Abstract
Objective:
To evaluate the efficacy of adenotonsillectomy (AT) in the treatment of children with obstructive sleep apnea (OSA) in a 3-y prospective, longitudinal study with analysis of risk factors of recurrence of OSA.
Study Design:
An investigation of children (6 to 12 y old) with OSA documented at entry and followed posttreatment at 6, 12, 24, and 36 mo with examination, questionnaires, and polysomnography.
Multivariate generalized linear modeling and hierarchical linear models analysis were used to determine contributors to suboptimal long-term resolution of OSA, and Generalized Linear Models were used for analysis of risk factors of recurrence.
Results:
Of the 135 children, 88 terminated the study at 36 months post-AT. These 88 children (boys = 72, mean age = 8.9 ± 2.7 yersus boys 8.9 ± 2.04 y, girls: 8.8 ± 2.07 y; body mass index [BMI] = 19.5 ± 4.6 kg/m2) had a preoperative mean apnea-hypopnea index (AHI0) of 13.54 ± 7.23 and a mean postoperative AHI at 6 mo (AHI6) of 3.47 ± 8.41 events/h (with AHI6 > 1 = 53.4% of 88 children). A progressive increase in AHI was noted with a mean AHI36 = 6.48 ± 5.57 events/h and AHI36 > 1 = 68% of the studied group. Change in AHI was associated with changes in the OSA-18 questionnaire.
The residual pediatric OSA after AT was significantly associated with BMI, AHI, enuresis, and allergic rhinitis before surgery. From 6 to 36 mo after AT, recurrence of pediatric OSA was significantly associated with enuresis, age (for the 24- to 36-mo period), postsurgery AHI6 (severity), and the rate of change in BMI and body weight.
Conclusions:
Adenotonsillectomy leads to significant improvement in apnea-hypopnea index, though generally with incomplete resolution, but a worsening over time was observed in 68% of our cases.
Citation:
Huang YS; Guilleminault C; Lee LA; Lin CH; Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. SLEEP 2014;37(1):71-76.
Keywords: adenotonsillectomy, comorbidity, obstructive sleep apnea, polysomnography, treatment outcomes
INTRODUCTION
Obstructive sleep apnea (OSA) syndrome is a highly prevalent condition in children and characterized by snoring, witnessed apnea, unrefreshing sleep, and excessive daytime sleepiness.1,2 Children with OSA experience recurrent periods of elevated upper airway resistance during sleep due to partial or complete upper airway obstruction, which results in snoring, episodic oxyhemoglobin desaturation, hypercapnia, and repeated arousals.3,4 The respiratory disturbance of recurrent hypoxia-reoxygenation episodes during the night is associated with an increased risk of suboptimal growth, poor sleep quality, neurocognitive dysfunction, behavioral problems, overweight status, and cardiovascular disease in childhood.5–8 The prevalence of OSA is approximately 2-3% in children,9,10 and current studies have evaluated the influence of OSA on various associated morbidities5–8,11 and tried to identify the factors predicting poor treatment outcome.12
The choice of therapy for OSA is predicated on the etiology, severity, and individual history of the increased upper airway resistance. The timely diagnosis and appropriate treatment of OSA is especially important, because untreated OSA can account for markedly increased health care costs.13–15 Adenotonsillar hypertrophy is considered an important factor in the development of OSA in otherwise healthy children. Adenotonsillectomy (AT) is recommended as the first line of treatment for childhood OSA by the American Academy of Pediatrics,1 and the effectiveness of AT in the treatment of children with OSA has been confirmed by several studies.15–17 However, some studies examining the efficacy and outcomes of AT in pediatric OSA showed incomplete resolution of OSA after surgery.12–17 For example, nonobese children with severe OSA and chronic asthma were found to be at higher risk of residual OSA in a recent multicenter retrospective study.12 In addition, few studies have evaluated the overall long-term efficacy of AT in the treatment of children with OSA. Thus, this 3-y prospective, longitudinal study aimed to delineate factors contributing to potential post-AT OSA through careful examination of demographic and pre- and post-AT polysomnography (PSG) information. In our geographical location, the mean age for AT at the time of the study was approximately 8 y, and such age for surgery was based on two factors: usual recommendations made by ear, nose, and throat (ENT) surgeons when AT was considered, and reluctance of parents in our culture to have their children undergo nonemergency surgery.
