Abstract
Study Objectives:
While many studies have examined the association between insomnia and depression, no studies have evaluated these associations (1) within a narrow time frame, (2) with specific reference to acute and chronic insomnia, and (3) using polysomnography. In the present study, the association between insomnia and first-onset depression was evaluated taking into account these considerations.
Design:
A mixed-model inception design.
Setting:
Academic research laboratory.
Participants:
Fifty-four individuals (acute insomnia [n = 33], normal sleepers [n = 21]) with no reported history of a sleep disorder, chronic medical condition, or psychiatric illness.
Interventions:
N/A.
Measurements and Results:
Participants were assessed at baseline (2 nights of polysomnography and psychometric measures of stress and mood) and insomnia and depression status were reassessed at 3 months. Individuals with acute insomnia exhibited more stress, poorer mood, worse subjective sleep continuity, increased N2 sleep, and decreased N3 sleep. Individuals who transitioned to chronic insomnia exhibited (at baseline) shorter REM latencies and reduced N3 sleep. Individuals who exhibited this pattern in the transition from acute to chronic insomnia were also more likely to develop first-onset depression (9.26%) as compared to those who remitted from insomnia (1.85%) or were normal sleepers (1.85%).
Conclusion:
The transition from acute to chronic insomnia is presaged by baseline differences in sleep architecture that have, in the past, been ascribed to Major Depression, either as heritable traits or as acquired traits from prior episodes of depression. The present findings suggest that the “sleep architecture stigmata” of depression may actually develop over the course transitioning from acute to chronic insomnia.
Citation:
Ellis JG; Perlis ML; Bastien CH; Gardani M; Espie CA. The natural history of insomnia: acute insomnia and first-onset depression. SLEEP 2014;37(1):97-106.
Keywords: Acute insomnia, natural history, first-onset depression, chronic insomnia, short-term insomnia, precipitating factors
INTRODUCTION
Despite the DSM-5 suggesting insomnia in its acute or short-term form (i.e. meeting full criteria for Insomnia Disorder but with duration less than three months) may still warrant clinical attention, little is actually known about the early phase of insomnia. Understanding the full course of insomnia is not only important to the arena of preventative sleep medicine but is underscored by accumulating evidence suggesting insomnia, in its chronic form, is a risk factor for a range of psychiatric and physical morbidities.1–5 The most compelling case for insomnia being a risk factor for future morbidity is with regard to Major Depression.6–10 It has been shown that insomnia often precedes the onset of depression11 is present throughout its developmental course,12 and treating insomnia in those with depression results in a reduction in depressive symptoms.13 To that end, a recent meta-analysis suggests that chronic insomnia confers a two-fold risk for developing depression.14 What is unknown, however, is where this increased risk for depression occurs in the natural history of insomnia, as all the aforementioned studies have examined this link within the timeframe of chronic insomnia.
While a wealth of theoretical perspectives and cross-sectional studies exist on the topic of the natural history of insomnia, there are only a handful of prospective longitudinal studies that provide data on this subject.15–21 The primary limitations of these seminal studies pertain to (1) the clear delineation of what constitutes acute insomnia, (2) whether the index episode represents a first-onset or recurrence of insomnia, (3) the mode of assessment used (in most cases non-standard instruments and often single-item questions), (4) the timeframes used for assessments (in most cases either at annual or bi-annual intervals), and (5) the lack of objective sleep measures like polysomnography. For example, with regard to the timeframes between assessments, one study retrospectively assessed insomnia symptoms at six time points, the shortest follow-up being 2 years,15,18 one retrospectively assessed once at 5 years,20 and two others17,21 annually; one for 3 consecutive years and the other at one time point. As such, it would be difficult to reliably track the transitions between normal sleep, acute insomnia, and chronic insomnia, based upon the time between accounts, while also relying heavily on memory. Furthermore, differing criteria have been used in each study to define episodes of both acute (poor sleep/brief insomnia) and chronic insomnia (ranging between ≥ 1 month to ≥ 1 year), making definitive conclusions about the transition to chronic insomnia difficult. Interestingly, the only study with a follow-up period that could track the progression from acute insomnia to chronic insomnia as it occurred (3 months) found that rates of chronic insomnia, in a sample of hospitalized individuals, almost doubled over the period, from 10% to 19%,16 suggesting a 3-month incidence rate of approximately 9%. Moreover, these figures fit with the only epidemiological study that uses the DSM-5-defined criteria for acute insomnia (i.e., a 9.15% incidence over 3 months).22
From a theoretical standpoint, the most well-established conceptualization of the pathogenesis of insomnia comes from Spielman and colleagues.23–25 Spielman's model suggests that predispositional characteristics exist making some individuals more prone to insomnia than others. These characteristics, in combination with precipitating events, such as life stressors that may be biopsychosocial in nature, serve to initiate an acute episode of insomnia. An acute episode, in turn, may resolve, in tandem with the precipitating event, or evolve into chronic insomnia. The evolution to chronic insomnia is thought to be largely, if not wholly, mediated by perpetuating factors that are behavioral in nature, relating to how the individual manages their insomnia. As such, the initiation of insomnia and its acute phase is thought to occur in response to life-event stress. This viewpoint, although adopted in several subsequent models of insomnia,26–29 and having received some support,30–32 appears to be more complex than a simple stress-diathesis model. In essence, it may not be the life event per se that drives the insomnia, but the ability to manage the stress.33–36 Therefore, a potentially better indicator of the precipitating dimension of the Spielman model may well be the perception of stress as opposed to the number of stressful life-events experienced. Moreover, to date there has been no examination of the Spielman model in terms of whether the circumstances surrounding the precipitating event (e.g., the severity of either the stressor or the initial sleep disruption) influence whether an individual will transition from acute or short-term insomnia to chronic insomnia.
From an empirical standpoint, the most comprehensive study of the natural history of insomnia comes from Morin and colleagues.21,37,38 Here the emphasis was placed on changes over time in the number of reported symptoms (i.e., characterizing subjects over time as syndromal or subsyndromal for insomnia). Among the major findings from this programme of work are: (1) subsyndromal insomnia may persist for years and not progress to a full syndrome; (2) about 34% of subjects with subsyndromal or syndromal insomnia will exhibit full remission within 2 years; and (3) once syndromal, the insomnia tends to be persistent, with 70% of subjects remaining ill within 2 years. Because this approach does not embrace the concepts of acute and chronic insomnia, however, it does not allow, by design, a systematic study of how the individual transitions between these disease stages. Finally, only one study to date has incorporated polysomnography (PSG) into a natural history study.19 A single-night of PSG, at baseline revealed sleep latency as the only “biological” marker of the transition from “poor sleep” to chronic insomnia over a single follow-up period of, on average, seven and a half years. However, as the sample also included those with existing physical and psychiatric morbidities, including depression, at baseline and did not account for whether this was the first episode or a recurrent episode of insomnia, it would be difficult to determine whether this finding relates to other comorbidi-ties, the current episode of insomnia, or previous exposure(s) to insomnia. Further, as life-event stress or perceived stress was not assessed, their contribution to the development of insomnia in this population remains unknown.
