Abstract
Background and Study Objective:
Telomere length provides an estimate of cellular aging and is influenced by oxidative stress and health behaviors such as diet and exercise. This article describes relationships between telomere length and sleep parameters that included total sleep time (TST), wake after sleep onset (WASO), and self-reported sleep quality in a sample of adults with chronic illness.
Design and Participants:
Cross-sectional study of 283 adults (74% male, 42% Caucasian) infected with human immunodeficiency virus (HIV) while living in the San Francisco Bay area, CA, USA. Ages ranged from 22-77 y.
Measurements and Results:
TST and WASO were estimated with wrist actigraphy across 72 h; self-reported sleep quality was assessed with the Pittsburgh Sleep Quality Index. Relative telomere length (RTL) in leukocytes was estimated by quantitative polymerase chain reaction assays. Shorter RTL was associated with older age, and RTL was shorter in males than females. RTL was unrelated to HIV disease characteristics. RTL was not associated with WASO or self-reported sleep quality. Participants with at least 7 h sleep had longer RTL than those with less than 7 h, even after controlling for the effects of age, sex, race, education, body mass index, metabolic hormones (i.e., leptin, ghrelin, adiponectin, and resistin), depression and anxiety, and sleep quality.
Conclusion:
Results suggest that sleep duration is associated with preserving telomere length in a population of human immunodeficiency virus-infected adults. Getting at least 7 hours of sleep at night may either protect telomeres from damage or restore them on a nightly basis.
Citation:
Lee KA; Gay C; Humphreys J; Portillo CJ; Pullinger CR; Aouizerat BE. Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. SLEEP 2014;37(1):157-166.
Keywords: Actigraphy, body mass index, HIV, metabolic hormones, telomere
INTRODUCTION
Telomeres are repetitive DNA sequences that cap the ends of chromosomes and protect their integrity.1 Leukocyte telo-mere length is a useful biological marker of the aging process as telomere repeats are lost with cell division. Telomere length is a product of one's genetic constitution and environmental exposures to stress. Even stress experienced in utero has been associated with shortened telomeres in young adults.2 Telomeres shorten with each year of life as a function of oxidative stress, but shortening can be exacerbated by obesity, smoking, and poor health.3 Measures to moderate the effects of oxidative stress on telomere length include healthy diet and regular physical exercise.2
Sleep has been evaluated as a correlate of telomere length, but findings have been contradictory. Telomere length was not associated with sleep duration in a sample of healthy women after controlling for body mass index (BMI), activity, stress, and smoking.3 However, sleep duration was estimated with only one self-report item for average hours of sleep during the prior 6 w, and the sample consisted of healthy women under the stressful condition of having a sister in treatment for breast cancer. Another study based on self-reported sleep duration found that sleeping an average of more than 7 h per night was associated with longer telomeres among older men, but not among older women.4 In a study of healthy midlife women, the Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality, and shorter telomere length was associated with self-reported poorer sleep quality.5 Time in bed, sleep onset latency, and sleep duration were not associated with telomere length in that large sample of healthy women; BMI was the only significant predictor of telomere length after controlling for age, race, and income.5 However, these two studies did not include other indicators of dietary behavior, such as waist and hip circumferences, intake of caffeine or alcohol, smoking, or plasma values of metabolic hormones involved in dietary intake (e.g., leptin, ghrelin, adiponectin, and resistin), even though such factors are related to both sleep and BMI.6–8 There were also no measures of depression or anxiety, which are often associated with poor sleep quality and BMI.
Human immunodeficiency virus (HIV) infection is a type of chronic illness that initially activates the immune system and cell turnover processes before the immunosuppression phase seen in acquired immunodeficiency syndrome (AIDS). Studies of telomere length in the HIV-infected population have been ongoing since the 1990s and have focused on telomere length and rate of disease progression9–11 or effects of antiretroviral therapy.12 In a small sample of young HIV-infected adults (31-41 y of age), telomere length was associated with CD4 cell count, and the 16 patients with CD4 cell counts less than 200 cells/mm3 had significantly shorter telomeres compared with healthy age-matched controls.13 However, the relationship between telomere length and sleep parameters in HIV-infected adults has not been examined. The potential influence from dietary factors known to be associated with both sleep parameters and telomere length was not addressed.