METHODS
Starting in August 2007, children between the ages of 6 and 12 y with signs and symptoms of a sleep disturbance, including snoring, mouth breathing, and witnessed breath holding, lasting for at least 3 mo, were evaluated prospectively using a standardized history and physical examination, neurocognitive and psychological testing, and PSG in the sleep center of Chang Gung Memorial Hospital (CGMH). The CGMH sleep clinic is a multidisciplinary clinic with expertise in the management of pediatric sleep apnea and is the only accredited pediatric sleep laboratory in Taiwan.
The inclusion criteria for subjects, based on PSG, were an obstructive apnea-hypopnea index (AHI, the number of apnea and hypopnea events per hour of sleep) greater than one event/h or a respiratory disturbance index (RDI) of more than five events/h with clinical symptoms. Both patients and parents agreed to participate in this longitudinal study and were willing to sign informed consent.
Exclusion criteria for the children were previous AT, cranio-facial abnormalities, neuromuscular disease, or other significant medical, psychiatric, or genetic disorders, and obesity (obesity was defined based on Taiwan general public health tables, taking into consideration age and body mass index (BMI) in kg/m2).18
This prospective study was approved by the institutional review board of CGMH, and the caregivers (parents) signed informed consent before their children were enrolled for study.
Procedure
All subjects underwent a routine medical history and physical examination by an otolaryngologist, a craniofacial surgeon, a pediatrician, and a child psychiatrist for assessment of associated comorbidities.
A standardized datasheet for patient demographic data, including age, sex, height, weight and all systemic comorbidi-ties, was checked by child psychiatrists.
The ENT examination was performed by an otolaryngolo-gist and a craniofacial surgeon. Tonsillar size was graded as follows: (1) small tonsils confined to the tonsillar pillars; (2) tonsils that extended just outside the pillars; (3) tonsils that extended outside the pillars but did not meet at the midline; (4) large tonsils that met at the midline.19 Adenoid tissue was examined with a lateral x-ray film of the neck or a flexible fiberoptic endoscope. The amount of obstruction was categorized into four grades (grade 0 = 0-25%, grade 1 = 25-50%, grade 2 = 50-75%, and grade 3 = 75-100%). Allergic rhinitis was confirmed by a specific immunoglobulin E (IgE) blood test (ImmunoCAP® 100; Phadia, Uppsala, Sweden), and duration and persistence of symptoms and comorbidities according to the Allergic Rhinitis and its Impact on Asthma (ARIA) classification.
Questionnaire evaluation: Parents completed the obstructive sleep disorder questionnaire (OSA-18).20,21 It is a subjective questionnaire that contains 18 items and covers quality of life. The items are divided into six domains: sleep disturbance, physical suffering, emotional distress, daytime problems, care-giver concerns, and total quality of life.20,21
During the evaluation period, children underwent other mental and cognitive tests not presented here.
Once the clinical evaluation was complete, a standard overnight PSG with simultaneous video recording was used for each subject in the hospital sleep laboratory preoperatively and 6 mo (AHI6), 12 mo (AHI12), 24 mo (AHI24), and 36 mo (AHI36) after AT. All children were instructed to discontinue any medication (or PSG was scheduled at least 7 days after discontinuation of medications prescribed for acute health problems prior to PSG). A family member was required to be present for all nocturnal PSG recordings. Sleep and wake were scored using the international criteria of Rechtschaffen and Kales22 with identification of stages 3 and 4, and identification of the onset of abnormal behavior to a specific sleep stage. EEG arousal was defined according to the guidelines of the American Sleep Disorders Association.23 Abnormal breathing events during sleep were analyzed according to the definitions of an apnea and hypopnea as outlined by the American Academy of Sleep Medicine (AASM),23 and the definition of flow limitation with abnormal increase in respiratory effort leading to arousals as outlined by Lin and Guilleminault.24 Based on these definitions, the AHI and a respiratory disturbance index (RDI, the number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep) were calculated. Periodic limb movement (PLM) was defined according to the AASM scoring rules,23 with a PLM index (PLMI) > 5 per hour being considered abnormal. PLMs associated with breathing events were not scored, and only those independent of apnea/hypopnea were considered. Abnormal behavior on video was noted when present. PSG scoring was performed by a technician blind to the clinical status of the child.
