Abstract
Study Objectives:
Despite the high prevalence of insomnia, daytime consequences of the disorder are poorly characterized. This study aimed to identify neurobehavioral impairments associated with insomnia, and to investigate relationships between these impairments and subjective ratings of sleep and daytime dysfunction.
Design:
Cross-sectional, multicenter study.
Setting:
Three sleep laboratories in the USA and Australia.
Patients:
Seventy-six individuals who met the Research Diagnostic Criteria (RDC) for Primary Insomnia, Psychophysiological Insomnia, Paradoxical Insomnia, and/or Idiopathic Childhood Insomnia (44F, 35.8 ± 12.0 years [mean ± SD]) and 20 healthy controls (14F, 34.8 ± 12.1 years).
Interventions:
N/A.
Measurements and Results:
Participants completed a 7-day sleep-wake diary, questionnaires assessing daytime dysfunction, and a neurobehavioral test battery every 60-180 minutes during an afternoon/evening sleep laboratory visit. Included were tasks assessing sustained and switching attention, working memory, subjective sleepiness, and effort. Switching attention and working memory were significantly worse in insomnia patients than controls, while no differences were found for simple or complex sustained attention tasks. Poorer sustained attention in the control, but not the insomnia group, was significantly associated with increased subjective sleepiness. In insomnia patients, poorer sustained attention performance was associated with reduced health-related quality of life and increased insomnia severity.
Conclusions:
We found that insomnia patients exhibit deficits in higher level neurobehavioral functioning, but not in basic attention. The findings indicate that neurobehavioral deficits in insomnia are due to neurobiological alterations, rather than sleepiness resulting from chronic sleep deficiency.
Citation:
Shekleton JA; Flynn-Evans EE; Miller B; Epstein LJ; Kirsch D; Brogna LA; Burke LM; Cremer E; Murray JM; Gehrman P; Lockley SW; Rajaratnam SMW. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. SLEEP 2014;37(1):107-116.
Keywords: Insomnia, fatigue, functional impairment, cognitive performance, sleep, sleepiness, depression
INTRODUCTION
Current diagnostic criteria for insomnia require the self-report of non-restorative sleep, and/or a difficulty initiating or maintaining sleep, along with a complaint of daytime impairment or distress.1–3 For many insomnia patients, the daytime complaints are the most salient consequence,4–6 with fatigue or psychological symptoms ultimately compelling them to seek treatment.7 A diagnosis of insomnia is unequivocally associated with decreased quality of life,8 emphasizing the negative impact of the daytime symptoms.9 Insomnia is also associated with workplace injuries10,11 and significant economic costs, through absenteeism and lost productivity,12 which are a likely consequence of daytime impairments related to the disorder.
Despite complaints of daytime functional impairment being a diagnostic criterion for insomnia,1,2 the specific nature of these impairments is not well understood. One approach places daytime complaints into the three categories of fatigue, physical health, and mental health symptoms.13 This approach is supported by focus group and large population studies that have also linked self-reported poor sleep quality with complaints of reduced psychological well-being and self-reported cognitive difficulties.5,14 Treatment studies have shown improvements in self-reported daytime functioning following improved sleep quality.15,16
Early studies reported that objective measures of sleepiness and neurobehavioral performance do not indicate deficits in individuals with insomnia.17 One reason for these negative findings is that the deficits are likely subtle and limited to more complex cognitive processes.18 A more recent meta-analysis concluded that insomnia patients show small to moderate deficits on working memory, episodic memory, and problem-solving tasks.19 Echoing previous concerns,18 the authors acknowledged the heterogeneity of insomnia participants pooled across studies and the variability of diagnostic and screening methods utilized, and concluded that poor sensitivity of previous studies may mask consistent but small impairments on complex reaction time and selective attention tasks.19
Given the difficulty in capturing neurobehavioral impairments using objective tests, relatively few studies have investigated the link between the sleep and neurobehavioral impairment. A large population-based study reported that insomnia patients with short sleep duration (< 6 h), as measured by polysomnography, showed deficits on a complex (switching attention) task, whereas those insomnia patients who slept more than six hours per night showed no such deficits.20 Furthermore, both insomnia and control participants with short sleep duration exhibited deficits on a processing speed task compared to rested controls. This finding suggests a direct relationship between sleep duration and daytime performance in individuals with insomnia. The nature of the insomnia complaint in this population was not well described, however, and it is unclear whether a recognized diagnostic criterion was used and if individuals were required to complain of daytime impairment. Other studies have reported only weak associations between cognitive impairments on a switching attention task and measures of sleep duration.21 A previous study reported that sleep quality of individuals with primary insomnia and those with insomnia secondary to a medical condition showed no association with subjective daytime symptoms, suggesting functional independence across the two spheres.22
Limited understanding of the nature of the daytime consequences of insomnia remains a barrier to characterizing the etiology of the disorder and to objectively evaluating treatments aimed at ameliorating daytime dysfunction. Furthermore, the extent to which neurobehavioral performance is related to sleep disturbance and/or impaired psychological well-being in insomnia patients is not well understood. The aim of this study was to investigate the neurobehavioral deficits associated with insomnia in a well-characterized and otherwise healthy group of patients as compared to controls. A secondary aim was to investigate the relationships between neurobehavioral performance and measures of sleep disturbance, daytime sleepiness, and daytime dysfunction in the insomnia group and the control group.
METHODS
Participants
Participants were recruited from the general community to 1 of 3 study sites: Boston, USA (n = 71); Melbourne, Australia (n = 20); or Philadelphia, USA (n = 5). From an initial sample of 80 individuals with insomnia and 21 healthy control subjects, 3 patients with insomnia and 1 healthy control did not complete the laboratory component, and 1 patient with insomnia was excluded after completing the study due to a high body mass index (> 30 kg/m2). The final sample consisted of 76 individuals (44 women) aged 35.75 ± 12.40 years (mean ± SD) who met criteria for insomnia (see below). Twenty individuals reporting healthy sleep (14 women) aged 34.75 ± 12.05 years were recruited from the general community in Boston, USA, and were age-matched (± 5 years) and gender-matched to 20 of the first 23 insomnia patients enrolled in the study. The study was approved by the Partners Human Research Committee, the Alfred Human Research Ethics Committee, and the University of Pennsylvania Institutional Review Board. All participants gave written informed consent.
Participants were aged 18 to 65 years; reported low nicotine, caffeine, and alcohol use; no history of drug abuse or medications likely to affect sleep or alertness; and no recent shift work or transmeridian travel (see supplemental material). We included participants with a body mass index between 18 and 30 kg/m2, and to further reduce the likelihood of sleep apnea, we excluded those with a positive screening result on the Berlin Questionnaire.23 Pregnancy or lactation as well as perimenopausal or menopausal symptoms were exclusionary. Health was assessed by medical history, physical examination, electrocardiography, blood biochemistry, hematology, urinalysis and urine toxicology, and examination by a physician with specialist training in sleep medicine. Participants were excluded if they reported the current use of medications for a psychiatric disorder or the presence of major depressive disorder or other severe psychopathological condition.
Several self-report questionnaires were included to evaluate sleep, physical health, and mental health, as well as cognitive and behavioral aspects of insomnia. The following questionnaires were administered prior to the laboratory visit: Beck Depression Inventory II,24 State-Trait Anxiety Inventory-Trait version,25 Pittsburgh Sleep Quality Index,26 Fatigue Severity Scale,27 Athens Insomnia Scale,28 Sleep Hygiene Questionnaire,29 Dysfunctional Beliefs and Attitudes About Sleep,30 Epworth Sleepiness Scale,31 Short Form Health Survey of Medical Outcomes (SF-36),32 and the Functional Assessment of Chronic Illness Therapy (FACT-Cog).33
Insomnia Group
Insomnia participants were eligible for inclusion if they met the Research Diagnostic Criteria (RDC) for Insomnia Disorder as well as the Research Diagnostic Criteria for any of the following subtypes: Primary Insomnia, Psychophysiological Insomnia, Paradoxical Insomnia, and/or Idiopathic Childhood Insomnia (see supplemental material for detailed diagnostic criteria).3 Primary insomnia is characterized by the persistence of insomnia symptoms for at least one month either with no current or past mental, psychiatric, or medical disorder, or where such is present, the temporal course of the insomnia shows some independence from that of the other condition(s) and no evidence of another insomnia subtype. In addition to the features of primary insomnia, psychophysiological insomnia is characterized by symptoms indicating conditioned sleep difficulties and/or heightened arousal in bed. With paradoxical insomnia, there is a marked discrepancy between sleep duration and sleep efficiency as recorded by polysomnography and what is reported by the patient. Finally, for idiopathic childhood insomnia, the insomnia disorder began during childhood (i.e., before age 10) without an identifiable precipitant and has persisted since that time.
