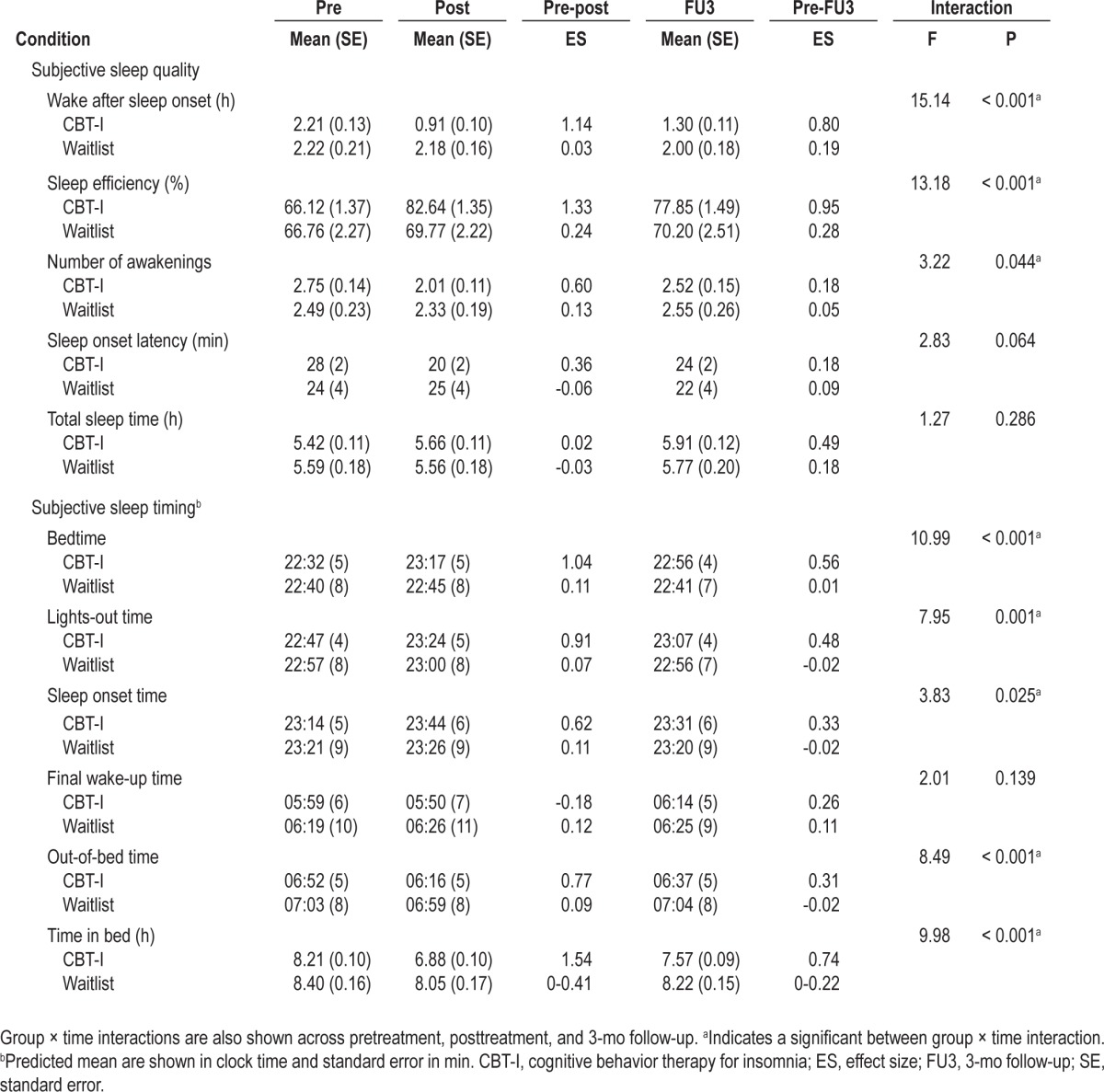
Immediately following treatment, participants in the CBT-I group reported a large reduction in wake after sleep onset, which was significantly greater than those reported by the wait-list group (P < 0.001). This improvement was accompanied by a substantial gain in sleep efficiency, which was also significantly greater than that reported by the waitlist group (P < 0.001). Improvements of wake after sleep onset and sleep efficiency were maintained at 3-mo follow-up for the CBT-I group when compared to the waitlist group (all P ≤ 0.026).