The aims of the present study were to: (1) assess whether individuals with acute insomnia exhibit more life events and/or greater perceived stress, anxiety, and depression than normal sleepers; (2) evaluate how subjective and objective sleep continuity varies between normal sleepers and those with acute insomnia; (3) determine if and how sleep architecture differs between normal sleepers and individuals with acute insomnia; (4) delineate whether subjects who exhibit a remission from insomnia differ from those that develop chronic insomnia on the above measures at baseline; and (5) evaluate first-onset cases of depression and how these occur in association with normal sleep, remitted insomnia, and chronic insomnia. In line with Spielman, it was hypothesized that those with acute insomnia would report significantly more stressful life events, score higher on perceived stress, and poorer on measures of mood than their normally sleeping counterparts. Further, those with acute insomnia would report subjectively and demonstrate objectively poorer sleep than normal sleepers. Finally, based on accumulating data that insomnia is a risk factor for depression, it was hypothesized that there would be significantly more “cases” of first-onset depression in those who went on to develop chronic insomnia compared to those who either remitted from their insomnia or remained normal sleepers. Due to a lack of prior evidence, there were no specific hypotheses in relation to which baseline characteristics (stress, mood, or sleep) would differentiate between those who would go on to develop chronic insomnia compared to those who would remit.
METHODS
Recruitment and Procedure
Prospective subjects were recruited, as part of a larger study, from a regional media campaign, which included a television interview on the evening news with the PI of the study (JGE) and several local newspaper articles about insomnia. The television segment and newspaper articles specifically asked for volunteers to take part in a study on insomnia and provided contact details.
Potential subjects were contacted for an initial clinical interview by telephone to determine eligibility for the larger study. The clinical interview began with a brief description of the study including the time commitment required (up to 25 min). Interested individuals provided informed consent and began the interview with 5 questions allowing for the diagnosis of Insomnia Disorder, as defined by DSM-5 criteria.39 This included 2 questions regarding (a) the individual's principal sleep complaint of dissatisfaction with their sleep, and (b) whether this was a complaint of initial, middle, and/or late insomnia; one question regarding sleep opportunity to establish adequate opportunity for sleep; one question regarding insomnia frequency (3 nights per week minimum); and one question regarding the impact of insomnia on daytime functioning. Additional prompts were used, where necessary, to arrive at definitive responses to each question (e.g., a list of markers of distress or impairment including memory impairments, concentration difficul-ties, fatigue, daytime sleepiness, distress, irritability, impaired occupational or psychosocial functioning, were provided if the subject was unsure whether their insomnia resulted in daytime dysfunction). Next, individuals were further screened for other intrinsic sleep disorders including narcolepsy, sleep related breathing disorders, parasomnias, circadian rhythm disorders, and restless legs syndrome and periodic limb movement disorder, as well as medical and psychiatric histories using DSM-IV-TR definitions.
If subjects did not meet criteria for insomnia (specifically, they had to report being satisfied with their sleep and no current difficulty in initiating sleep, maintaining sleep, or waking too early in the morning) or any other sleep disorder, they were classified as normal sleepers. If subjects met DSM-5 criteria for insomnia, a sixth question was asked “…for how long has this (the sleep problem) been going on?” This was to differentiate acute insomnia (meeting DSM-5 criteria for Insomnia Disorder but for a duration between 3 days and 3 months) from chronic insomnia (meeting DSM-5 criteria for Insomnia Disorder, including the duration threshold > 3 months).39,40 If a subject met the criteria for chronic insomnia, at this point they were thanked for their time and the interview was terminated. If the subject met the criteria for normal sleep or acute insomnia, they were asked if they had a history of acute or chronic insomnia. Finally, all subjects with acute insomnia and normal sleepers were asked if they would like to take part in further follow-up surveys about their sleep.
Those who completed the interview and agreed to take part in the follow-up surveys were sent login details for an online survey, or were mailed a paper copy of the survey the day following their telephone interview (baseline assessment). The survey contained a repetition of the telephone queries and additional information pertaining to sleep quality, quantity, timing and the subtype and severity of insomnia. Moreover, a series of standard measures were provided including instruments assessing life stress events, perceived stress, mood, and prospectively sampled sleep continuity using daily sleep diaries. Follow-up surveys, composed of the same measures, were generated and delivered to subjects at 1 month, 3 months, and 6 months following the baseline assessment. To ensure recurrent episodes of acute insomnia were not mistaken for the diagnosis of chronic insomnia at follow-up points, subjects were also asked in each survey whether there sleep had changed since the last assessment. This was corroborated with the response provided to item 6 from the central/core questionnaire which asked ‘‘…for how long has this (the sleep problem) been going on?” in each survey.
If at the end of the initial telephone interview a potential subject met the criteria for the main study (i.e., an individual with acute insomnia or a normal sleeper) and; (a) lived within the Greater Glasgow and Clyde region of Scotland; (b) reported no prior history of a sleep problem (including insomnia), psychiatric illness (including depression), head injury, or chronic medical condition; and (c) they had not sought help or were on medication for insomnia, they were asked if they would like to take part in the in-lab component of the present study (a PSG study of the subject's sleep). If interested, subjects were asked to attend a briefing meeting at the University of Glasgow Sleep Centre. The briefing meeting was scheduled approximately 15 days prior to the first scheduled overnight (Visit 1 = Day 15) and consisted of completing informed consent for the overnights and to deliver their completed baseline assessment pack.
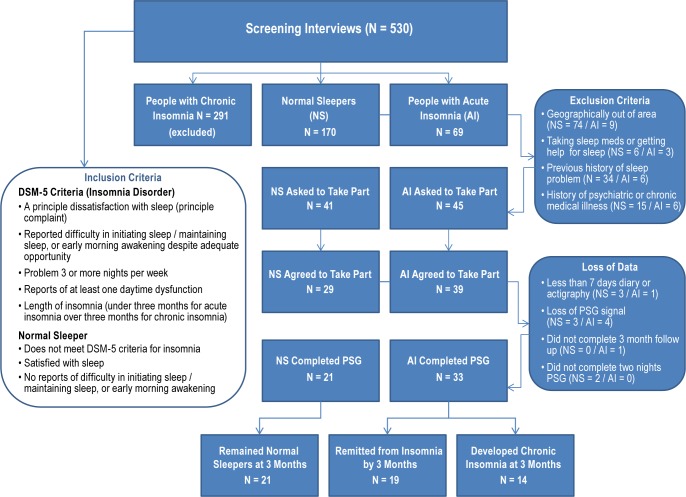
As can be seen in Figure 1, 530 telephone screening interviews were conducted; 291 individuals were excluded based on meeting criteria for chronic insomnia. Of the remaining 239 respondents, 170 were designated as normal sleepers (NS) and 69 as individuals with acute insomnia (AI). Of those, 86 met the additional inclusion criteria for the in-lab study and were asked to participate: 68 were enrolled, and 54 subjects completed the present study (21 normal sleepers and 33 subjects with acute insomnia). There were no differences in attrition between normal sleepers and those with acute insomnia between being asked to take part in the PSG assessment and completion of the study (Χ2(1) = 1.04, P = 0.31).