Determination of insomnia diagnosis was made through clinical history and interview by a certified sleep clinician. Where participants met diagnostic criteria for more than one insomnia disorder, both were recorded. Participants were not eligible if, based on clinical assessment, they met the Research Diagnostic Criteria for insomnia related to any of the following: Periodic Limb Movement Disorder, Sleep Apnea, Drug or Substance and/or Medical Condition. Insomnia participants were required to score from 10 to 28 inclusive on the Insomnia Severity Index.34 Past diagnosis of a comorbid psychiatric disorder was also exclusionary, per the RDC for Insomnia Disorder.
Control Group
Participants in the control group were included if they met Research Diagnostic Criteria for Normal Sleepers,3 reported habitual sleep duration > 6.0 h and < 9.5 h, and scored < 8 on the Insomnia Severity Index.34
Pre-Laboratory Protocol
Following screening and until study completion, participants were asked not to take any new prescription or non-prescription medications or supplements and refrain from using recreational drugs. This was verified by urine toxicology tests during the screening visit and on admission to the laboratory. Participants were instructed not to modify their caffeine, nicotine, or alcohol intake from the levels reported at screening. For the 48 h prior to their laboratory visit, participants were instructed to abstain from caffeine and nicotine.
Participants maintained their usual sleep-wake pattern in their home environment for ≥ 7 days prior to the laboratory visit, verified by daily sleep/activity diaries, visual inspection of actigraphy records (Actiwatch-L, Minimitter, Bend, OR), and daily call-in to a time-stamped voicemail service at bedtime and at wake time. Based on the daily call-in times, each participant's habitual average bedtime across the 7 days prior to the laboratory visit was calculated.
Laboratory Protocol
Participants attended a ∼12-h laboratory visit at the Intensive Physiologic Monitoring (IPM) Unit, Brigham and Women's Hospital (BWH; Boston, MA), the Monash University Sleep Laboratory (Melbourne, Australia), or the Clinical and Translational Research Center Sleep Laboratory (University of Pennsylvania, Philadelphia, PA). To minimize the potential confounding effects of variations in performance levels due to time of day and duration of prior wakefulness, laboratory events were scheduled relative to each participant's mean habitual bedtime. Participants were admitted to the laboratory approximately 9 h prior to their habitual bedtime. On arrival, participants completed practice sessions of neurobehavioral tests and were administered the Wechsler Test of Adult Reading (WTAR).35
During the laboratory visit, participants remained in a light-proof, sound-attenuated, and temperature-controlled suite. Ambient light levels were maintained at < 10 lux when measured in the horizontal plane at a height of 72 inches. A modified constant posture protocol was imposed; participants were seated ≥ 20 minutes prior to and during testing; food intake was standardized for timing and content; and wakefulness was monitored by direct observation by research staff throughout the laboratory visit. Participants were free to engage in sedentary activities at times when they were not required to undertake study procedures.
Neurobehavioral Performance, Sleepiness, and Alertness
The following tests were included in the neurobehavioral test battery: (1) auditory psychomotor vigilance task (PVT)36 and PVT-two tone (PVT-TT),37 to assess sustained attention with and without response choice, respectively; (2) Switching Attention Task (SAT), with 3 parts21,38; (3) N-Back task to assess working memory39; (4) Performance Evaluation and Effort Scale (PEERS) to assess perceived level of performance and effort40; and (5) Karolinska Sleepiness Scale (KSS)41 and Visual Analogue Scale-Alertness (VAS-A) to assess subjective sleepiness and alertness, respectively. See supplemental material for task details. The KSS and VAS-A were administered every hour from 5 h before to 2 h after habitual bedtime. A brief test battery consisting of the PVT and the PVT-TT was presented 5 h before habitual bedtime and then hourly from 3 h before habitual bedtime until 2 h after habitual bedtime, and a full test battery (brief test battery plus N-Back, SAT) presented at 5 and 3 hours before habitual bedtime and 1 h after habitual bedtime. Tasks were administered using the E Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA, USA) software system (unless specified) on a laptop computer.
Data Analysis
SPSS Statistics Version 20.0 (IBM, Armonk, New York) was used for all data analysis. Due to computer malfunction or error, some trials for some participants were not available for inclusion in the final analysis. For statistical analysis, reaction time (msec) on the PVT and PVT-TT were each divided by 1,000 and then reciprocally transformed (1/RT) as described previously.42 The transformed values were then averaged. The number of PVT lapses (RT > 500 msec) was transformed to normalize the distribution for statistical analysis ((√n) + (√n+1)).43 Appropriate normative data to convert raw scores on the Wechsler Test of Adult Reading (WTAR) into standardized scores and estimated IQ were only available for US participants; therefore, Australian subjects were excluded from the between-groups IQ analysis. For the daytime functioning questionnaire data, category totals are not equal to population totals where questionnaire total scores could not be calculated due to missing data (i.e., participants failing to respond to singles items or when responses to single items were illegible).
Age and gender were entered as covariates in each analysis, as these factors were expected to differentially affect sleep, neurobehavioral performance, and daytime functional impairment measures. Sleep diary and self-reported daytime impairment measures were analyzed using one-way analysis of covariance (ANCOVA). Neurobehavioral performance measures were analyzed using mixed model ANCOVA, with group as the between subjects factor (insomnia, control), and time relative to habitual bedtime was the within subjects factor, with 3 (-5, -2, +1), 6 (-5, -2, -1, 0, +1, +2), or 8 levels (-5, -4, -3, -2, -1, 0, 1, +2), depending on frequency of administration of the test.
Pearson partial correlation, controlling for age and gender, was used to investigate the relationships between neurobehavioral performance (sustained attention) and subjective daytime complaints, as well as sleep diary outcomes and state sleepiness and alertness measures. For these correlational analyses, we used PVT mean reciprocal reaction time and lapses as measures of sustained attention based on their established sensitivity to the effects of sleepiness and sleep loss.36,44 The subjective measures were chosen as they each capture a single domain of daytime dysfunction reported by insomnia patients (depression, anxiety, fatigue, physical health-related quality of life) or reflect insomnia severity. ISI scores had a restricted range because this measure was used as an inclusion criterion; therefore, the AIS was used in the correlational analysis. We analyzed the KSS and VAS-A administered immediately prior to cognitive tests. Sleep onset latency, wake after sleep onset, and total sleep time from the sleep diary measure were chosen, as they are consistently shown to capture differences in self-reported sleep between insomnia patients and healthy controls.45
Bonferroni adjustments for multiple comparisons were made for the 7 sleep diary (week-long) outcome measures (α = 0.007) and for 12 self-reported daytime complaint measures (α = 0.0041). Where a task/measure had more than one outcome variable, Bonferroni corrections for multiple comparisons were made, e.g., PVT, 2 outcome measures (α = 0.025); PEERS, 3 outcome measures (α = 0.0017). No adjustments for multiple comparisons were made for correlation analyses, as these were deemed secondary analyses. All results are reported as mean ± standard deviation unless otherwise stated.