All children were given antileucotriene medication for 6 mo post-AT.
We followed the children posttreatment at 6, 12, 24, and 36 mo with the same examination, questionnaires, and PSG.
Statistical Analyses
Descriptive statistics were performed. Considering the missing data in longitudinal studies and the evolution of AT over time, multivariate generalized linear model (GLM) and hierarchical linear models (HLM) analyses were used to determine contributors to residual sleep apnea at various time points post-AT. GLM statistics were used to analyze the risk factors for OSA recurrence after AT looking at the entire follow-up period. We used HLM analyses to investigate the changes during the different follow-up points (AT6,12,24,36): because there were different numbers of subjects at each follow-up-point, a two-level model was used. We analyzed fixed effects in level one, and random effects in level two. The coefficient of level one (fixed effects) was the intraclass correlation (ICC) which was, in our model, the proportion of group level variance from the total variance.
RESULTS
During the study period, 135 pediatric OSA subjects who underwent AT were enrolled. Eighty-eight children (64.6%) completed all preoperative and postoperative evaluations and were included in the analysis. The dropout rate was 17.8% in the first year, 28.9% in the second year, and 35.4% in third year, indicating the reluctance of parents to participate in long-term follow-up. The mean age at the time of screening was 8.9 ± 2.7 y. Boys were predominant in this sample (n = 72, 81.8% mean age 8.9 ± 2.04). The mean AHI before AT (AHI0), was 13.53 ± 7.23 events/h and the mean BMI was 19.54 ± 4.64 kg/m2. The BMI-z score revealed that our subjects were not obese. We compared the characteristics of subjects that completed the study and those that did not, and found there was no significant difference between these two groups (Tables 1 and 2).
Table 1.
Clinical characteristics of children completing the longitudinal study versus those who dropped out during the 3 y of follow-up

Table 2.
Comorbidities of children completing the longitudinal study versus those who dropped out during the 3 y of follow-up

A comparison of PSG data before and after AT showed that the baseline AHI0 (13.53 ± 7.23 events/h; median = 6.85, range 1.2 to 49.4 events/h) had improved significantly at all post-AT yearly time points (AHI12 P < 0.001, AHI24 P < 0.001, AHI36 P = 0.004, respectively). Rapid eye movement (REM) sleep also showed a significant increase at all post-AT yearly time points (P = 0.04, 0.016, 0.003, respectively), as did the percentage of slow wave sleep (P = 0.03, 0.02, 0.006, respectively). Median AHI also was significantly improved at all post-AT yearly time points (Tables 3 and 4).
Table 3.
Weight and polysomnographic data before and after adenotonsillectomy (n = 88)

Table 4.
Sleep variables before and after adenotonsillectomy (n = 88)

Overall, these data indicate the positive results of surgery but do not represent the real postsurgical evolution of the AHI over time. Using a multivariate Generalized Linear Model (GLM) analysis we found a significant improvement in the AHI (mean AHI0 = 13.54 to mean AHI6 = 3.47 events/h) during “period 1” (pre-AT-AHI0-to 6 mo post-AT-AHI6-). The success rate of AT in this study at 6- mo postsurgery is therefore 46.6%. The remaining 53.4% of the children had an AHI6 greater than one event/h following surgery.