The CBT-I group reported significantly delayed bedtime, lights out time, and sleep onset time relative to the waitlist group immediately following treatment (all P ≤ 0.016). These delays were accompanied by a significantly earlier out-of-bed time reported by the CBT-I group compared with the waitlist group at posttreatment (P < 0.001). At follow-up, although bedtime, lights out time, and sleep onset time remained significantly delayed relative to the waitlist group (all P ≤ 0.011), out-of-bed time drifted slightly later and was comparable to the waitlist group (P = 0.191). Participants also reported large reductions of TIB, which were significantly greater than those reported by the waitlist group both immediately following treatment and at follow-up (all P < 0.001).
Objective Sleep Variables
Actigraphically measured wake after sleep onset, TST, and sleep efficiency are shown for each group across time in Table 2.
Table 2.
Predicted means, standard error and treatment effect sizes for objective sleep measures (from actigraphy) across time for each group
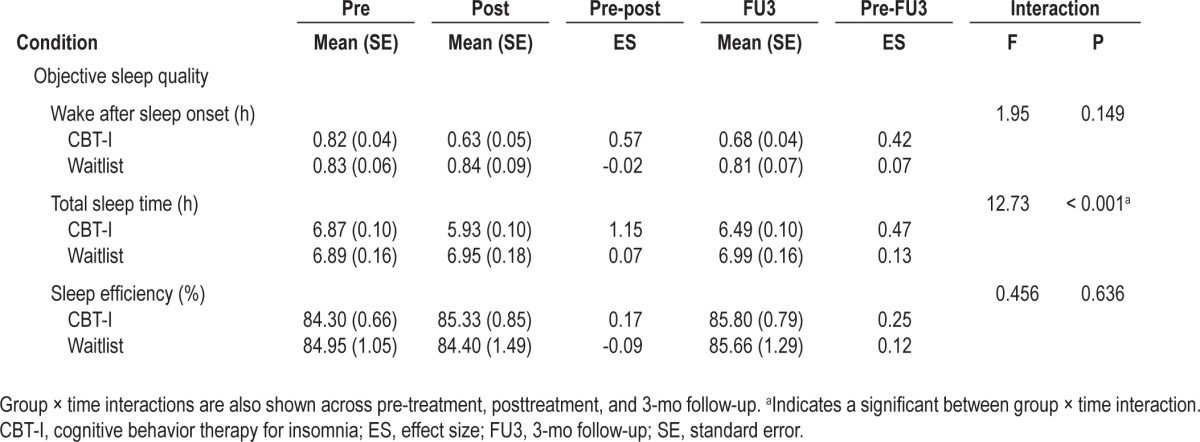
Immediately following treatment, participants in the CBT-I group had significant reductions in wake after sleep onset compared with the waitlist group (P ≤ 0.003). However by follow-up, the reduction reported by those in the CBT-I did not differ significantly from the waitlist group (P = 0.080). Participants in the CBT-I group also reported significant reductions in TST when compared with the waitlist group both immediately following treatment and at follow-up (all P ≤ 0.026).
Perceived Insomnia Severity, Daytime Functioning, and Confidence and Beliefs About Sleep
Table 3 shows the mean Insomnia Severity Index, Flinders Fatigue Scale, Epworth Sleepiness Scale, Daytime Feeling and Functioning Scale, Sleep Self-Efficacy, Dysfunctional Beliefs and Attitudes About Sleep, and Sleep Anticipatory Anxiety scores for both groups across the duration of the study.
Table 3.