RESULTS
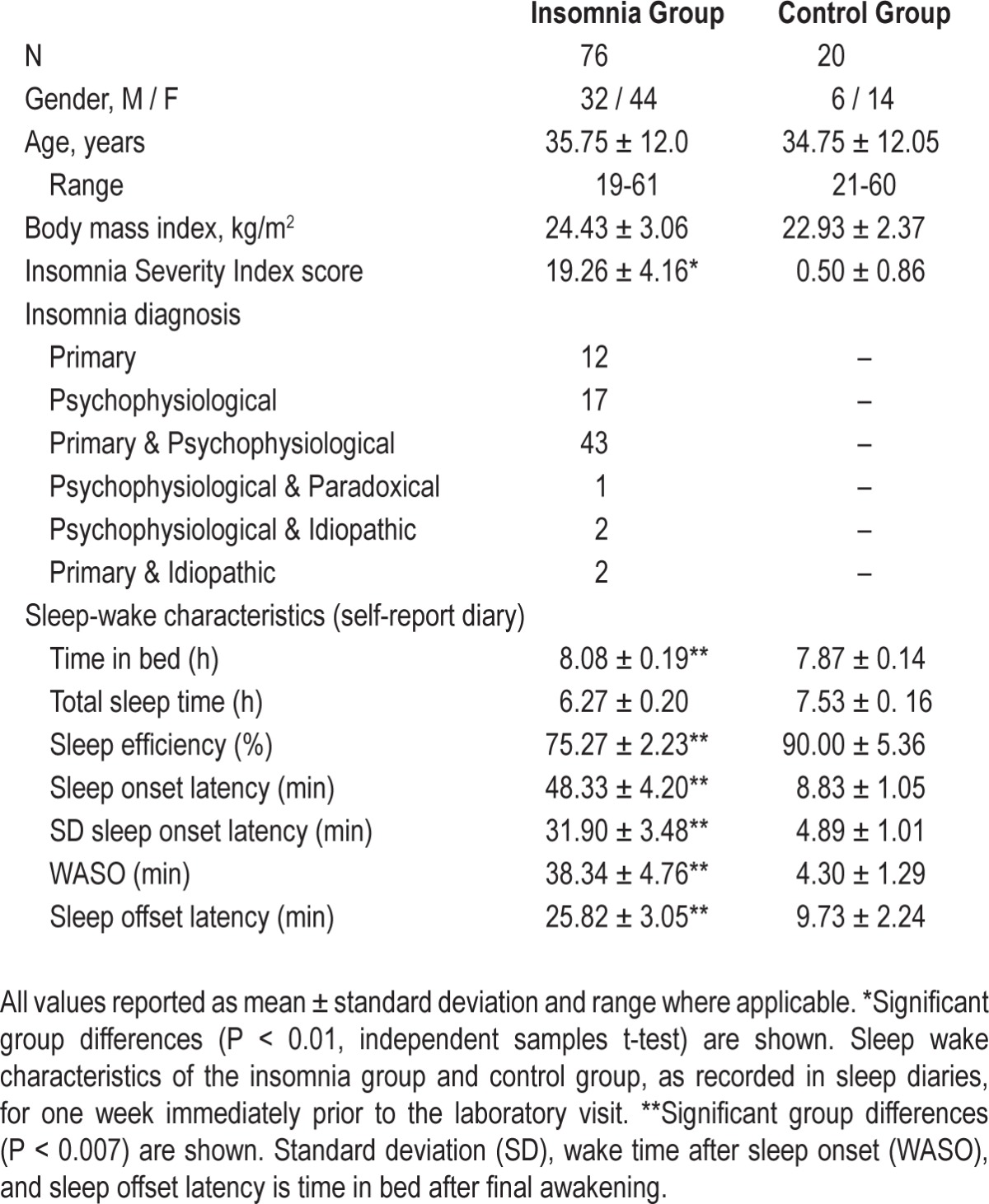
All participants in the insomnia groups met RDC for Insomnia Disorder.3 Most participants in the insomnia group met the criteria for the primary and psychophysiological insomnia subtypes (56%), followed by psychophysiological (22%) or primary (16%) insomnia only. The insomnia group had significantly higher scores on the Athens Insomnia Scale than the control group (P < 0.05; Table 1). The insomnia and control groups were not significantly different on age, gender, BMI, or estimated full-scale IQ score (P > 0.05).
Table 1.
Participant characteristics
We examined whether or not the primary sleep and neurobehavioral outcomes were consistent across study sites in the insomnia group. We found no differences among sites in total sleep time, sleep efficiency, wake after sleep onset, PVT mean reaction time, and PVT number of lapses (P > 0.05).
Self-Reported Sleep-Wake Assessment
Compared to the control group, the insomnia group reported significantly less total night sleep time and lower sleep efficiency, longer sleep onset latency, more wake after sleep onset, longer time in bed after their final awakening (sleep offset latency), and higher standard deviation of sleep onset latency (P < 0.007), the latter indicating increased night-to-night variability in sleep latency (Figure 1). No significant group difference in total time in bed was observed (P > 0.007).
Figure 1.
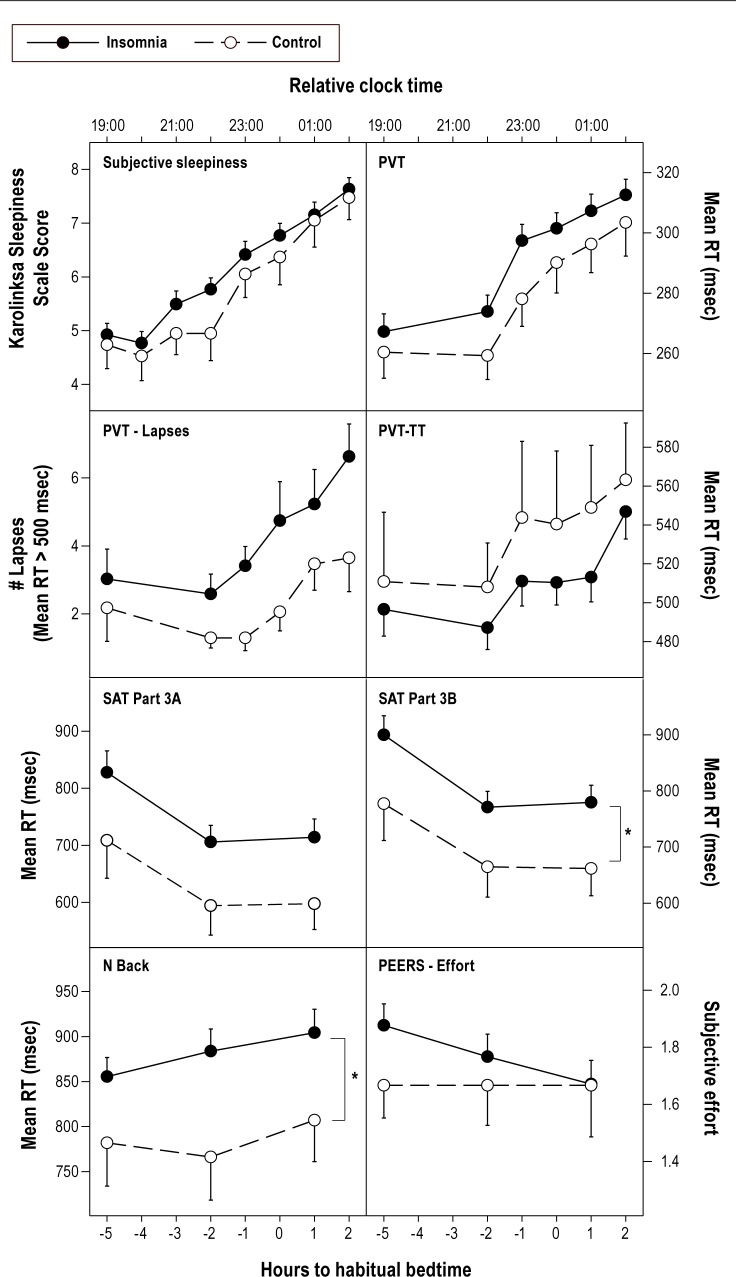
Neurobehavioral performance, subjective sleepiness and subjective task effort in the insomnia and control groups. Means and standard error of the mean are shown. Relative clock time is for a participant whose habitual bedtime was 12 midnight. PVT, psychomotor vigilance task; TT, two tone; RT, reaction time; SAT, Switching Attention Task; PEERS, Performance and Effort Evaluation Scale. For PVT MRT, PVT Lapses, and PVT-TT, raw means are shown here, but analysis was conducted on transformed RTs and lapses as described in the Data Analysis section. Only significant group differences are shown. *Significant group differences (main effect group), P < 0.05.