The purpose of this study was to describe the relationship between telomere length and sleep parameters using both subjective and objective sleep measures in a sample of HIV-infected men and women. Based on findings from earlier studies of healthy women, we hypothesized that both sleep duration and sleep quality would account for a significant amount of the variance in telomere length even after controlling for age, sex, race, income, education, clinical HIV status, symptoms of anxiety or depression, anthropometric measures, and metabolic hormones.
METHODS
Participants and Procedures
The Committee on Human Research at the University of California, San Francisco (UCSF) approved the study protocol, and 350 adults living with HIV in the San Francisco Bay area were recruited and enrolled using posted flyers at HIV-related clinical and community sites. Written informed consent was obtained prior to study participation. Eligibility criteria included English-speaking adults at least 18 y of age in whom HIV had been diagnosed for a minimum of 3 mo and who were not working nightshift schedules or pregnant within the prior 3 mo. Study visits were conducted at the UCSF outpatient Clinical Research Center (CRC). Participants provided a urine sample for toxicology screening using RediCup® (Redwood Toxicology Laboratory, Inc, Santa Rosa, CA, USA).
After excluding 32 participants who tested positive on urine sample screening for illicit drugs or who could not provide a specimen, 29 participants had missing data at random from actigraphy, and six participants had missing data for telomere length. The analysis for this study includes 283 adults with complete data for actigraphy and telomere length. A description of the complete sample's demographic, symptom, and clinical data is reported elsewhere.14
Measures
In addition to completing demographic and sleep questionnaires, participants wore a wrist actigraph monitor for 72 h continuously during weekdays, and anthropometric measures were obtained for calculation of BMI and waist/hip ratio. Blood pressure was also recorded and a fasting blood sample was obtained to measure plasma levels of leptin, ghrelin, adiponectin, and resistin. Daily diaries were used to record caffeine, alcohol, and tobacco intake, and use of sleep aids, as well as bedtimes, wake times, and daytime naps. Number of comorbid health conditions was assessed by self-report. A copy of the participants' most recent laboratory results for CD4+ T-cell count and viral load was obtained from their medical record, and antiretroviral therapy (ART) and other medication usage was determined by review of the participants' self-report of current medications.
Relative Telomere Length
Mean relative telomere length (RTL) is measured as the ratio of telomeric product/single copy gene (T/S) obtained using a quantitative polymerase chain reaction (qPCR). The qPCR assay amplifies the telomere repeats; therefore, the longer the telomeres are in each sample, the more qPCR products will be generated in qPCR reactions using primers specific for the telomeric DNA. The single copy gene serves to normalize the number of genome equivalents (i.e., cells) represented in a given biospecimen genomic DNA sample.
Briefly, two separate qPCR experiments were performed for each sample (i.e., T and S). Using methods previously detailed,15–17 telomeric sequence was amplified using primers tel1b and tel2b and the human beta-globin gene (HBG) served as the single copy gene, which was amplified using primers hbg1 and hbg2 (Invitrogen Inc., Life Technologies, Carlsbad, California). A standard curve using a reference DNA was constructed for each qPCR experiment to enable estimates of participants' telomere lengths and single copy gene quantities.
All samples were measured in quintuplicate and the mean of at least three valid replicates was used in subsequent analyses. Outlier estimates (defined as ≥ 0.3 standard deviation units) were excluded. If three replicates could not be retained, the qPCR sample was repeated. For each reaction, 4 ng of input DNA template was used. For each qPCR experiment, one intra-plate and one interplate control sample was included to assess for intraplate and interplate variability. The coefficient of variation across all qPCR experiments was 2.4% (range 1.1-4.9%) for the telomere qPCR target and 1.2% (range 0.6-2.7%) for the single copy gene (HBG) qPCR target.
Metabolic Hormones
For selected plasma biomarker measures of energy metabolism (leptin, ghrelin, adiponectin, and resistin), blood samples were centrifuged and plasma was stored at -80°C until samples were assayed using the Luminex xMAP multiplex platform by Millipore, Inc (BioMarker Services, Millipore, St. Charles, MO). Samples were assayed in duplicate, and the mean of the two assays were used for subsequent analyses. The within and between assay coefficients of variation were acceptable for leptin (< 10% and < 20%), ghrelin (< 10% and < 20%), adiponectin (< 10% and < 15%), and resistin (< 10% and < 15%).