Figure 1.
Subjects spent 2 consecutive nights in the sleep center undergoing PSG study of their sleep. Subjects were instructed to refrain from alcohol, drugs, excessive caffeine and nicotine before arriving at the sleep laboratory at around 8:00 pm each night for study preparation (e.g. electrode placement, bio-calibrations). Bedtime was determined according to sleep diary reported bedtime. Total recording period was for ≥ 8 h for all subjects, although time out of bed in the morning was recorded and subjects could leave the bedroom during the remainder of the recording period (ambulatory PSG). The PSG data from the screening/adaptation first night was examined the next morning to confirm the absence of other comorbid sleep disorders using the American Academy of Sleep Medicine (AASM) scoring criteria.41 All subjects completed a sleep diary upon awakening. After electrode removal, subjects were free to leave and continue their day, as usual. For all in-lab meetings and assessments subjects were provided taxis to and from the laboratory. Subjects received an honorarium of £80 for their participation.
The central and supplementary questions from the follow-up surveys, including the question about sleep changes since the last assessment, were used to determine sleep status at the 3-month point. Subjects who met all the DSM-5 criteria at the 3-month point and had not remitted between time points were classified as people with chronic insomnia. People who at baseline had acute insomnia but no longer met DSM-5 criteria at either 1 month or 3 months (i.e., they had to report being satisfied with their sleep and no current difficulty in initiating sleep, maintaining sleep, or waking too early in the morning) were classified as natural remitters. Finally, those who reported normal sleep at baseline and continued to report normal sleep at 1 month and 3 months (i.e., they had to report being satisfied with their sleep and no current difficulty in initiating sleep, maintaining sleep, or waking too early in the morning) were classified as normal sleepers. The protocol for both the survey and in-lab study received ethics approval from the University of Glasgow Ethics Committee and the National Health Service, and conformed to the Declaration of Helsinki's ethical principles.
Measures
Psychological Self-Report Measures
The Social Readjustment Rating Scale: SRRS42 measures the number of major life events experienced over the previous 12 months. Each of 42 events provided is weighted by impact (e.g., 100 points for death of a spouse vs. 11 for minor violation of the law), and the sum of each weighting is calculated. Scores between 0-149 are generally considered to confer a low susceptibility to stress-related illness, 150-299 a medium susceptibility, and 300 points or more a high susceptibility to stress-related illness.
The Perceived Stress Scale: PSS43 is a 14-item scale measuring individuals' appraisal of levels of stress over the past month. Responses to each item are scored on a 5-point Likert-type scale (0-4), and scores range between 0 and 56, with higher scores indicating higher levels of perceived stress.
The Hospital Anxiety and Depression Scale: HADS44 is a 14-item scale that measures symptoms of depression and anxiety in clinical and nonclinical populations (7 depression items and 7 anxiety items). Each item is rated on a 4-point Likert-type scale (0-3) and scores range from 0-21 for each subscale. It is generally considered that scores ≥ 11 are indicative of a “case” of depression and/or anxiety in a general population sample.45
Measures of Sleep
Subjective Sleep: A standard sleep diary46 was used to derive core measures of subjective sleep continuity (time in bed [TIB], sleep latency [SL], wake after sleep onset [WASO], number of awakenings [NWAK], total sleep time [TST], and to calculate sleep efficiency [SE]) over a period ≥ 7 continuous days to a maximum of 14 days. Subjects were instructed to complete the diary each morning upon waking. Mean values were derived for each variable based upon the number of nights completed (mean continuous completion 13.37 ± 1.39 days).
Objective Sleep: Polysomnography (PSG) was carried out over 2 consecutive nights and was recorded on a 33-channel SomnoScreen plus (S-Med, Birmingham, UK). The first night served as a screening/adaptation night and consisted of an extended EEG montage (including C3-A2; C4-A1), submental and anterior tibialis electromyograms (EMG), bilateral electrooculogram (EOG), heart rate, thoracic and abdominal respiratory effort, airflow (by nasal-oral thermocouple and nasal thermistor), and oxygen saturation via finger pulse oximetry. The second night of PSG (used for the between group assessment) was a reduced montage and consisted of the same EEGs, EMG (submental only), EOG, and heart rate measurements. On both nights, percentages of and latency to each stage of sleep (Wake, N1, N2, N3, and REM) as well as clinical measure of SL, WASO, NWAK, TST, and TIB were documented. Sleep efficiency (SE) scores were derived by dividing total TST by TIB and multiplying by 100 to achieve a percentage.
Blinding Procedures and Data Analysis
PSG data were blind scored, using AASM criteria,41 independently by a RPSGT qualified technician from another laboratory to ensure no experimenter bias. Identifying information was removed prior to electronic transfer and scorers were unaware of group assignments. A random sample (50%) of the PSG data was later scored by the corresponding author (JGE) to corroborate the scoring. Group differences were examined using χ2 analyses for dichotomous data and independent t-tests (2 groups) or ANOVAs (3 groups) with post hoc Scheffe tests for continuous data. For all analyses a significance level of P < 0.05 was chosen. Missing data were treated by mean substitution when < 5% of that scale or measure was missing. More than 5% of data missing from a measure resulted in casewise deletion. Percentages and percentage differences are reported for clinical caseness. For the present analysis, data on sleep (acute insomnia or normal sleeper) stress and mood (life events, perceived stress, anxiety, and depression) at baseline are reported first. Then, data on sleep status (normal sleeper, individual with chronic insomnia, or natural remitter) and levels of depression derived from the 3-month follow-up survey are reported.
RESULTS
Final Sample Composition
The final sample consisted of 18 males and 36 females. As a history of a psychiatric illness or insomnia was exclusion criteria, neither group had a current or a past history of depression or a past history of insomnia. The mean age of the sample was 33.4 ± 12.8. There were no significant between group differences in age (P = 0.77) or gender (P = 0.55) (Table 1). Additionally, none of the subjects had an apneahypopnea index ≥ 15, ruling out sleep apnea. Similarly, none of the subjects met AASM criteria for an objective diagnosis of periodic limb movement disorder, bruxism, or an underlying parasomnia.