Predicted means, standard error and treatment effect sizes for perceived severity of insomnia, daytime functioning, and confidence and beliefs about sleep across time for each group
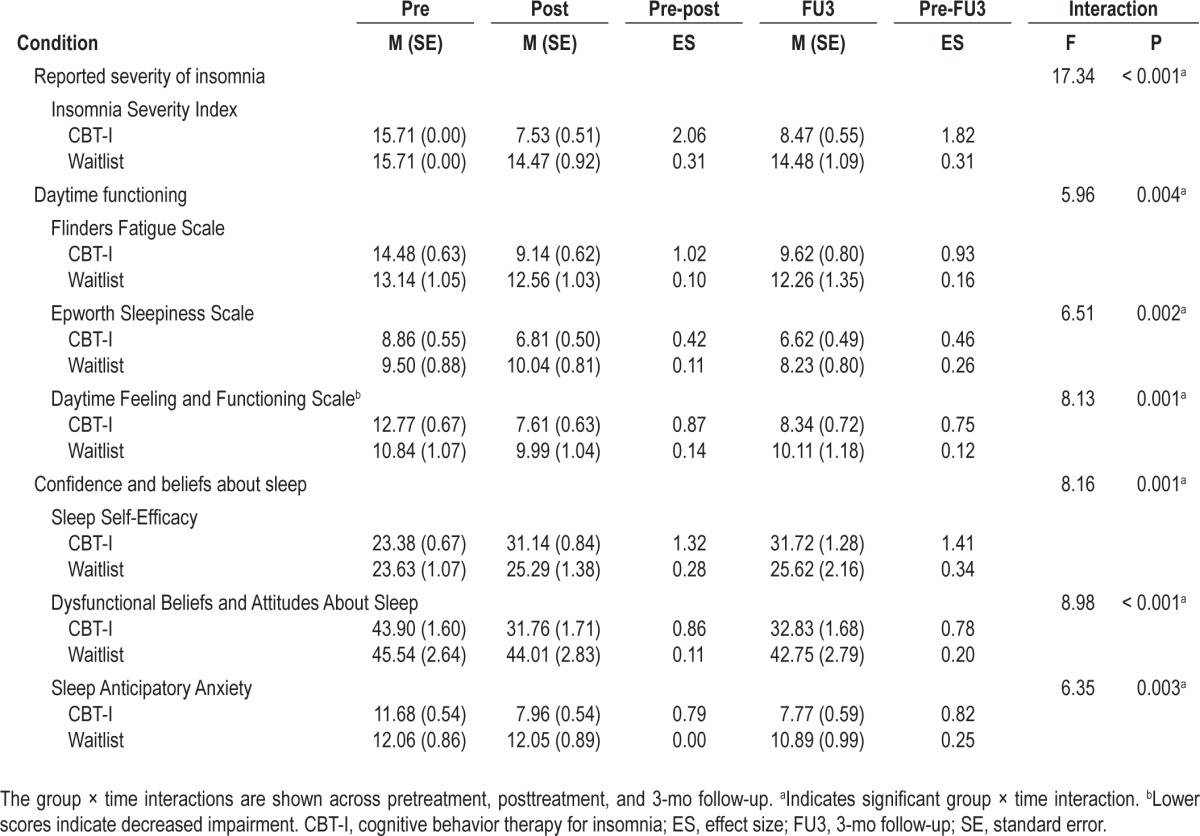
Immediately following treatment, perceived insomnia severity was significantly reduced for those in the CBT-I group (P < 0.001) and was maintained at follow-up (P < 0.001).
Participants in the CBT-I group reported large reductions in fatigue, daytime sleepiness, and impaired daily feelings and functioning immediately following treatment. These reductions were significantly greater than those reported by the waitlist group (all P ≤ 0.009). CBT-I participants continued to report a significant reduction in impaired daily feelings and functioning at 3-mo follow-up when compared to waitlist (P = 0.011).
Immediately following treatment, participants in the CBT-I group reported a substantial improvement in sleep self-efficacy from pretreatment. This improvement was accompanied by reductions in dysfunctional beliefs and attitudes about sleep, and sleep anticipatory anxiety. These improvements were significantly greater than those reported by the waitlist group immediately following treatment (all P ≤ 0.004) and at follow-up (all P ≤ 0.038).