Self-Reported Daytime Impairments
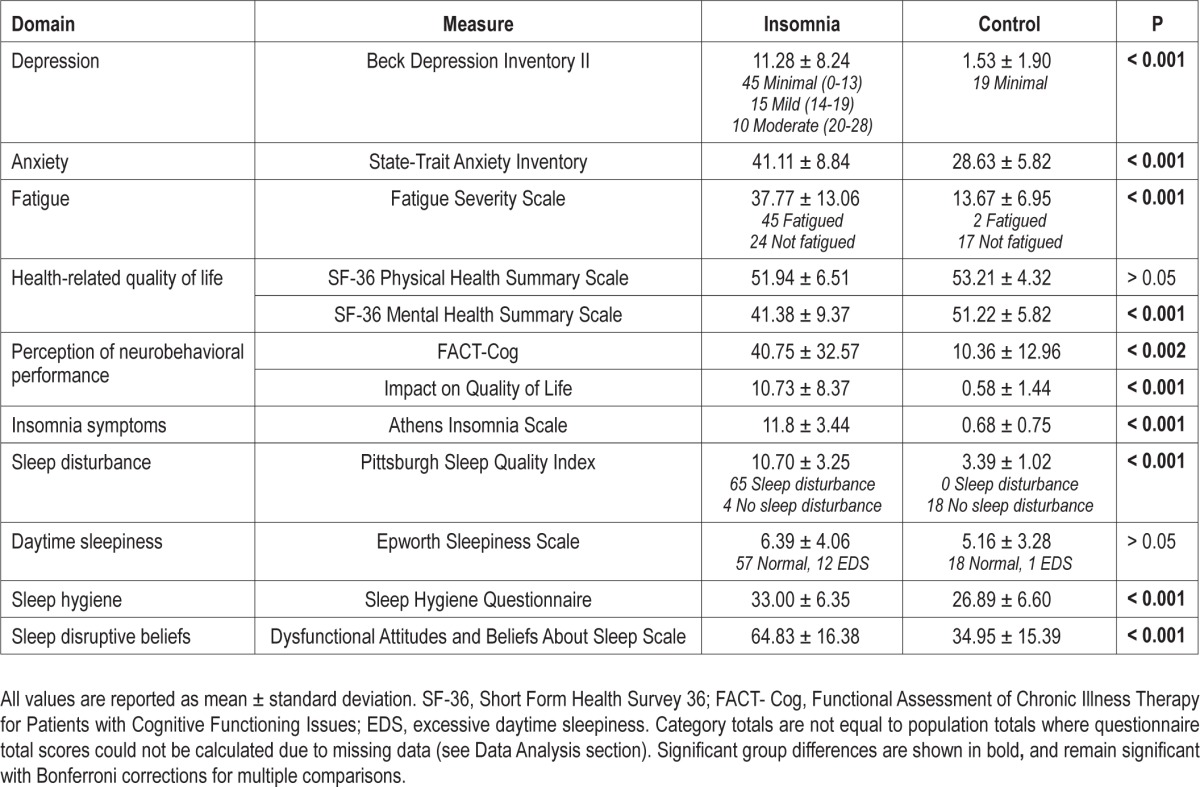
Participants in the insomnia group reported significantly higher levels of fatigue, depression, anxiety, and sleep disturbance, as well as poorer mental health-related quality of life than those in the control group (P < 0.002). Insomnia patients reported significantly poorer neurobehavioral performance in everyday day life (P < 0.0038), which they also reported as reducing their quality of life (P < 0.0038). There were no significant group differences on physical health-related quality of life or daytime sleepiness (P > 0.0038; Table 2).
Table 2.
Subjective measures of daytime impairment, sleep quality, and sleepiness
Laboratory Assessment
Both groups showed an increase in sleepiness (KSS score) with time of day (P < 0.05; Figure 2). No significant difference between groups or interaction effects with time were observed on the KSS or VAS-A (P > 0.05). Likewise, both groups showed slower mean reaction times on the PVT and PVT-TT with time into the laboratory session (P < 0.025; P < 0.05). For PVT lapses, however, no effect of time was observed (P > 0.05). For the PVT (lapses and mean reaction time) and PVT-TT, no overall difference between groups and no time by group interaction effects were observed (P > 0.05; Figure 2).
Figure 2.
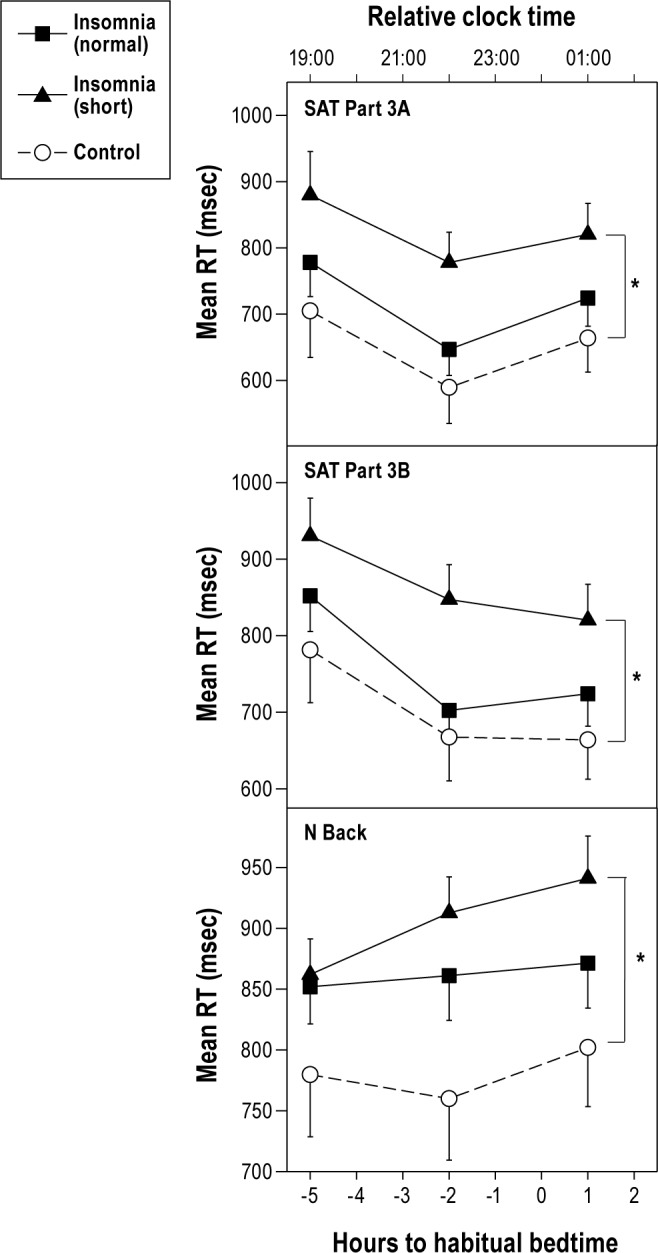
Neurobehavioral performance in the insomnia with normal (≥ 6 h) sleep duration group, insomnia with short (< 6 h) sleep duration group and control group. Means and standard error of the mean are shown. See Figure 1 for key. *Significant differences are only between the insomnia short sleep duration group and the control group (P < 0.05).
Overall, mean reaction time on the Switching Attention Task Part 3B was significantly slower in the insomnia group compared to the control group (P < 0.05). A trend towards significantly slower mean reaction times on Part 3A (P < 0.06) was also observed (Figure 2). No main effect of time or time by group interaction was observed (P > 0.05). The insomnia groups showed significantly slower mean reaction times on the N-Back task compared to controls (P < 0.05; Figure 2). There was no main effect of time and no time by group interaction effect (P > 0.05).
On the self-reported performance and effort rating scale (PEERS), both groups rated themselves as performing increasingly worse with time (P < 0.0167). No significant main effect of group and no time by group interaction differences were found on self-reported effort or perceived ability to improve performance (P > 0.0167; Figure 2).