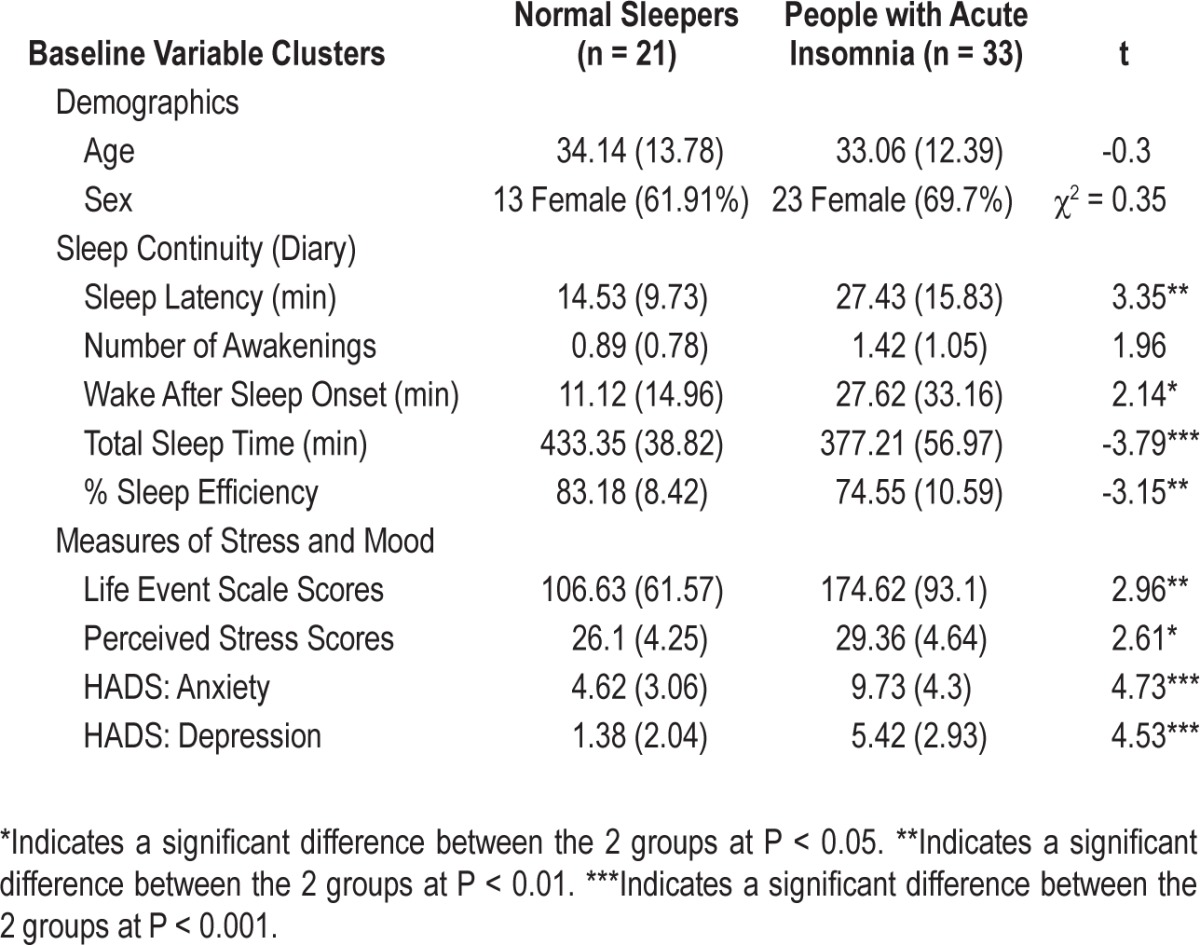
Table 1.
Baseline group differences in demographics and self-report measures
Baseline Profiles of Normal Sleepers vs. those with Acute Insomnia (Stress and Mood Measures)
As expected, normal sleepers and those with acute insomnia differed on all the stress and mood measures; Social Readjustment Rating Scale Scores (P < 0.005), Perceived Stress Scale Scores (P < 0.012), and both anxiety (P < 0.001) and depression scores (P < 0.001). In each case, those with acute insomnia reported higher stress and poorer mood than normal sleepers.
Sleep Profiles in Normal Sleepers vs. those with Acute Insomnia
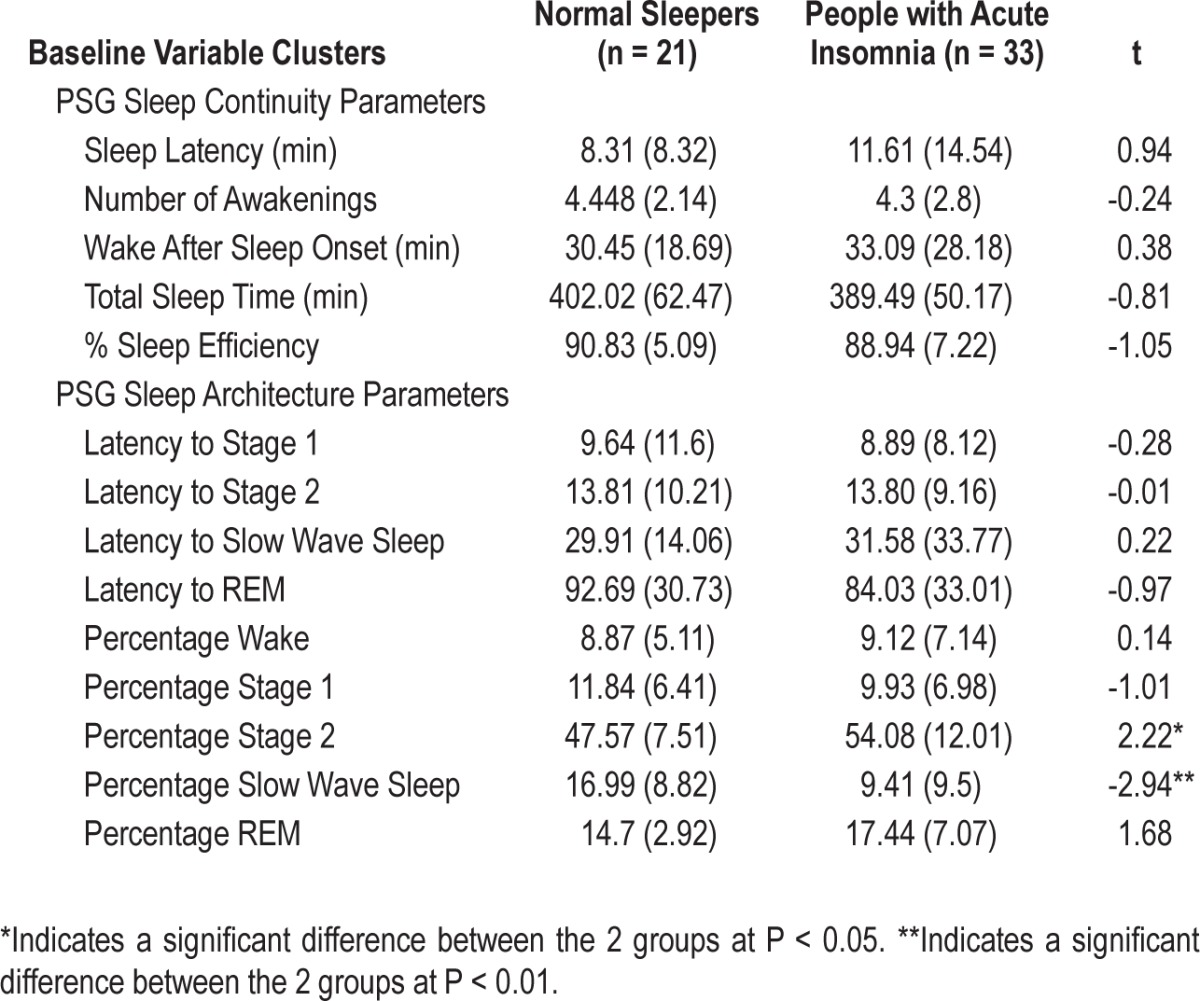
The next analysis examined sleep continuity variables from the sleep diaries. As expected, normal sleepers and those with acute insomnia differed in terms of SL (P < 0.005), WASO (P < 0.037), TST (P < 0.001), and SE (P < 0.005). The groups tended to differ with respect to NWAK, but this was not signifi-cant (P = 0.056). On each variable, those with acute insomnia reported sleeping worse than normal sleepers. No significant between group PSG differences were observed for any of the standard 5 sleep continuity variables; SL, NWAK, WASO, TST, or SE (all at P > 0.05) (Table 2). Further, there were no differences in terms of latency to any stage of sleep; N1, N2, N3, and REM (all at P > 0.05). Significant differences for sleep architecture were observed between normal sleepers and those with acute insomnia, with those with acute insomnia showing greater percentages of N2 (P < 0.03) and reduced percentages of N3 (P < 0.005). There were no differences between the groups in terms of percentages of Wake, N1, or REM (all at P > 0.05).