Clinical Significance of Change in Subjective Sleep Quality and Reported Insomnia Severity Following Treatment
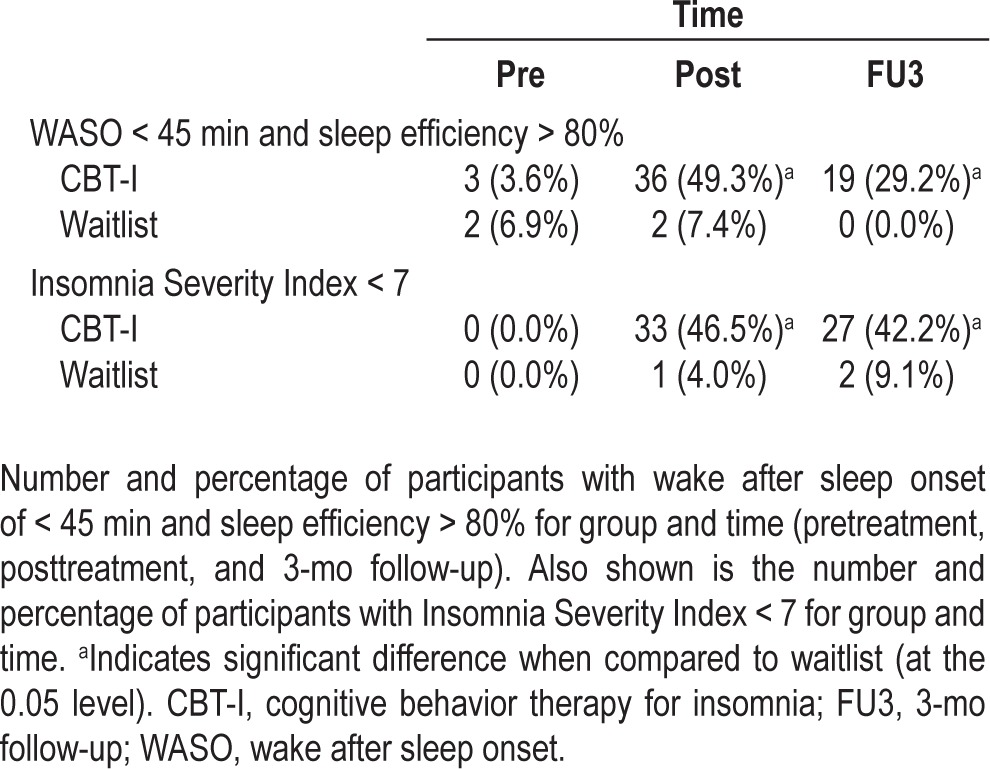
Table 4 indicates the proportion of individuals who no longer met the diagnostic criteria for insomnia at pretreatment, posttreatment, and 3-mo follow-up. Of those participants who received treatment, 49% reported wake after sleep onset of < 45 min and a sleep efficiency of ≥ 80% immediately following treatment, whereas 46.5% of participants reported nonclinical levels of insomnia severity. These proportions were largely maintained at 3-month follow-up with 29% and 42% of participants, respectively, continuing to report sleep quality and insomnia severity outside the diagnostic range. The proportion of participants who reported sleep quality and insomnia severity outside the diagnostic range was significantly greater for those who received CBT-I compared with the waitlist group, both immediately following treatment and at follow-up (P < 0.05).
Table 4.
DISCUSSION
The current study evaluated the efficacy of a brief 4-w group-administered treatment program of CBT-I for older adults. CBT-I produced robust and durable improvements in sleep quality, daytime functioning, and sleep timing in comparison with the waitlist group.
Participants reported improvements in sleep quality across the majority of outcome measures, including substantial reductions of wake after sleep onset and increases in sleep efficiency. These changes were supported by large effect sizes (ES = 1.14-1.54) and were significantly greater than the wait-list group both immediately following treatment and at 3-mo follow-up. There were initial reductions in number of awakenings and sleep onset latency with CBT-I; however, these were not sustained at 3-mo follow-up. Participants also reported a trend toward greater TST following treatment. It is likely this trend reflects a reciprocal change in TST and TIB whereby TIB was reduced immediately following therapy but relaxed to some extent by 3-mo follow-up.
The improvements in sleep quality reported in the current study, particularly for wake after sleep onset and sleep efficiency, are robust when compared to earlier studies investigating the use of CBT-I for late-life insomnia.11,38,39 The effect sizes for improvements in wake after sleep onset and sleep efficiency in the current study (ES = 1.14-1.54) are nearly double those reported by these earlier studies (ES = 0.39-0.61) and are also larger than those typically reported following the use of individualized CBT-I14 and CBT-I in healthy young adults (ES = 0.57-1.00).11
Similar to the current findings, earlier studies have reported average reductions in the number of awakenings of 0.7 and very little change in sleep onset latency immediately following treatment in older adults.38 The small increase in TST reported in the current study mirror those of previous studies immediately following treatment but have demonstrated superior durability. Earlier studies have reported modest improvements in TST of approximately 14 min.38 However, unlike previous studies that report participants generally relapsing to pretreatment levels fairly quickly,38 by 3-mo follow-up there was a further mild increase in TST in the current study.