During period 2 (6 to 36 mo post-AT) there was an AHI elevation beginning at the 6-mo time point: from mean AHI6 = 3.47 to mean AHI36 = 6.48 events/h (range: AHI6 = 0 to 35.5 events/h to AHI36 = 0 to 42.3 events/h). Though all values were significantly less than the baseline AHI (AHI0), the mean AHI showed a significant increase from 6 to 36 mo posturgery (Tables 3 and 4 and Figure 1). Using GLM analysis, we found persistence of a significant difference between presurgery and postsurgery PSG data, but this effect varied between different time sequences: With HLM analysis and the two-level model calculations, the coefficient in level one (fixed effects) showed again that the value of the AHI was significantly reduced during the period (AHI0 to AHI6), and that the significant positive gains covered not only AHI but also apnea index (AI), mean oxygen saturation, sleep latency, wake after sleep onset (WASO), and percentage of REM sleep. But in period 2, the proportion of the AHI was significantly increased: from 6 mo posttreatment to later time points (up to AHI36). This AHI rebound starting 6 mo postsurgery was associated with a worsening of nocturnal sleep (WASO, sleep latency, and sleep efficiency).
Figure 1.
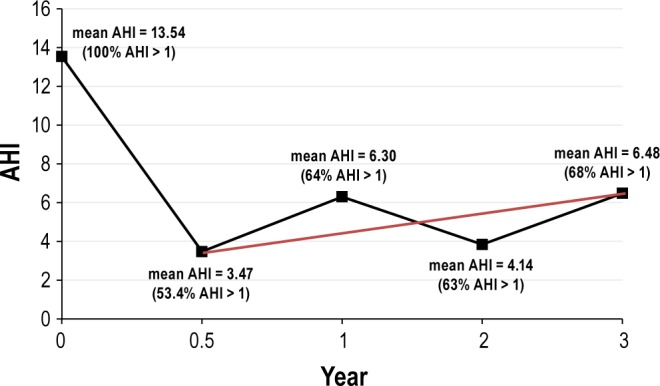
Change of apnea-hypopnea index (AHI) after adenotonsillectomy using Multivariate generalized linear modeling (GLM) and the hierarchical linear model (HLM); the straight thin line indicates the significant linear increase. Period 1 (from before AT -AHI0- to 6 months post AT surgery-AHI6): results showed a significant improvement in AHI (from a mean AHI0 = 13.54 to a mean AHI6 = 3.47 events/h). Period 2 (from 6 mo postsurgery-AHI6 to 36 mo postsurgery -AHI36): the mean AHI significantly increased between 6 mo postsurgery to 36 mo postsurgery (mean AHI6 = 3.47 to mean AHI36 = 6.48 events/h). This increase was associated with recurrence in 68% of subjects followed for 36 mo postsurgery.
Subgroup Analyses
We divided the post-AT patients into two groups. Group 1 (the successful group, n = 41) had an AHI6 < 1 at AT+6 mo. The analysis of this subgroup, considered as initially fully treated, revealed that the recurrence rate was 35.3% after AT+12 mo, 50% after AT+24 mo, and 66.7% after AT+36 mo. Group 2 (the nonsuccessful group, n = 47) had an AHI6 > 1 at AT+6 mo and also showed a progressive increase in the residual AHI at +36 mo. Overall, when considering worsening and recurrence, 68% of the children followed had an abnormal AHI and abnormal sleep at AT+36 mo. The mean AHI36 was 6.48 ± 5.57events/h.
Determination of risk factors for OSA recurrence after AT was based on GLM of repeated measure analysis: AHI6 was significantly associated with BMI, body weight, AHI, and the presence of enuresis and allergic rhinitis before surgery: the risk factor of residual pediatric OSA 6 mo after AT was significantly related to BMI and body weight, severity of pediatric OSA, enuresis and rhinitis before surgery. The analyses performed in period 2 (from post-AT+6 mo to +36 mo) showed that the recurrence of pediatric OSA was significantly associated with age, persistence of enuresis, the AHI post-AT+6 mo, and a fast and abnormal increase of BMI and body weight (from post-AT+6 mo to +12 mo, from +12 mo to +24 mo, and from +24 mo to +36 mo) (Table 5).
Table 5.