Table 2.
Baseline group differences in objective sleep parameters
Sub-Group Composition (Normal Sleepers vs. Natural Remitters vs. those with Chronic Insomnia)
Of the 33 people with acute insomnia at baseline: 14 (42%) met DSM-5 criteria for insomnia at 3 months and reported no changes in sleep status over the previous 3 months were classified as subjects with chronic insomnia; and 19 (58%) no longer met the criteria for insomnia, either reporting remission by 1 month (n = 15) with no new onset by 3 months or remission by 3 months (n = 4), and were classified at natural remitters. None of the original 21 normal sleepers reported the onset of a sleep problem at 1 or 3 months and were still classified as normal sleepers.
Differences between Groups (Normal Sleepers vs. Natural Remitters vs. those with Chronic Insomnia) on Baseline Measures of Stress, Mood, and Sleep
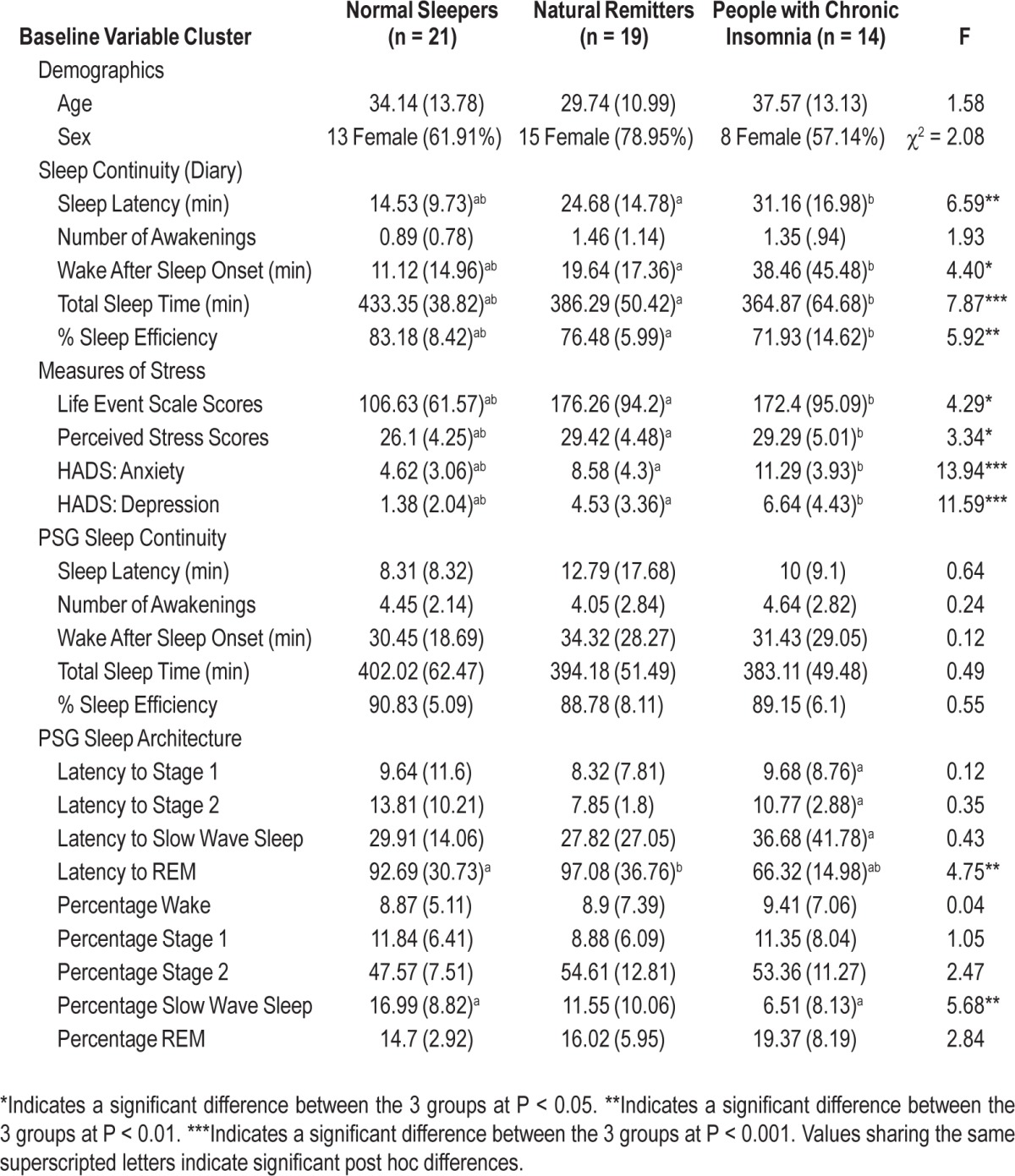
The 3 groups (normal sleeper, natural remitter, and those with chronic insomnia), while continuing not to differ with respect to Age and Sex (all at P > 0.05), differed on 4 of the 5 baseline self-report sleep continuity measures; SL (P < 0.003), WASO (P < 0.017), TST (P < 0.001), and SE (P < 0.005), but not NWAK (P = 0.16) where the natural remitters and chronic insomnia groups exhibited greater morbidity than normal sleepers but did not differ from each other (i.e., no differences between those with chronic insomnia and natural remitters). Similarly, the 3 groups differed on all the baseline measures of stress and mood, but again the natural remitter and chronic insomnia groups did not differ on these measures (see Table 3). The 3 groups did not differ with respect to PSG assessed sleep continuity (all at P > 0.05). In terms of comparisons on sleep architecture, differences were evident within REM latency (P < 0.01) and percentage of N3 (P < 0.006): The chronic insomnia group exhibited a reduced REM latency as compared to normal sleepers (P < 0.05) and natural remitters (P < 0.05) and those with chronic insomnia exhibited reduced N3 compared to normal sleepers (P < 0.05).