The current study reports few objective therapeutic effects following treatment. The initial declines and subsequent rebound by follow-up of actigraphically measured wake after sleep onset and TST in the CBT-I group relative to the unchanging waitlist group is likely a reflection of the imposition of bedtime restriction therapy and its relaxation by follow-up. These findings are similar to earlier studies using actigraphy to assess the efficacy of CBT-I interventions.12,18,38,40,41 Research has consistently demonstrated low correlations between self-reported and actigraphically assessed sleep,42,43 with actigraphy producing different estimates of sleep time, number of awakenings, and sleep onset latency than sleep diaries.44,45 Furthermore, a recent study reported polysomnographically derived TST, sleep latency, wake after sleep onset, and sleep efficiency could not discriminate adults with primary insomnia from good sleepers.46
Participants in the current study reported improvements in perceived severity of insomnia, daytime functioning, and confidence in and beliefs about sleep. All improvements were statistically larger than those reported by the waitlist group and were clinically effective, particularly for perceived insomnia severity, fatigue, sleep self-efficacy, and dysfunctional beliefs and attitudes about sleep (ES = 0.86-2.06). These improvements were all durable with participants continuing to report greater improvements at 3-mo follow-up when compared to the waitlist group, with the exception of fatigue and sleepiness.
Very few studies investigating the use of CBT-I in older adults have included outcome measures of daytime functioning, instead focusing on demonstrating therapeutic effects in terms of sleep quality. Lichstein and Morin26 included outcome measures of fatigue, sleepiness, dysfunctional beliefs and attitudes about sleep, and insomnia severity when evaluating the use of individually administered sleep compression in late-life insomnia. Immediately following treatment, participants reported moderate reductions in these measures with small effect sizes (ES = 0.04-25). Although the current study reported moderate reductions in sleepiness, there were large reductions in fatigue, dysfunctional beliefs and attitudes about sleep, and insomnia severity.
Morin and colleagues17 included the Insomnia Severity Index as an outcome when assessing the efficacy of a 10-w group-based program of CBT-I in 24 older adults (mean age = 61 y) with chronic insomnia. Morin and colleagues17 reported a reduction following treatment similar to the current study using only about half the number of treatment sessions.
In a more recent study, Buysse and colleagues14 included the Epworth Sleepiness Scale as an outcome measure when assessing the benefits of an individually administered brief behavioral intervention for insomnia in older adults, which was based on sleep restriction and education. Unlike the current study, which showed a moderate effect-size reduction of daytime sleepiness following treatment (mean reduction of 2.06), Buysse and colleagues14 reported that daytime sleepiness remained relatively unchanged following their intervention (mean reduction of 0.31).
After CBT-I, participants were going to bed, turning their light off, and falling asleep later than before treatment. Participants who received CBT-I also reported getting out of bed earlier after treatment, resulting in less TIB following treatment and at follow-up when compared with the waitlist group. These changes in sleep timing were generally large in effect (ES = 0.62-1.04) and differed significantly from changes reported by the waitlist group.
The standardized nature of the current program ensures effective administration of the program by individuals who are not extensively trained. This program has the potential to be administered by health care workers, nurses,47 or even via media such as the Internet, to allow self-guided treatment by the patient.
There are several noteworthy limitations of the current study. Despite the beneficial effects demonstrated, the high rate of attrition reflects the difficulties associated with enrollment and delivery of group-based interventions. The waitlist control group did not receive any placebo control. Therefore, the therapeutic effect of nonspecific treatment effects, such as social support and observational learning, remain unclear. The current study also did not use any objective assessment of daytime functioning (i.e., test of executive function), but would be recommended for future research.
When interpreting the findings of the current study, it is important to acknowledge this sample comprised community-dwelling individuals who had relatively unimpaired daytime functioning, volunteered for an intensive university-based study, and had no comorbid medical or psychiatric conditions. Therefore, caution should be used when attempting to generalize the current findings to more typical clinical populations and individuals who are medically ill, dependent on hypnotic agents, frail, or institutionalized. The evaluation of the efficacy of brief group-based CBT-I programs for these populations should be a primary aim of further research.
In summary, the brief group-administered treatment program used in the current study has demonstrated clinical effectiveness at least equal to that of other longer, more individualized programs. It promises to be a brief and inexpensive answer to the effective treatment of insomnia in the older population. Many of the changes in sleep quality and daytime functioning reported immediately following treatment were larger in magnitude than those demonstrated for older adults in the past. This was particularly the case of wake after sleep onset and sleep efficiency. The magnitude of these benefits is at least comparable even to those that have been reported following treatment in healthy younger adults. Although there is a lack of published literature examining the durability of changes following treatment in older adults, the current study demonstrates substantial (3-mo) durability of improvements.
DISCLOSURE STATEMENT
This research was supported by a grant through the National Health and Medical Research Committee. The authors have indicated no financial conflicts of interest. There was no off-label or investigational use in this study.