Comparison of Neurobehavioral Performance in Insomnia Patients with Short versus Normal Sleep Duration
Based on the findings of others,20 the insomnia group was separated using sleep diary data into short sleep duration (self-reported total sleep time < 6 h) and normal sleep duration (self-reported total sleep time > 6 h). All control participants reported total sleep time > 6 h. When compared to the control group, the insomnia-short sleep duration group, but not the insomnia-normal sleep duration group, were significantly slower on the Switching Attention Task Part 3A and 3B, as well as the N-Back Task (P < 0.05; Figure 2). No main effect of time or time by group interaction was observed for any measure (P > 0.05). There was no main effect for group, time, or time by group interaction effect (P > 0.05) for the PVT mean reaction time or PVT number of lapses.
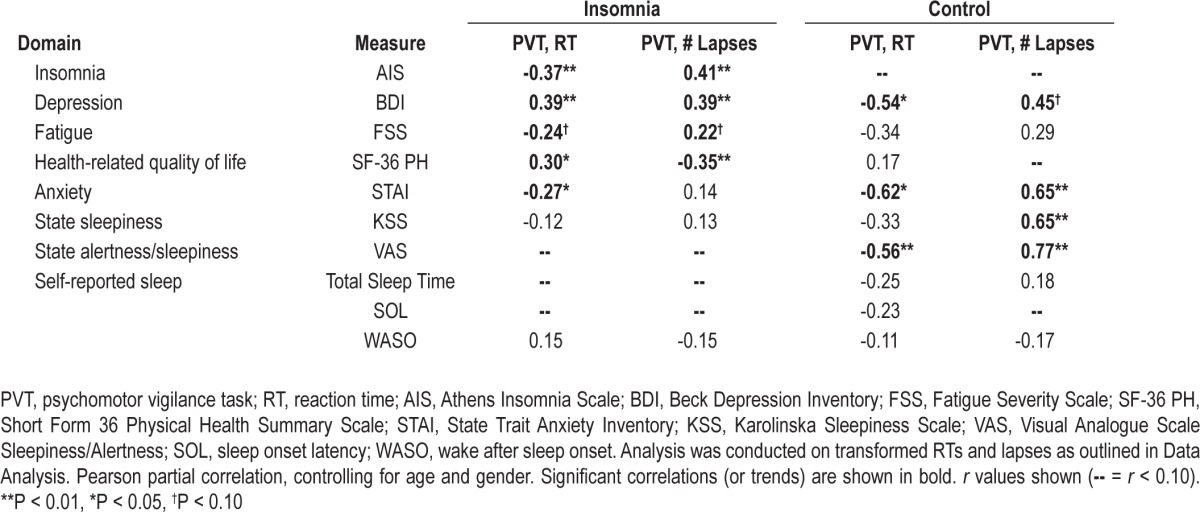
Correlates of Neurobehavioral Performance
In the insomnia group, significant correlations were found between poor performance on the PVT (mean 1/ RT, lapses) and increased scores on measures of depression, anxiety, and insomnia severity, as well as lower physical health-related quality of life (P < 0.05; Table 3). In contrast, in the control group, significant correlations were found between poor performance on the PVT (mean 1/RT, lapses) and increased state sleepiness and alertness, as well as increased levels of anxiety and depression (P < 0.05; Table 3).
Table 3.
Correlations between cognitive performance and trait daytime complaints and state sleepiness/alertness measures and sleep assessment measures
DISCUSSION
This study demonstrated that, compared to healthy controls, patients with insomnia disorder and no other medical or psychiatric conditions exhibited significant performance impairments on tasks of working memory and switching attention, but not sustained attention. Insomnia patients also exhibited significant impairments in subjective measures of daytime functioning, but did not differ from controls on measures of state or trait sleepiness. Neurobehavioral performance impairments in the insomnia group were associated with insomnia severity, fatigue, anxiety, depression, and physical health-related quality of life, but were not associated with measures of state sleepiness or alertness, or with the characteristics of sleep in the week prior to testing. Conversely, in control participants, neurobehavioral performance was strongly associated with state and trait measures of sleepiness. The insomnia group was more likely than the control group to report that cognitive impairments contributed to their reduced quality of life, highlighting the clinical significance of performance impairments in this population.
This study used a well-characterized population of insomnia patients who reported significantly less total nighttime sleep duration compared to healthy controls, despite no difference in the duration of time in bed. Consistent with their diagnosis, the insomnia group reported significantly longer sleep onset latency, more wake after sleep onset, and poorer sleep efficiency. The insomnia group also showed a significantly higher variability in their sleep onset latency, indicating night-to-night variability in their sleep disturbance.46,47 The insomnia group also reported poorer sleep quality, poorer sleep hygiene, and elevated levels of dysfunctional beliefs about the consequence of poor sleep, consistent with previous studies.30,46 Thus, there was considerable consistency across measures of different aspects of the insomnia experience.
Patients with insomnia showed poorer performance on a task of working memory (N-Back task), consistent with a recent meta-analysis.19 Insomnia patients were also shown to have significant impairments on a Switching Attention Task (Part 3B), supporting the findings of others.21,38 One of these previous studies,21 however, also reported significant performance impairments on Part 3A of the task. Part 3B of the task can be considered more difficult than Part 3A, as shown in the relatively longer response times. The insomnia group therefore only showed deficits on the higher level cognitive tasks included in the test battery.
There is considerable cortical overlap between the two cognitive functions which were found to be significantly impaired in insomnia patients—shifting attention and working memory.48 Functional neuroimaging studies show a strong link between performance on spatial N-Back working memory tasks and prefrontal cortical activation.49 Similarly, switching (or shifting) attention processes are thought to be a function of the prefrontal cortex,50 which is supported by functional neuroimaging studies.48 Deficits common to these two tasks may therefore be due to functional and/or structural changes in the prefrontal cortex that have been reported in patients with insomnia, including reduced orbitofrontal volume (MRI),51 hypometabolism in the prefrontal cortex during wake (PET),52 and hypoactivation in prefrontal (and medial) areas during a prototypical executive task (fMRI).53 Focal injuries to dorsomedial prefrontal areas are associated with moderate to severe insomnia symptoms (sleep initiation and maintenance),54 and reduced orbitofrontal volume has been found to be associated with symptom severity.51 The current study, together with others,51–54 collectively indicate that cognitive deficits in insomnia patients may be due to altered functioning of the pre-frontal cortex, which has also been implicated in the sleep disturbances characterizing the disorder, rather than simple sleepiness resulting from chronic sleep deficiency.
Additional support for the finding that the neurobehavioral performance deficits in insomnia patients are qualitatively different from those observed following sleep loss comes from the finding that the insomnia participants who self-reported short sleep duration (total sleep time < 6 h) did not show performance deficits on the sustained attention task (PVT) compared to controls. This is despite this task being widely recognized for its sensitivity to the effects of sleepiness.36 In contrast, insomnia patients who reported short sleep duration did show performance deficits on the working memory and switching attention tasks, while those reporting normal sleep duration did not show such deficits. These findings are consistent with those of others who have linked objectively determined short sleep duration in insomnia patients with the presence of cognitive deficits.20
Importantly, in the insomnia group there were no significant associations between state sleepiness measures and performance on a task known to be sensitive to sleepiness (PVT). In contrast, in the control group, subjective sleepiness and alertness measures explained up to two-thirds of the variance in performance on this task. In the insomnia group, neurobehavioral performance was strongly associated with insomnia severity, depression, fatigue, anxiety, and physical health-related quality of life. These results suggest that objectively measured daytime performance among insomnia patients is related to their experience of other daytime impairments such as dysphoric mood and fatigue, which perhaps share a common mechanism.55 Daytime performance was not correlated with sleepiness or alertness immediately prior to testing, or to sleep disturbances in the week prior to testing. This finding supports previous studies showing weak relationships between measures of sleep and performance among insomnia patients.21 Cognitive impairments in insomnia patients do not seem to be directly related to experiences of sleepiness or sleep disturbance, but instead to other domains of daytime dysfunction.17,18
Despite a reduction in self-reported total sleep time and sleep efficiency, the level of daytime sleepiness in insomnia patients did not differ from that of the control group. Neither did the insomnia group show elevated sleepiness under controlled laboratory conditions, when potentially stimulating environmental factors were highly controlled. The insomnia group did, however, reported experiencing significantly higher levels of daytime fatigue. This finding confirms prior assertions that the term fatigue rather than sleepiness may better capture the daytime experience of insomnia patients,18 and should therefore be included in standardized assessments of the disorder.45
This study demonstrated that sustained attention (PVT) in the insomnia group was not impaired relative to controls, even when the cognitive load was increased (PVT-TT). While the majority of previous studies have shown that insomnia is not associated with slowed reaction times on simple or complex attention tasks,19 one study reported that patients with insomnia perform faster than controls on a simple sustained attention task but slower than controls on a complex sustained attention task.37 This finding was not replicated here, perhaps due to differences in task modalities (i.e., visual stimuli in a previous study37 versus auditory stimuli here), the substantially older age group used in the previous study (60.6 years versus 35.6 years in the present study), or the tasks used in the present study lacked sensitivity to detect subtle attention deficits.