Table 3.
Group differences based on baseline characteristics (sleep parameters, stress and mood)
Assessment of First-onset Depression (Normal Sleeper vs. Natural Remitter vs. those with Chronic Insomnia at Follow Up)
At the 3-month follow-up there was a significant difference between the three groups in terms of depression scores from the HADS (F2,51 = 8.61, P < 0.001). Post hoc tests revealed no significant differences between those who had remitted (6.16 ± 3.34) and those who remained normal sleepers (3.14 ± 2.92) or those who became chronic (7.79 ± 4.06), but a significant difference between the normal sleepers and those who had developed chronic insomnia (P < 0.05). Using the cutoff scores for a “clinical” case of depression (i.e., HADS scores ≤ 11), 0 of the normal sleepers had a case of depression at baseline, 0 at 1-month, and 1 at the 3-month follow-up. As such there was an overall increase of 1 case of depression between baseline and 3 months in the normal sleeping group. For the natural remitters, at baseline there were 0 cases, 1 case at one month, and still one case at the 3-month follow-up; thus there was an overall increase of 1 case over the 3-month period. For the subjects with chronic insomnia, there were 0 cases at baseline, 2 cases at one month, and 5 cases at follow-up, suggesting an increase of 5 cases between baseline and 3-month follow-up.
DISCUSSION
The primary aims of this study were to determine whether differences existed in terms of life events, perceived stress, mood, and sleep, both subjectively and objectively, between those with acute insomnia and normal sleepers. Additionally, we aimed to determine whether any baseline characteristics differentiated those who would remit from insomnia from those who would transition to chronic insomnia. The final aim of the study was to examine whether those who developed chronic insomnia would report more cases of first-onset depression compared to those who were normal sleepers or remitted before the insomnia became chronic.
The first finding, that individuals with acute insomnia exhibited more life events, greater perceived stress, anxiety, and depression than normal sleepers, supports Spielman's model, in that insomnia appears to be precipitated by stress. Importantly, where previous research has shown experimentally induced stress, prior to sleep onset to demonstrate this relationship,36,47–50 the present study now documents this association in a naturalistic way during the acute phase of insomnia. Interestingly it also appears that the number of life events experienced, levels of perceived stress, and levels of negative mood had no bearing on whether an individual would go on to develop chronic insomnia or not. As such, it appears that the severity and impact of the initial stressor are unlikely to be the main drivers in the progression from acute to chronic insomnia.
The finding that there were reliable subjective differences in most sleep continuity dimensions (SE, SL, WASO, and TST) is unsurprising, as subjective reports of difficulties in initiating or maintaining sleep are central criteria for a diagnosis of both acute and chronic insomnia, although an examination of the means and standard deviations of these variables, particularly SL and WASO, shows that, like chronic insomnia, acute insomnia is not a homogeneous phenomenon and is characterized by extreme between-subject variability in terms of overall severity and presenting subtype. Normal sleepers and individuals with acute insomnia did not exhibit objective sleep continuity differences but did exhibit sleep architecture differences. The lack of PSG sleep continuity findings is, to some extent, surprising. Given an adaptation night, at least small observable differences would be expected between the normal sleeper and acute insomnia groups. The absence of such findings may be a reflection of within-subject night-to-night variability in insomnia severity51–53 and further evidence that multiple days or weeks of data are required for stable and reliable sleep continuity estimates. Alternatively, it may be that the perceptual aspect of insomnia (the inability to perceive sleep as sleep) develops in advance of frank sleep initiation and maintenance problems, or there is a sequence of effects that cannot be resolved with the present “sampling rate.” Whatever the case, the lack of sleep continuity findings further underscores the wisdom of the AASM recommendation that PSG is not required for a diagnosis of insomnia and allows for the prospect of early intervention. That is, one is not required to wait until objective findings are present to initiate treatment.
The PSG data revealed two differences between normal sleepers and subjects with acute insomnia with respect to sleep architecture. Acute insomnia was characterized by “lighter” sleep (lower percentages of N3 and higher percentages of N2 sleep). These findings are consistent with the perspective that the sleep of acute insomnia may be less perceptible as sleep, or at least perceived as less restorative.27 Specifically, previous studies have shown that individuals with chronic insomnia, woken successively during N2 sleep, are more likely to perceive the period before awakening as wakefulness as opposed to sleep.54,55 Moreover, one study demonstrated “abnormal” ERP responses during N2 sleep in individuals with chronic insomnia, compared to controls, suggesting that cortical arousal protective processes are impaired in this population.56 Whether the current findings simply reflect two biological markers specific to this population or an increased opportunity for enhanced information processing during sleep (i.e., a vulnerability inherent in acute insomnia as well as chronic insomnia) remains to be seen but is worthy of future enquiry. Certainly, the fact that those with acute insomnia reported poorer sleep on almost all sleep continuity dimensions compared to normal sleepers but did not demonstrate any objective differences hints towards the latter hypothesis.