Objective Sleep
Objective sleep duration (total sleep time, TST) and quality (wake after sleep onset, WASO) were estimated with a noninvasive battery-operated wrist actigraph microprocessor that detects acceleration and motion with a piezoelectric beam (Mini Motionlogger Actigraph, AAM-32 Ambulatory Monitoring, Inc., Ardsley, NY, USA). Actigraphy provides continuous movement counts and data were sampled in 30-sec epochs using zero-crossing mode. The actigraphy monitor was worn continuously on the nondominant wrist for 72 h on 3 consecutive weekdays between Monday and Friday to control for potential weekend variability and reduce participant burden in this patient population. Wrist actigraphy has been validated with polysomnography measures of sleep and wake time for healthy and disturbed sleepers.18–20
Bedtime and final wake times were determined by one or two approaches: participants pressing the event marker on the actigraph for “lights out” and “lights on”; or diary entry of clock times that matched with a 50% change in movement during the same 10-min block of time on actigraphy. If these times were not available, the sleep onset time was deemed missing for that night and the mean value for 2 nights was used. The Cole-Kripke algorithm was used to calculate TST in min and percent time spent awake after falling asleep (WASO) using an automatic sleep scoring program (Action4® Software Program, Ambulatory Monitoring Inc.) to reduce researcher scoring bias. The mean for 3 nights of complete data was used for TST and WASO. To estimate daytime sleep, the time after final awakening to the next bedtime was examined and sleep diary notations of nap times were noted. Daytime min of sleep were calculated after excluding any time when the monitor was off the participant's wrist. The mean for 3 complete days was used for analysis of min of daytime sleep.
Subjective Sleep
The PSQI was used to estimate general perception of sleep quality and self-reported sleep duration.21 The PSQI asks participants how often they experienced seven categories of sleep related problems, each on a scale of 0 (not during the past month) to 3 (three or more times a week) and has adequate validity with laboratory polysomnography. Scores can range from 0 to 21, and scores greater than 5 indicate substantial sleep disturbance.21 Specific questions address such things as usual bedtime and waking up time, hours of actual sleep, and an overall rating of sleep quality from 0 (very good) to 3 (very bad). Based on the responses, an estimate of habitual sleep efficiency is also calculated. The seven component scores representing overall sleep quality were internally consistent with a Cronbach alpha coefficient of 0.67 in this sample.
Anxiety and Depressive Symptoms
The Profile of Mood States (POMS) nine-item subscale for tension-anxiety was used to assess severity of anxiety in the past week. Scores can range from 0 to 36 with higher scores indicating a more severe level of anxiety. The POMS has well-established concurrent and construct validity.22 In this study, the subscale was internally consistent with a Cronbach alpha coefficient of 0.86.
The Center for Epidemiological Studies-Depression Scale (CES-D) was used to assess frequency of depressive symptoms during the past week. The 20-item CES-D score can range from 0 to 60, with higher scores indicating more frequent symptoms of depression. The CES-D has well-established concurrent and construct validity.23 In this study, internal consistency reliability (Cronbach alpha coefficient) was 0.88.
Statistical Analysis
Descriptive statistics were used to summarize demographic and clinical characteristics. The 72 h of continuous actigraphy data were analyzed for each individual using Action4® software (Ambulatory Monitoring, Inc.). This software allows for automatic sleep scoring using Cole-Kripke algorithm for adults. These data were entered and analyzed using IBM SPSS Statistics Version 20 software (SPSS, Inc, Chicago IL, USA) for group analyses. Actigraphy data for TST and WASO were averaged over the 3 nights of monitoring. The average total sleep time in 24 h was also computed from the 72 h of actigraphy data. Fasting metabolic hormone values were transformed (square root) to meet statistical assumptions. Demographic and clinical group differences in RTL length were evaluated using independent sample t-tests or analysis of variance. Associations between RTL length and continuous variables were assessed using Pearson correlations (r), and Spearman correlations (rho) were used for ordinal variables (education and PSQI sleep quality item). Significance for all statistical tests was set at P < 0.05 for all analyses.
Hierarchical linear regression analysis was used to determine the unique contribution of sleep duration to RTL after controlling for salient demographic and clinical characteristics. Potentially confounding demographic and clinical variables related to sleep or RTL in prior research were included in the model: age, sex, race, education, anthropometrics, metabolic hormones, and depression and anxiety symptoms as well as medications to treat these symptoms. Two-way interactions were also evaluated and those with P ≤ 0.10 were included in the final model. Sleep parameters significantly associated with RTL in bivariate analyses were also included in the model. Given the association between sleep quality and RTL in prior research, objective actigraphy and self-report measures of sleep quality were also evaluated in the final model.