The risk factors for OSA recurrence after AT

Six months after AT, the OSA-18 analyses showed signifi-cant improvement in the items of sleep disturbance (mean: 4.06 ± 1.52 to 2.40 ± 1.22), physical suffering (mean: 3.82 ± 2.02 to 2.79 ± 1.92), daytime problems (mean: 4.12 ± 1.98 to 3.33 ± 2.03), caregiver concerns (mean: 4.44 ± 1.78 to 3.01 ± 1.91) and total quality of life (mean: 5.12 ± 2.08 to 6.01 ± 2.95) (higher scores indicated greater severity, except the item of total quality of life, which was the reverse). However, the items of sleep disturbance, daytime problems, and caregiver concerns worsened again at +36 mo post-AT. The results of the sleep questionnaire were similar to the results of the PSG.
DISCUSSION
The limitations of this study include: (1) a somewhat small sample size, ending with 88 children and a male predominance; the dropout rate was 36% with only 88 subjects completing the 36-mo follow-up; (2) age (6 to 12 y) was taken into consideration when AT would usually be performed in Taiwan; (3) because of our institutional review board, this was not a randomized controlled trial; (4) the lack of obese children (a deliberate choice); and (5) the fact that craniofacial structure imaging data (i.e., three-dimensional computed tomography) was not performed on every child. Although a 1-y follow-up study of sleep disordered breathing with AT25 has discussed the recurrence issue, our study, to the best of our knowledge, is the first prospective 3-y longitudinal study of pediatric OSA after AT. Also, the long-term follow-up of these patients was performed in the same hospital sleep center using the same recording techniques and PSG scoring criteria. Clinical evaluation was a systematic evaluation that was based on criteria established prior to the beginning of the study, and repeated psychiatric and neurocognitive testing was administered by the same team.
AT is the treatment of choice for pediatric OSA and our results showed significant improvements post-AT. However, incomplete resolution of pediatric OSA was noted in many of our children. Our results show that post-AT OSA does not spontaneously remit, and although the effect size was relatively small (66.7% of subjects), AHI did worsen over time, even if surgery was successful at 6 months posttreatment. A recent study has reported some mechanisms by which this worsening may occur.12 Asthma was not a predictive factor for post-AT OSA in our study group, but our rate of asthma was low at the outset. This difference may be related to differing rates of asthma and triggers, differences in OSA phenotype or subtype compared to other study populations, or overall adequacy of treatment and lung function during the study period. In this study, the predictive factors of residual pediatric OSA after AT-HI6 were BMI and body weight, severity of pediatric OSA, and enuresis and rhinitis before surgery. The recurrence and persistence of pediatric OSA was associated with enuresis, age (post-AT+24 mo to post-AT+36 mo), AT-AHI6 (the severity of residual pediatric OSA after AT) and the change (with a fast increase) in BMI and body weight (from post-AT-AHI6 to AHI12, from post-AT-AHI12 to AHI24, and from post-AT-AHI24 to AHI36). Increases in BMI and body weight are common post-AT.17 Also, there is reluctance in our own pediatric field to perform AT with young children, and surgical treatment acceptance varies depending on culture and the pediatrician's education. At times, surgery may be delayed, and this is related to the issues that delay the treatment of OSA. Therefore, education will be an important issue to physicians in the future. Moreover, the GLM analysis in our study showed that age was a risk factor for recurrence of OSA after AT. Age not only has an effect on oral-facial growth, with 60% of the adult face already formed by 4 y of age,26 but it may favor incomplete surgical results and secondary worsening posttreatment, as age at the time of surgery was a significant variable predicting incomplete resolution of pediatric OSA. Our study supports the need to perform AT at an earlier age than it is often done in our culture and some other cultures.27 Independent of age, AT overall improved OSA, but even if it appeared successful initially, as demonstrated by recordings at 6 mo postsurgery, recurrence of abnormal breathing within 1 to 3 y is important. Overall, 68% of children treated with adenotonsillectomy presented a mean AHI of 6.48 events/h. This polysomnographic finding is associated with a subjective and objective demonstration of poor sleep, and a demonstration of the worsening of symptoms associated with attention and daytime hyperactivity.