Despite the findings regarding stress as a precipitating factor for acute but not chronic insomnia is valuable, it may well be that the PSG data and follow-up depression data are the most suggestive. The two sleep architecture differences (i.e., reduced N3 and decreased REM latency in those who become chronic) have the potential to provide an insight into the natural history of insomnia and its association with depression. Traditionally in pure cross-sectional designs, decreased REM latency and decreased N3 are not seen in chronic insomnia.57 However, this pattern is commonly observed in other psychiatric disorders, most notably preceding and during an episode of depression.58–62 This would suggest, as is commonly held, that reduced N3 and decreased REM latency are features of depression (expressed or unexpressed). In the present study, it seems unlikely that these differences relate to the number of life events experienced, levels of perceived stress, mood, or severity of sleep complaint, as these factors did not differ between those who remitted and those who transitioned to chronic insomnia. Further, given our observation that subjects with chronic insomnia disproportionately exhibited first-onset depression (i.e., a 9.26% increase compared to 1.85% in normal sleepers and 1.85% in those who naturally remitted), this suggests, as one possibility of many, that acute insomnia may represent the “initial wound” to future depression and this, in combination with an unknown factor, results in reduced REM latency and decreased N3 in subjects that develop chronic insomnia. Once chronic, both the insomnia and the reduced REM latency and N3 represent risk factors for first-onset depression. These speculations should however be treated with a certain degree of caution as the mean REM latency, at baseline, in those who went on to develop chronic insomnia (mean of 66 min) was higher than what is generally considered in the region for primary depression (50-65 min).63,64 However, a REM latency of 70 minutes has been shown to confer a sensitivity of 82% and specificity of 69% to detect “all-cause” depression,65 and several studies have demonstrated a mean REM latency, similar to the data presented here (i.e., mean REM latencies of 65, 68, and 69 minutes),66–68 in patients with primary depression. Finally, to our knowledge REM latency has never been examined in first-onset depression, so it is unknown whether the observed REM latencies in this population are outside the norm. As such, of the many questions that remain here are (1) do the two phenomena (reduced N3 and shorter REM latencies) represent the same risk or different risks that may interact? (2) do the reduced REM latency and N3 observations represent a trait or state vulnerability? and (3) do reduced REM latency and N3 continue to be present as a “scar” and a risk factor for depression if the chronic insomnia remits or is treated? Certainly, in terms of the first question, qEEG would be a useful addition and would also help determine whether this reduced REM Latency signifies increased REM pressure and/or a weakening of the NREM system.69–71 Moreover, in terms of the second question, the assessment of a gene/REM latency interaction would add to the existing literature which currently leans towards a trait vulnerability for depression, at least in terms of reduced REM latency.72–74 Clearly these issues await natural history studies that adopt some of the strategies deployed here but in substantially larger samples.
Limitations
The findings should be with interpreted with caution. This is the first study of its kind in terms of examining objective and subjective sleep in naturally occurring acute insomnia and examining baseline characteristics with a short temporal resolution. Moreover, psychiatric illnesses, current status and history, were only assessed by self-report, which may have been subject to a self-presentation bias. As such, replication and extension (e.g., having access to medical and psychiatric records) are vital to confirm the validity of the present findings. The results are also limited in terms of the nature or type of the insomnia. As with most insomnia studies, the sample was not classified in terms of presenting type of insomnia (e.g., idiopathic, paradoxical). Similarly, there were no individuals recruited who objectively demonstrated a co-occurring sleep disorder. While these findings do strengthen our understanding of “pure” acute insomnia and fit with what has been observed in the prevalence and incidence of acute insomnia (i.e., a higher prevalence and incidence of “pure” cases compared to complex cases),22 the extent to which these findings are replicable to those with complex acute insomnia in unknown.
CONCLUSION
The present study sought, for the first time, to characterize stress, sleep, and mood during acute insomnia and to determine whether any of the factors present at the onset of insomnia differentiated those who would transit to chronic insomnia from those who would remit. Further, whether those who developed chronic insomnia were also more likely to develop first-onset depression, compared to those who remitted or remained normal sleepers. The findings suggest acute insomnia is characterized by stress (the number of life events experienced and the perception of stress), poorer mood, and poorer subjective sleep. Moreover, acute insomnia appears to be characterized by longer periods of lighter sleep. Finally, there is a substantial literature suggesting insomnia is a risk factor for depression.6–11 The present data tentatively suggest that it is acute insomnia that is associated with the vulnerability for first-onset depression. Interestingly, the figures of those who developed first-onset depression over the acute period were over twice the estimated annual incidence,75 reflecting the findings from a recent meta-analysis.15
DISCLOSURE STATEMENT
This was not an industry-funded study. This study was funded by the Economic and Social Research Council (RES-061-25-0120-A). The funders had no role in any aspect of the study or production of the manuscript. The PI (Dr. Ellis) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Ellis has received an educational grant and speaking honoraria from UCB Pharma, and a research grant from Transport for London. He has also consulted for the British Broadcasting Corporation. Professor Bastien, Dr. Gardani, Dr. Perlis report no conflicts of interest. Professor Espie has consulted and/or had speaking engagements for UCB Pharma, Boots UK, Novartis. He is clinical and scientific director for Sleepio Ltd and has used equipment for research on agreement from Philips Respironics. None of these conflicts of interest are related to the current manuscript.
REFERENCES
- 1.Vahtera J, Pentti J, Helenius H, Kivimaki M. Sleep disturbances as a predictor of long-term increase in sickness absence among employees after family death or illness. Sleep. 2006;29:673. doi: 10.1093/sleep/29.5.673. [DOI] [PubMed] [Google Scholar]
- 2.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1:227–47. doi: 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz SW, Cornoni-Huntley J, Cole SR, Hays JC, Blazer DG, Schocken DD. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8:384–92. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- 5.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford D, Kamerow D. Epidemiologic study of sleep disturbance and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 7.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for the onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 8.Pigeon WR, Hegel M, Unutzer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31:481–8. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riemann D, Voderholzer U. Primary Insomnia: A risk factor to develop depression. J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 10.Riemann D, Berger M, Voderholzer U. Sleep and depression- results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 11.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–67. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 13.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Vollrath M, Wicki W, Angst J. The Zurich Study VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths MF, Peerson A. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49:245–53. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 17.Jansson-Frojmark M, Linton S. The course of insomnia over one-year: A longitudinal study in the general population of Sweden. Sleep. 2008;31:881–6. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35:689–97. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahtera J, Kivimäki M, Hublin C, et al. Liability to anxiety and severe life events as predictors of new-onset sleep disturbances. Sleep. 2007;30:1537–46. doi: 10.1093/sleep/30.11.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3 year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 22.Ellis JG, Perlis ML, Neale LF, Espie CA, Bastien CH. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;10:1278–85. doi: 10.1016/j.jpsychires.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Spielman AJ. Assessment of insomnia. Clin Psychol Rev. 1986;6:11–25. [Google Scholar]
- 24.Spielman AJ, Caruso L, Glovinsky PB. A behavioural perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 25.Spielman AJ, Nunes J, Glovinsky PB. Insomnia. Neurol Clin. 1996;14:513–43. doi: 10.1016/s0733-8619(05)70272-3. [DOI] [PubMed] [Google Scholar]
- 26.Espie CM. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 27.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 28.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–87. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallieres A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2:50–62. doi: 10.1207/s15402010bsm0201_5. [DOI] [PubMed] [Google Scholar]
- 31.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Geng FU, Fan F, Mo L, Simandl I, Liu X. Sleep problems among adolescent survivors following the 2008 Wenchuan earthquake in China: a cohort study. J Clin Psychiatry. 2013;74:67–74. doi: 10.4088/JCP.12m07872. [DOI] [PubMed] [Google Scholar]
- 33.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 34.Ellis J, Cropley M. An examination of thought control strategies employed by acute and chronic insomniacs. Sleep Med. 2002;3:393–400. doi: 10.1016/s1389-9457(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 35.Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychol. 2004;23:542–5. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- 36.Germain A, Buysse DJ, Omao H, Kupfer DJ, Hall M. Psychophysiological reactivity and coping styles influence the effects of acute stress exposure on rapid eye movement sleep. Psychosom Med. 2003;65:857–64. doi: 10.1097/01.psy.0000079376.87711.b0. [DOI] [PubMed] [Google Scholar]
- 37.LeBlanc M, Beaulieu-Bonneau S, Merette C, Savard J, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–66. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors for insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Proposed 5th Edition. Accessed 10/08/2012. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=65.