In addition to fatigue, individuals with insomnia also reported poorer mental health-related quality of life, as well as elevated symptoms of anxiety and depression. This is consistent with previous studies that have used stringent screening protocols to include only individuals with primary insomnia, yet find significant and widespread areas of daytime dysfunction in the same domains reported here.56,57
Limitations of this study should be acknowledged. We did not use polysomnography to exclude those with comorbid sleep disorders, although all participants were assessed by a sleep physician and excluded if a comorbid sleep disorder was thought to be present. The correlational approach used in this study is unable to determine causal relationships between daytime dysfunction and cognitive performance. We acknowledge that elevated depression and anxiety symptoms, which are common in patients with insomnia, also have an impact on cognitive function.19 While it will be difficult for future research studies to establish the extent to which the daytime symptom burden is due to sleep disturbance, or whether it may be due to other psychological issues, a necessary first step in attributing causality is to measure accurately each daytime symptom using standardized questionnaires. This approach would allow studies to better monitor the chronicity and temporal characteristics of these symptoms relative to the sleep disturbance, thereby providing clearer and more complete evidence of causality.
This study adds to the growing body of literature demonstrating that patients with insomnia do indeed experience objective deficits in cognitive function. This is consistent with the experience of patients who consistently report difficulties with their ability to think clearly during the day. The fact that differences were found on tasks of shifting attention and working memory compared to healthy sleepers, and not on simple or complex tasks of sustained attention, suggests that patients with insomnia may be able to maintain a reasonable level of performance and seem to be relatively unimpaired on low demand tasks. Differences are more likely to emerge as cognitive demands of a task increase. This is likely one reason for the reduced work productivity reported for those with insomnia.12 As this consistent pattern of findings begins to emerge, it may become possible to develop a standardized cognitive assessment that could be used in the assessment of insomnia and its daytime consequences. Of note, the findings of the current study indicate that the daytime consequences of insomnia are not simply caused by sleep loss and the resulting increase in sleepiness, but instead by a disturbance of brain functioning that results in contemporaneous sleep disturbance and reduced cognitive functioning. This distinction lends further weight to the statement that insomnia is not equivalent to sleep deprivation. Future studies are needed to determine the nature and mechanisms of the disturbance in brain function that underlies insomnia.
DISCLOSURE STATEMENT
The study was funded by an investigator-initiated research grant from Philips Respironics to Drs. Lockley, Rajaratnam, and Epstein. Dr. Lockley has received investigator-initiated research grants from Philips Respironics, Bioilluminations LLC, and Philips Lighting, and sponsor-initiated research contracts with Vanda Pharmaceuticals; has received consulting fees from Naturebright, Sound Oasis, Thomas Jefferson University, and Wyle Integrated Science and Engineering (NASA); unrestricted equipment gifts from Bioilluminations LLC, Bionetics Corporation, and Philips Lighting; an unrestricted monetary gift to support research from Swinburne University of Technology; and a fellowship gift from Optalert, Pty, Melbourne, Australia; he reports holding equity in iSLEEP, Pty, Australia; has received advance author payment and royalties from Oxford University Press and payment for editing a textbook section from Elsevier; has received honoraria and/or travel and accommodation support for invited seminars, conference presentations or teaching from 8th International Conference on Managing Fatigue, Harvard University, Lighting Science Group Corp, Ontario Association of Fire Chiefs, Rio Tinto, Woolcock Institute of Medical Research, Wyle Integrated Science and Engineering; holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women's Hospital; and has also served as a paid expert on behalf of public bodies in arbitration hearings related to sleep, circadian rhythms and work hours. Dr. Shekleton was supported by an Australian Postgraduate Award from Monash University. Dr. Flynn-Evans is a co-investigator on a sponsored clinical trial supported by Vanda Pharmaceuticals, and a member of the clinical advisory board at Isis Parenting. Dr. Epstein is Chief Medical Officer for Sleep HealthCenters, a sleep medicine services provider. Dr. Kirsch has participated in research for Philips Respironics and Fisher-Paykel, for which he was not directly compensated. Dr. Rajaratnam has served as a consultant through his institution to Vanda Pharmaceuticals, Philips Respironics, EdanSafe, The Australian Workers' Union, and National Transport Commission; has through his institution received research grants and/or unrestricted educational grants from Philips Respironics, Vanda Pharmaceuticals, Cephalon, and ResMed Foundation, and reimbursements for conference travel expenses from Vanda Pharmaceuticals; his institution has received equipment donations or other support from Optalert, Compumedics, and Tyco Healthcare; and he has also served as an expert witness and/or consultant to shift work organizations. The other authors have indicated no conflicts of interest in relation to this study.
SUPPLEMENTAL MATERIAL
Recruitment
The insomnia and control participants self-referred to the study by responding to community advertising, including on-line, poster, and television advertising. Individuals in the community who were either experiencing symptoms of insomnia or who considered themselves to be healthy sleepers (control group) made an initial inquiry about participation requirements and, if interested, underwent preliminary assessment of eligibility. Study advertising predominantly targeted individuals with insomnia complaints, rather than healthy sleepers, and as a result the majority of inquiries were received from individuals with insomnia.
Participant Characteristics
Participants reported not having smoked more than 5 cigarettes per week or used other tobacco products more than once per week in the previous 3 months. Participants reported habitual caffeine consumption < 300 mg per day on average, and moderate alcohol intake (for men an average ≤ 4 standard drinks per day, ≤ 28 standard drinks per week, and ≤ 6 standard drinks in any one day in the past 1 month; and for women an average ≤ 2 standard drinks per day, ≤ 14 standard drinks per week, and ≤ 4 standard drinks in any one day in the past 1 month). Participants were excluded if they reported night shift work in the past 2 years (≥ 6 hours of work between 10 pm and 8 am), transmeridian travel (≥ 2 time zones) in the past 2 months, and/or a habitual sleep onset time earlier than 9 pm or later than 3 am.
Participants were required to have no history of drug or alcohol abuse in the past 12 months, no current use of any drugs or medications likely to affect sleep, alertness, circadian rhythms or melatonin (including β-blockers, nonsteroidal anti-inflammatory drugs), or if appropriate, were willing to discontinue the medication(s) for ≥ 2 weeks prior to enrolment and throughout the study with consent from their treating clinician.
Participants in the insomnia groups met the Research Diagnostic Criteria (RDC) for Insomnia Disorder as well as the Research Diagnostic Criteria for any of the following subtypes: Primary Insomnia, Psychophysiological Insomnia, Paradoxical Insomnia, and/or Idiopathic Childhood Insomnia (see below).1
A total of 154 participants signed the screening consent form. Of these individuals, 53 were excluded for the following reasons: laboratory test (electrocardiography, blood biochemistry, hematology, urinalysis) result outside normal range (n = 22), comorbid sleep disorder (n = 9), non-compliance with pre-study sleep-wake protocol (n = 8), psychiatric disorder (n = 6), BMI outside range (n = 3), positive drug screen (n = 3), smoking (n = 1), and habitual bedtime outside study limits (n = 1).
Diagnostic Criteria for Insomnia and Insomnia Subtypes (reproduced with permission from Edinger et al.1).