RESULTS
Sample Characteristics
Two participant outliers (one male and one female) had RTL values more than 5 standard deviation units above the mean RTL value for their sex; the RTL distribution was normalized by truncating them to the next highest value for their sex. RTL values by demographic and clinical characteristics for the 283 participants included in this analysis are presented in Tables 1 and 2. The sample was ethnically diverse and predominantly male, reflecting the local population of adults living with HIV infection. Most participants had been living with HIV for many years, 70% were currently receiving ART, 51% had received an AIDS diagnosis at some point in the past, and most were unemployed (86%) or receiving medical disability assistance (75%). Their overall symptom experience has been reported elsewhere.14 Anthropometric measures and metabolic hormones are known to differ by sex and are thus reported separately for males and females in Table 3.
Table 1.
Relative telomere length by sample demographic characteristics (n = 283)
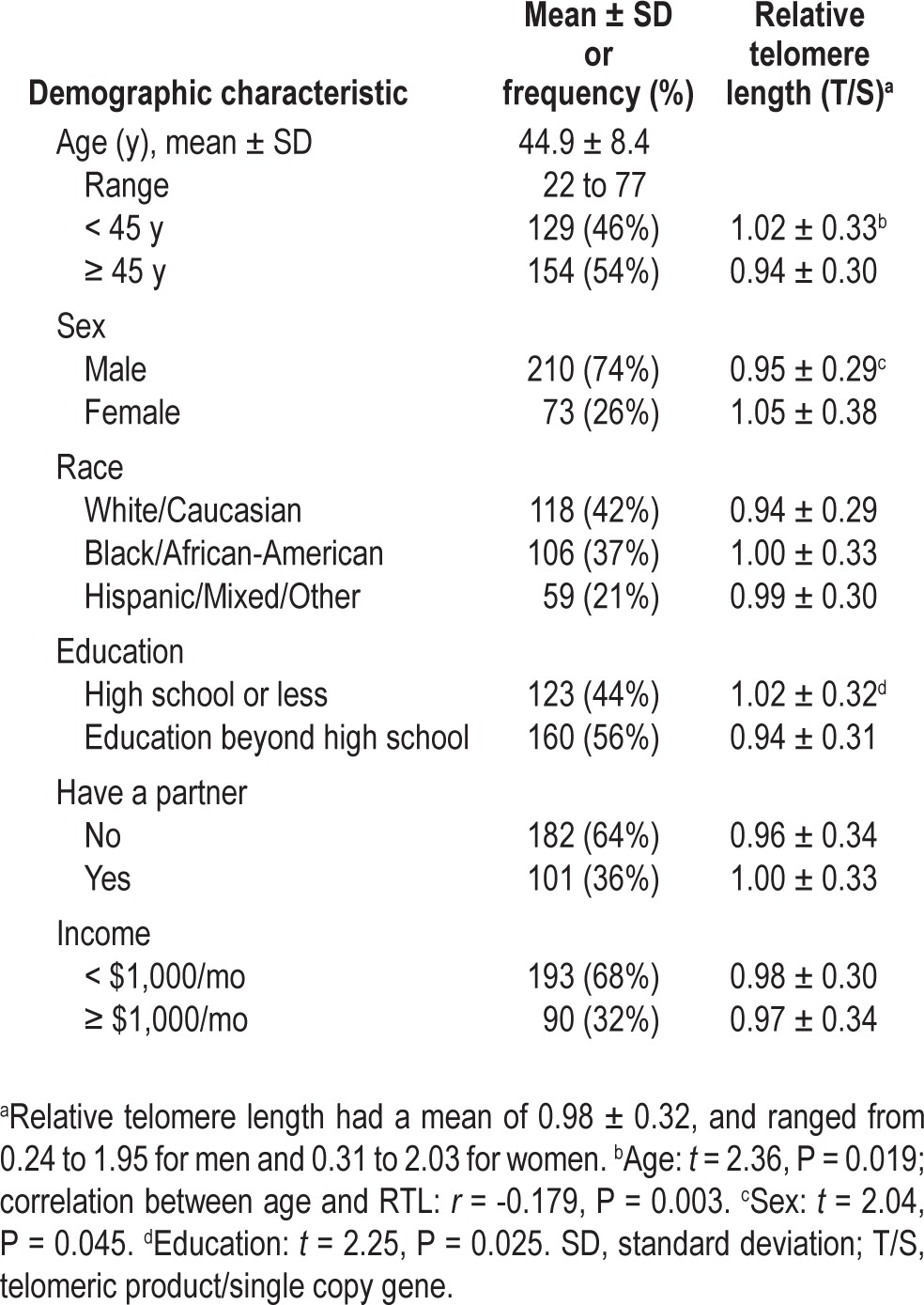
Table 2.