ACKNOWLEDGMENTS
The authors acknowledge the trainee assistants (Michelle Short, Neralie Cain, Anna Johnson, Jason Gill, and Vickie Simos) for their involvement in this research.
REFERENCES
- 1.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–16. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Morin C. Insomnia: psychological assessment and management. New York: The Guilford Press; 1993. [Google Scholar]
- 4.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Badlioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Dis. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 national sleep foundation survey. II. Sleep. 1999;22:354–8. [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–71. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 8.National Sleep Foundation. 2003 Sleep in America Poll. 2003. http://www.sleepfoundation.org.
- 9.Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65:33–40. [PubMed] [Google Scholar]
- 10.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2:403–6. [PubMed] [Google Scholar]
- 11.Irwin M, Cole J, Nicassio P. Comparative meta-analysis of behavioural interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioural treatment programs for comorbid geriatric insomnia. Health Psychol. 2002;17:288–98. [PubMed] [Google Scholar]
- 13.Backhaus J, Hohagen F, Voderholzer U, Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251:35–41. doi: 10.1007/s004060170066. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR. Dose response effects of behavioural insomnia therapy: Final report. Paper presented at the Associated Professional Sleep Societies; 2004; Philadelphia, PA. [Google Scholar]
- 16.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clinical Psychol. 1993;61:137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 18.Espie CA, MacMahon KMA, Kelly H-L, et al. Randomized clinical effectiveness trial of nurse-administered small group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 21.Yesavage J, Brink T. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Lovibond S, Lovibond P. Manual for the Depression anxiety stress scales. 2nd ed. Sydney: Psychological Foundation; 1995. [Google Scholar]
- 23.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 24.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 25.Morin CM, Espie C. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 26.Lichstein K, Morin C. Treatment of late-life insomnia. London: Sage Publications; 2000. [Google Scholar]
- 27.Lichstein KL. Behavioral intervention for special insomnia populations: Hypnotic-dependent insomnia and comorbid insomnia. Sleep Med. 2006;7S1:S27–31. [Google Scholar]
- 28.Lacks P. Behavioral treatment for persistent insomnia. New York: Pergamon Press; 1987. [Google Scholar]
- 29.Gradisar M, Lack L, Richards H, Harris J, Gallasch J, Boundy M, Johnston A. The Flinders Fatigue Scale: Preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3:722–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Johns M. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 31.Gradisar M, Lack L, Harris J, et al. Psychometric properties of two new scales for measuring daytime functioning for insomnia. Sleep. 2006;29:A339. Abstract Supplement. [Google Scholar]
- 32.Lacks P. Behavioral treatment for persistent insomnia. New York: Pergamon Press; 1987. [Google Scholar]
- 33.Bootzin R, Shoham V, Kuo T. Sleep Anticipatory Anxiety Questionnaire: a measure of anxiety about sleep. Sleep Res. 1994;23:88. [Google Scholar]
- 34.Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastien C, Morin C, Ouellet M, Bais F, Bouchard S. Cognitive-behavior therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72:653–9. doi: 10.1037/0022-006X.72.4.653. [DOI] [PubMed] [Google Scholar]
- 36.Kendall PC, Grove WM. Normative comparisons in therapy outcome. Behav Assessment. 1988;10:147–58. [Google Scholar]
- 37.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev. 2004;8:47–62. doi: 10.1016/S1087-0792(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 39.Pallesen S, Nordhus IH, Kvale G. Nonpharmacological interventions for insomnia in older adults: a meta-analysis of treatment efficacy. Psychotherapy. 1998;35:472–82. [Google Scholar]
- 40.Broomfield NM, Espie CA. Initial insomnia and paradoxical intention: an experimental investigation of putative mechanisms using subject and actigraphic measurement of sleep. Behav Cogn Psychother. 2003;31:313–24. [Google Scholar]
- 41.Friedman L, Benson K, Noda A. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 42.Jean-Louis G, Kripke D, Ancoli-Israel S. Sleep and quality of well being. Sleep. 2000;23:1115–21. [PubMed] [Google Scholar]
- 43.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: toward a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 44.Guilleminault C, Clerk A, Black J, Labanowski N, Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia. Ann Intern Med. 1995;155:838–44. [PubMed] [Google Scholar]
- 45.Wilson KG, Watson St, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;64:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 46.Edinger JD, Ulmer CS, Means MK. Sensitivity and specificity of polysomnography criteria for defining insomnia. J Clin Sleep Med. 2013;9:481–91. doi: 10.5664/jcsm.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]