Research Diagnostic Criteria for Insomnia Disorder
A. The individual reports one or more of the following sleep related complaints: 1. difficulty initiating sleep; 2. difficulty maintaining sleep; 3. waking up too early; or 4. sleep that is chronically non-restorative or poor in quality. B. The above sleep difficulty occurs despite adequate opportunity and circumstances for sleep. C. At least one of the following forms of daytime impairment related to the nighttime sleep difficulty is reported by the individual: 1. fatigue/malaise; 2. attention, concentration, or memory impairment; 3. social/vocational dysfunction or poor school performance; 4. mood disturbance/ irritability; 5. daytime sleepiness; 6. motivation/energy/initiative reduction; 7. proneness for errors/accidents at work or while driving; 8. tension headaches, and/or gastrointestinal symptoms in response to sleep loss; and 9. concerns or worries about sleep.
Research Diagnostic Criteria for Primary Insomnia
A. The individual meets the criteria for insomnia disorder. B. The insomnia noted in A has been present for at least one month. C. One of the following two conditions applies: 1. There is no current or past mental or psychiatric disorder. 2. There is a current or past mental or psychiatric disorder, but the temporal course of the insomnia shows some independence from the temporal course of the mental or psychiatric condition. D. One of the following two conditions applies: 1. There is no current or past sleep-disruptive medical condition. 2. There is a current or past sleep-disruptive medical condition, but the temporal course of the insomnia shows some independence from the temporal course of the medical condition. E. The insomnia cannot be attributed exclusively to another primary sleep disorder (e.g., sleep apnea, narcolepsy, or parasomnia) or to an unusual sleep/ wake schedule or circadian rhythm disorder. F. The insomnia cannot be attributed to a pattern of substance abuse or to use or withdrawal of psychoactive medications.
Research Diagnostic Criteria for Psychophysiological Insomnia
A. The individual meets the criteria for insomnia disorder. B. The insomnia noted in A has been present for at least one month. C. The patient has evidence of conditioned sleep difficulty and/or heightened arousal in bed as indicated by one or more of the following: 1. Excessive focus on and heightened anxiety about sleep. 2. An inability to fall asleep in bed at the desired bedtime or during planned naps but relative ease falling asleep during other relatively monotonous activities (e.g., watching TV, reading, etc.) when not intending to sleep. 3. Being able to sleep better away from home than at home. 4. Mental arousal in bed characterized either by intrusive thoughts or a perceived inability to volitionally cease sleep-preventing mental activity. 5. Heightened somatic tension in bed reflected by a perceived inability to relax the body sufficiently to allow the onset of sleep. D. One of the following two conditions applies: 1. There is no current or past mental disorder. 2. There is a current or past mental disorder, but the temporal course of the insomnia shows some independence from the temporal course of the mental disorder. E. One of the following two conditions applies: 1. There is no current or past sleep-disruptive medical condition. 2. There is a current or past sleep-disruptive medical condition, but the temporal course of the insomnia shows some independence from the temporal course of the medical condition. F. The insomnia cannot be attributed solely to another primary sleep disorder (e.g., sleep apnea, narcolepsy, or parasomnia) or to an unusual sleep/wake schedule or circadian rhythm disorder. G. The insomnia cannot be attributed to a pattern of substance abuse or to use or withdrawal of psychoactive medications.
Research Diagnostic Criteria for Paradoxical Insomnia
A. The individual meets criteria for insomnia disorder. B. The insomnia noted in A has been present for at least one month. C. Nocturnal polysomnography shows a sleep time > 6 hours and a sleep efficiency > 85%. D. One or more of the following applies: 1. The individual reports a chronic pattern of little or no sleep most nights with rare nights during which relatively normal amounts of sleep are obtained. 2. Sleep log data during one or more weeks of monitoring show an average sleep time well below published age adjusted normative values, often with no sleep at all indicated for several nights per week. Typically there is an absence of daytime naps following such nights. 3. The individual shows a consistent, marked mismatch between objective findings from polysomnography and subjective sleep estimates. E. The daytime impairment reported is consistent with that reported by other insomnia subtypes, but it is much less severe than expected given the extreme level of sleep deprivation reported. There is no report of intrusive daytime sleep episodes, disorientation, or serious mishaps due to marked loss of alertness/vigilance. F. One of the following applies: 1. There is no current or past mental disorder. 2. There is a current or past mental disorder, but the temporal course of the insomnia shows some independence from the temporal course of the mental condition. G. One of the following applies: 1. There is no current or past sleep-disruptive medical condition. 2. There is a current or past sleep-disruptive medical condition, but the temporal course of the insomnia shows some independence from the temporal course of the medical condition. H. The insomnia cannot be attributed solely to another primary sleep disorder (e.g., sleep apnea, narcolepsy, or parasomnia) or to an unusual sleep/wake schedule or circadian rhythm disorder. I. The insomnia cannot be attributed to a pattern of substance abuse or to use or withdrawal of psychoactive medications.
Research Diagnostic Criteria for Idiopathic (Childhood Onset) Insomnia
A. The individual meets the criteria for insomnia disorder. B. The insomnia noted in A began during childhood (i.e., before age 10) without an identifiable precipitant. C. The insomnia has been persistent and unrelenting since its onset. D. One of the following applies: 1. There is no current or past mental disorder. 2. There is a current or past mental disorder, but the temporal course of the insomnia shows some independence from the temporal course of the mental condition. E. One of the following applies: 1. There is no current or past sleep-disruptive medical condition. 2. There is a current or past sleep-disruptive medical condition, but the temporal course of the insomnia shows some independence from the temporal course of the medical condition. F. The insomnia cannot be attributed solely to another primary sleep disorder (e.g., sleep apnea, narcolepsy, or parasomnia) or to an unusual sleep/wake schedule or circadian rhythm disorder. G. The insomnia cannot be attributed to a pattern of substance abuse or to use or withdrawal of psychoactive medications.
Neurobehavioral Tasks
Participants completed the Karolinska Sleepiness Scale every hour from 5 hours before to 2 hours after habitual bedtime. In addition, participants completed a brief test battery (Psychomotor Vigilance Task, Psychomotor Vigilance Task Two Tone) 5 hours before habitual bedtime and then hourly from three hours before habitual bedtime until 2 hours after habitual bedtime, as well as a full test battery (brief test battery plus N-Back, Switching Attention Task) at 5 and 3 hours before habitual bedtime and one hour after habitual bedtime. Each of these tasks was chosen because of its sensitivity to insomnia and/or sleep loss in previous studies.
Psychomotor Vigilance Task (Auditory) (PVT)
The auditory PVT is a 10-minute task of sustained attention where participants are instructed to press the space bar with their dominant hand as quickly as they can in response to a tone played at random intervals. The auditory PVT is sensitive to the effects of sleep deprivation and circadian misalignment.2,3 Mean reaction time (RT) was averaged across the 10-minute trial and the number of lapses (RTs > 500 msec) occurring during the 10-minute trial was recorded.
Psychomotor Vigilance Task (Auditory) – Two Tone (PVT –TT)
The PVT-TT is a 10 minute task of sustained attention with a response choice. The PVT-TT includes Tone 1 (the same tone used in the PVT, high pitch tone) and Tone 2 (lower pitched tone). Participants were instructed to press the space bar with their dominant hand as quickly as possible in response to Tone 1 and were instructed to ignore Tone 2. The second tone was introduced into the traditional PVT task in order to increase its cognitive load, potentially making it more sensitive to subtle performance deficits.4 Mean reaction time (RT) to Tone 1 was averaged across the 10-minute trial.