- 40.Ellis JG, Gehrman P, Espie CA, Riemann D, Perlis ML. Acute insomnia: Current conceptualizations and future directions. Sleep Med Rev. 2012;16:5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 42.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–8. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 44.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 45.Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;4:429–34. doi: 10.1348/014466501163904. [DOI] [PubMed] [Google Scholar]
- 46.Morin CM. Insomnia: Psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 47.Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, Kupfer D, Thayer JF. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 48.Haynes SN, Fitzgerald SG, Shute G, O'Meary M. Responses of psychophysiologic and subjective insomniacs to auditory stimuli during sleep: a replication and extension. J Abnorm Psychol. 1985;94:338–45. doi: 10.1037//0021-843x.94.3.338. [DOI] [PubMed] [Google Scholar]
- 49.Haynes SN, Adams A, Franzen M. The effects of pre-sleep stress on sleep-onset insomnia. J Abnorm Psychol. 1981;90:601–6. doi: 10.1037//0021-843x.90.6.601. [DOI] [PubMed] [Google Scholar]
- 50.Gross RT, Borkovec TD. Effects of cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther. 1982;13:112–6. [Google Scholar]
- 51.Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–53. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 52.Perlis ML, Swinkels CM, Gehrman PR, Pigeon WR, Matteson-Rusby SE, Jungquist CR. The incidence and temporal patterning of insomnia: A pilot study. J Sleep Res. 2010;19:31–5. doi: 10.1111/j.1365-2869.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–71. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borkovec TD, Lane TW, VanOot PH. Phenomenology of sleep among insomniacs and good sleepers: wakefulness experience when cortically asleep. J Abnorm Psychol. 1981;90:607–9. doi: 10.1037//0021-843x.90.6.607. [DOI] [PubMed] [Google Scholar]
- 55.Mercer JD, Bootzin RR, Lack LC. Insomniacs' perception of wake instead of sleep. Sleep. 2002;25:564–72. [PubMed] [Google Scholar]
- 56.Yang C, Lo H. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep. 2007;30:585–92. doi: 10.1093/sleep/30.5.585. [DOI] [PubMed] [Google Scholar]
- 57.Feige B, Al-Shajlawi A, Nissen C, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 58.Perlis ML, Giles DE, Buysse DJ, Thase ME, Tu X, Kupfer D. Which depressive symptoms are related to which sleep electroencephalographic variables? Biol Psychiatry. 1997;42:904–13. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 59.Giles DE, Kupfer DJ, Roffwarg HP. EEG sleep before and after the first episode of depression. Sleep Res. 1990;19:161. [Google Scholar]
- 60.Benca RM, Okawa M, Uchiyama M, et al. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 61.Giles DE, Roffwarg HP, Schlesser MA, Rush AJ. Which endogenous depressive symptoms relate to REM latency reduction? Biol Psychiatry. 1986;21:473–82. doi: 10.1016/0006-3223(86)90189-7. [DOI] [PubMed] [Google Scholar]
- 62.Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio: a biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–5. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 63.Rush AJ, Erman MK, Giles DE, et al. Polysomnographic findings in recently drug-free and clinically remitted depressed patients. Arch Gen Psychiatry. 1986;43:878–84. doi: 10.1001/archpsyc.1986.01800090068009. [DOI] [PubMed] [Google Scholar]
- 64.Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: the influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. Eur Arch Psychiatry Clin Neurosci. 1994;243:279–90. doi: 10.1007/BF02191586. [DOI] [PubMed] [Google Scholar]
- 65.Akiskal HS, Lemmi H, Yerevanian B, King D, Belluomini J. The utility of the REM latency test in psychiatric diagnosis: a study of 81 depressed outpatients. Psychiatry Res. 1982;7:101–10. doi: 10.1016/0165-1781(82)90058-0. [DOI] [PubMed] [Google Scholar]
- 66.Rush AJ, Giles DE, Jarrett RB, et al. Reduced REM latency predicts response to tricyclic medication in depressed outpatients. Biol Psychiatry. 1989;26:61–72. doi: 10.1016/0006-3223(89)90008-5. [DOI] [PubMed] [Google Scholar]
- 67.Giles DE, Schlesser MA, Rush AJ, Orsulak PJ, Fulton CL, Roffwarg HP. Polysomnographic findings and dexamethasone nonsuppression in unipolar depression: A replication and extension. Biol Psychiatry. 1987;22:872–82. doi: 10.1016/0006-3223(87)90085-0. [DOI] [PubMed] [Google Scholar]
- 68.Rush AJ, Giles DE, Roffwarg HP, Parker CR. Sleep EEG and dexamethasone suppression test findings in outpatients with unipolar major depressive disorders. Biol Psychiatry. 1982;17:327–41. [PubMed] [Google Scholar]
- 69.Kupfer DJ, Ehlers CL. Two roads to rapid eye movement latency. Arch Gen Psychiatry. 1989;46:945–8. doi: 10.1001/archpsyc.1989.01810100087016. [DOI] [PubMed] [Google Scholar]
- 70.Buysse DJ, Kupfer DJ. Diagnostic and research applications of electroencephalographic sleep studies in depression conceptual and methodological issues. J Nerv Ment Dis. 1990;178:405–14. doi: 10.1097/00005053-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Berger M, Riemann D. REM sleep in depression—an overview. J Sleep Res. 1993;2:211–23. doi: 10.1111/j.1365-2869.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 72.Giles DE, Roffwarg HP, Rush AJ. REM latency concordance in depressed family members. Biol Psychiatry. 1987;22:910–4. doi: 10.1016/0006-3223(87)90090-4. [DOI] [PubMed] [Google Scholar]
- 73.Giles DE, Biggs MM, Rush AJ, Roffwarg HP. Risk factors in families of unipolar depression. I. Psychiatric illness and reduced REM latency. J Affect Disord. 1988;14:51–9. doi: 10.1016/0165-0327(88)90071-7. [DOI] [PubMed] [Google Scholar]
- 74.Giles DE, Kupfer DJ, Rush AJ, Roffwarg HP. Controlled comparison of electrophysiological sleep in families of probands with unipolar depression. Am J Psychiatry. 1998;155:192–9. doi: 10.1176/ajp.155.2.192. [DOI] [PubMed] [Google Scholar]
- 75.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]