Our study outlines some risk factors, such as severe pediatric OSA, obesity, and a large increase in BMI after AT, rhinitis, enuresis, and older age for recurrence of OSA, but we do not claim to have identified all of the risk factors. Finally, an obvious conclusion of our work is that children in whom OSA is diagnosed require long-term follow-up.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by Chang Gung Memorial Hospital: CMRPG 470011. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank professor Fan-Ming Hwang and Po-Yu Huang, PhD, for help with statistical analysis, and Shannon Sullivan, MD, for her editing of the manuscript.
REFERENCES
- 1.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 2.Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–8. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein NA, Pugazhendhi V, Rao SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics. 2004;114:33–43. doi: 10.1542/peds.114.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D, Wang M, Pope DM., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108:693–7. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 5.Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109:e55. doi: 10.1542/peds.109.4.e55. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126:e1161–7. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 7.Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D. Nocturnal enuresis and overweight are associated with obstructive sleep apnea. Pediatrics. 2009;124:e53–9. doi: 10.1542/peds.2008-2805. [DOI] [PubMed] [Google Scholar]
- 8.Suratt PM, Barth JT, Diamond R, et al. Reduced time in bed and obstructive sleep-disordered breathing in children are associated with cognitive impairment. Pediatrics. 2007;119:320–9. doi: 10.1542/peds.2006-1969. [DOI] [PubMed] [Google Scholar]
- 9.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Constantin E, Tewfik TL, Brouillette RT. Can the OSA-18 quality-of-life questionnaire detect obstructive sleep apnea in children? Pediatrics. 2010;125:e162–8. doi: 10.1542/peds.2009-0731. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–83. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 13.Reuveni H, Simon T, Tal A, Elhayany A, Tarasiuk A. Health care services utilization in children with obstructive sleep apnea syndrome. Pediatrics. 2002;110:68–72. doi: 10.1542/peds.110.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2007;175:55–61. doi: 10.1164/rccm.200604-577OC. [DOI] [PubMed] [Google Scholar]
- 15.Tarasiuk A, Simon T, Tal A, Reuveni H. Adenotonsillectomy in children with obstructive sleep apnea syndrome reduces health care utilization. Pediatrics. 2004;113:351–6. doi: 10.1542/peds.113.2.351. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117:1844–54. doi: 10.1097/MLG.0b013e318123ee56. [DOI] [PubMed] [Google Scholar]
- 17.Redline S, Amin R, Beebe D, et al. The childhood adenotonsillectomy trial (CHAT): rationale, design, and challenges of a randomized controlled trail evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–17. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. http://www.bhp.doh.gov.tw/BHPnet/Portal/Them_Show.aspx?Subject=200712250006&Class=0&No=200905050001.
- 19.Brodsky L, Moore L, Stanievich J. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13:149–56. doi: 10.1016/0165-5876(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 20.Franco RA, Rosenfeld RM, Rao M. Quality of life for children with obstructive sleep disorders. Otolaryngol Head Neck Surg. 2000;123:9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 21.Wang CH, Huang YS. Clinical utility of the Obstructive Sleep Apnea Questionaire-18 in children with obstructive sleep apnea syndrome. 2009 World Congress on Sleep Apnea (WCSA) Proceeding Korean Sleep Society; 2009; Seoul (South Korea). abstract-194. [Google Scholar]
- 22.Rechtschaffen A, Kales A. A manual of standardized terminology: techniques and scoring systems for sleep stages of human subjects. Los Angeles, CA: University of California, Los Angeles, Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 23.Amercian Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 24.Lin CH, Guilleminault C. Current hypopnea scoring criteria underscore pediatric sleep disordered breathing. Sleep Med. 2011;12:720–9. doi: 10.1016/j.sleep.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177:654–9. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enlow DH, Poston WR. Facial Growth. 3rd ed. Philadelphia, PA: Saunders; 1990. [Google Scholar]
- 27.Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion before and after adenotonsillectomy in children with obstructive sleep apnea. Somnology. 2012;16:125–32. [Google Scholar]