Switching Attention Task (SAT)
The Switching Attention Task (SAT) has three parts, with Part 3 shown to detect performance impairments in insomnia patients.5,6 In Part 1, participants were required to respond to either a square appearing on the left side of the screen by pressing the number 7 on an external number pad or a square on the right side of the screen by pressing 9. In Part 2, participants responded to an arrow appearing in the centre of the screen by pressing 7 if the arrow is pointing to the left or pressing 9 if the arrow was pointing to the right. In Parts 1 and 2, there were 12 practice items followed by 16 test items. In Parts 3A and B, the word SIDE or the word DIRECTION appeared on the screen, immediately followed by an arrow which appeared on either the left or the right side of the screen and pointed to either the left or the right. When the arrow was preceded by the word SIDE participants were required to press the key that corresponded to the side of the screen in which the arrow appeared. When the arrow was preceded by the word DIRECTION they were instructed to press the key that corresponded to the direction in which the arrow was pointing. There were 12 practice items and 48 test trials. The outcome of Part 3A was the mean reaction time to all SIDE stimuli and for Part 3B, it was the mean reaction time to all the DIRECTION stimuli. Data from the 16 test items from Parts 1 and 2 were not included in the analysis because these have not been shown to be consistently sensitive to insomnia-related deficits.5,6
N-Back Working Memory Task – 2-Back
The N-Back working memory task7 is a computerized, visually based task of working memory (Labview 8.5 software, National Instruments, North Ryde, NSW, Australia). Participants are required to match the position of the current stimulus with the position of the stimulus which was presented 2 trials previously. Similar tasks have been shown to be sensitive to performance deficits in an insomnia group8 and to sleepiness in healthy individuals.9 During the 4-minute task, one of 12 possible capital-letter stimuli appeared every 4.5 sec in one of 12 possible locations on a computer screen. A small fixation point was present in the centre of the grid. The capital-letter stimulus was presented for 200 msec. At 1.5 seconds prior to stimulus onset, a warning cue in the center of the screen appeared for 200 msec. The letter stimulus occurred 1.3 sec after the cue disappeared. Participants were instructed to respond as quickly and as accurately as possible by pressing N on the keyboard if the location of the letter did not match the location of the letter presented 2 trials earlier and M if the location did match. They were instructed to ignore the identity of the letter and to concentrate only on the location. Participants are required to complete 15 practice trials prior to the test trials, and were required to achieve ≥ 40 correct responses on at least one of these practice trials for test trials to proceed. Mean reaction time to all correct responses was recorded.
Performance Evaluation and Effort Scale (PEERS)
The PEERS10 comprises 3 questions, designed to assess a participant's perceived level of performance (1 to 7 scale), effort (1 to 4 scale), and ability to improve performance (1 to 3 scale).
Karolinska Sleepiness Scale (KSS)
The KSS is a 9-point scale requiring participants to rate how sleepy they have felt in the preceding 10 minutes, from 1-very alert to 9-very sleepy, fighting sleep, great effort to stay awake.11 The KSS is sensitive to state-dependent changes in sleepiness.12
Visual Analogue Scale – Alertness (VAS-A)
The VAS-A is a pen and paper task asking participants to mark on a 10 cm line how alert to sleepy they feel at that moment. The score is the distance in centimetres from an anchor point to their mark. Low scores indicate high levels of alertness.
REFERENCES
- 1.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 2.Jung CM, Ronda JM, Czeisler CA, Wright KP., Jr Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J Sleep Res. 2011;20:348–55. doi: 10.1111/j.1365-2869.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 4.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–43. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 5.Edinger JD, Glenn DM, Bastien LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiol Behav. 2000;70:127–34. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 6.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signal L, Van Den Berg M, Mulrine H, Gander P. Sleep intertia after naps is short-lived and not percieved. Sleep Biol Rhythms. 2010;8:A1–14. [Google Scholar]
- 8.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 9.Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–67. [PMC free article] [PubMed] [Google Scholar]
- 10.Dinges DF, Kribbs ND, Steinberg KN, Powell JW. Do we lose the willingness to perform during sleep deprivation? [abstract] Sleep Res. 1992;21:318. [Google Scholar]
- 11.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders, revised: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. 4th Text Revision ed. [Google Scholar]
- 3.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 4.Carey TJ, Moul DE, Pilkonis PA, Germain A, Buysse D. Focusing on the experience of insomnia. Beh Sleep Med. 2005;3:73–86. doi: 10.1207/s15402010bsm0302_2. [DOI] [PubMed] [Google Scholar]
- 5.Kyle SD, Espie CA, Morgan K. “…Not just a minor thing, it is something major, which stops you from functioning daily”: quality of life and daytime functioning in insomnia. Behav Sleep Med. 2010;8:123–40. doi: 10.1080/15402002.2010.487450. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. National Institutes of Health State of the Science Conference Statement: Manifestations and Management of Chronic Insomnia in Adults June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14:69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Espie CA, Kyle SD, Hames P, Cyhlarova E, Benzeval M. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the Great British Sleep Survey. J Clin Psychiatry. 2012;73:e1478–84. doi: 10.4088/JCP.12m07954. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia, comorbidity, and risk of injury among insured Americans: results from the America Insomnia Survey. Sleep. 2012;35:825–34. doi: 10.5665/sleep.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahly V, Berglund PA, Coulouvrat C, et al. The associations of insomnia with costly workplace accidents and errors: results from the america insomnia survey. Arch Gen Psychiatry. 2012;69:1054–63. doi: 10.1001/archgenpsychiatry.2011.2188. [DOI] [PubMed] [Google Scholar]
- 12.Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34:443–50. doi: 10.1093/sleep/34.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Relations between sleep, fatigue, and health-related quality of life in individuals with insomnia. J Psychosom Res. 2010;69:475–83. doi: 10.1016/j.jpsychores.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leger D, Partinen M, Hirshkowitz M, Chokroverty S, Touchette E, Hedner J. Daytime consequences of insomnia symptoms among outpatients in primary care practice: EQUINOX international survey. Sleep Med. 2010;11:999–1009. doi: 10.1016/j.sleep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Kyle SD, Morgan K, Spiegelhalder K, Espie CA. No pain, no gain: an exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med. 2011;12:735–47. doi: 10.1016/j.sleep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Means MK, Lichstein KL, Epperson MT, Johnson CT. Relaxation therapy for insomnia: nighttime and day time effects. Behav Res Ther. 2000;38:665–78. doi: 10.1016/s0005-7967(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 17.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4:277–98. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- 18.Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichstein KL, Durrence HH, Bayen UJ, Riedel BW. Primary versus secondary insomnia in older adults: subjective sleep and daytime functioning. Psychol Aging. 2001;16:264–71. doi: 10.1037//0882-7974.16.2.264. [DOI] [PubMed] [Google Scholar]
- 23.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 25.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 28.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–60. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 29.Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006;29:223–7. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 30.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs' attributions. psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. J Psychosom Res. 2000;48:141–8. doi: 10.1016/s0022-3999(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M, Keller SK. SF-36 physical and mental health summary scales: A user's manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 33.Cella D. Functional Assessment of Chronic Illness Therapy (FACT-Cog) Elmhurst, IL: FACIT; 2008. [Google Scholar]
- 34.Morin CM, Belleville G, Belanger L. Validation of the insomnia severity index. Sleep. 2006;29:A258–9. Abstract Supplement. [Google Scholar]
- 35.Psychological Corporation. Wechsler Test of Adult Reading. New York: Psychological Corporation; 2001. [Google Scholar]
- 36.Jung CM, Ronda JM, Czeisler CA, Wright KP., Jr Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J Sleep Res. 2011;20:348–55. doi: 10.1111/j.1365-2869.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–43. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 38.Edinger JD, Glenn DM, Bastien LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiol Behav. 2000;70:127–34. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 39.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 40.Dinges DF, Kribbs ND, Steinberg KN, Powell JW. Do we lose the willingness to perform during sleep deprivation? [abstract] Sleep Res. 1992;21:318. [Google Scholar]
- 41.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 42.Basner M, Dinges DF. Maximizing sensitivity of the Psychomotor Vigilance Test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson C, Wales AW, Horne JA. PVT lapses differ according to eyes open, closed, or looking away. Sleep. 2010;33:197–204. doi: 10.1093/sleep/33.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 45.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 46.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Ortuno MM, Edinger JD, Wyatt JK. Daytime symptom patterns in insomnia sufferers: is there evidence for subtyping insomnia? J Sleep Res. 2011;20:425–33. doi: 10.1111/j.1365-2869.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 49.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 50.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 51.Altena E, Vrenken H, Van Der Werf YD, Van Den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 53.Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. J Neurosci. 2010;30:16041–3. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riemann D, Kloepfer C, Berger M. Functional and structural brain alterations in insomnia: implications for pathophysiology. Eur J Neurosci. 2009;29:1754–60. doi: 10.1111/j.1460-9568.2009.06721.x. [DOI] [PubMed] [Google Scholar]
- 56.Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orff HJ, Drummond SPA, Nowakowski S, Perlis ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30:1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]