Relative telomere length by sample clinical characteristics (n = 283)
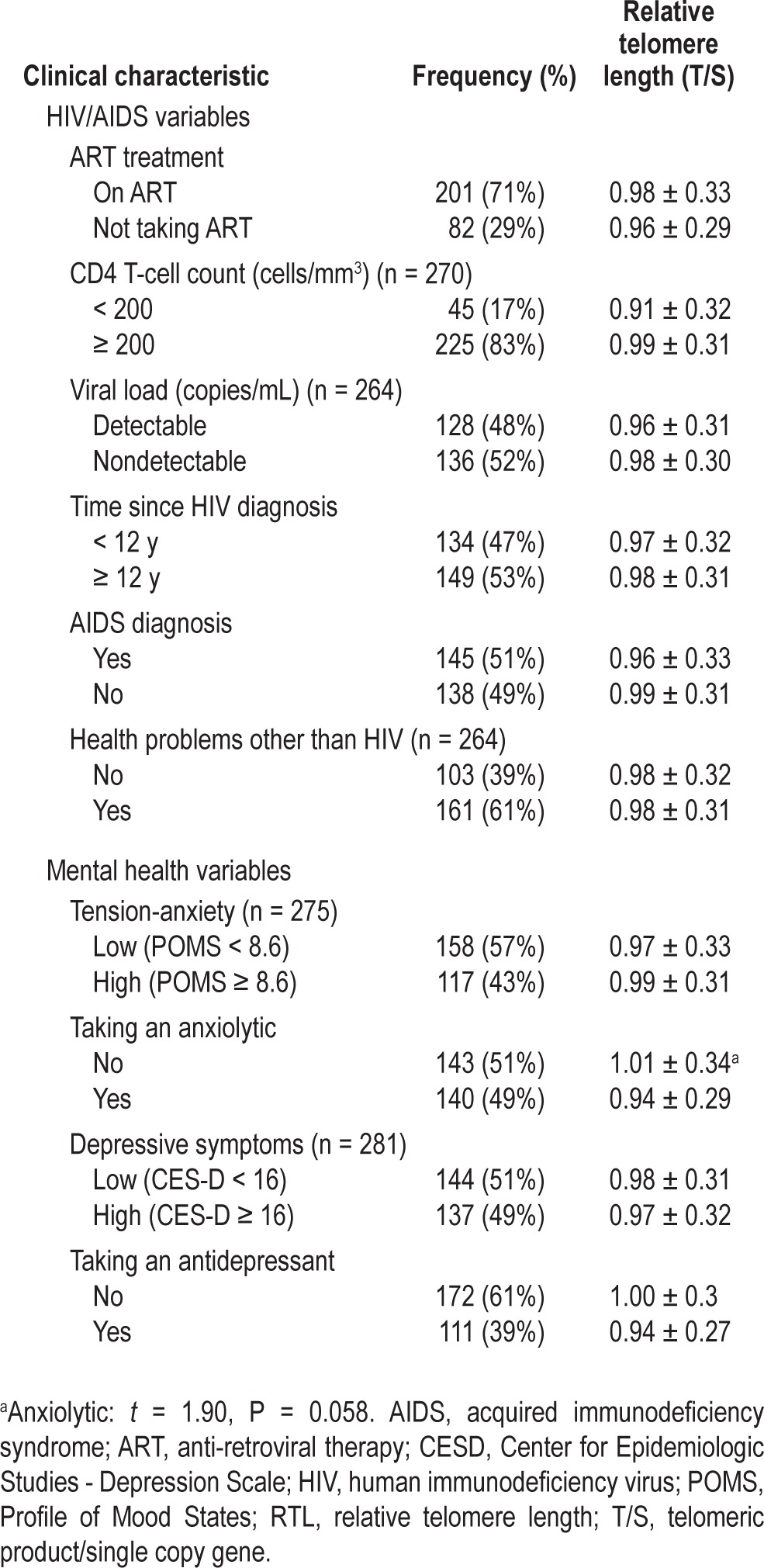
Table 3.
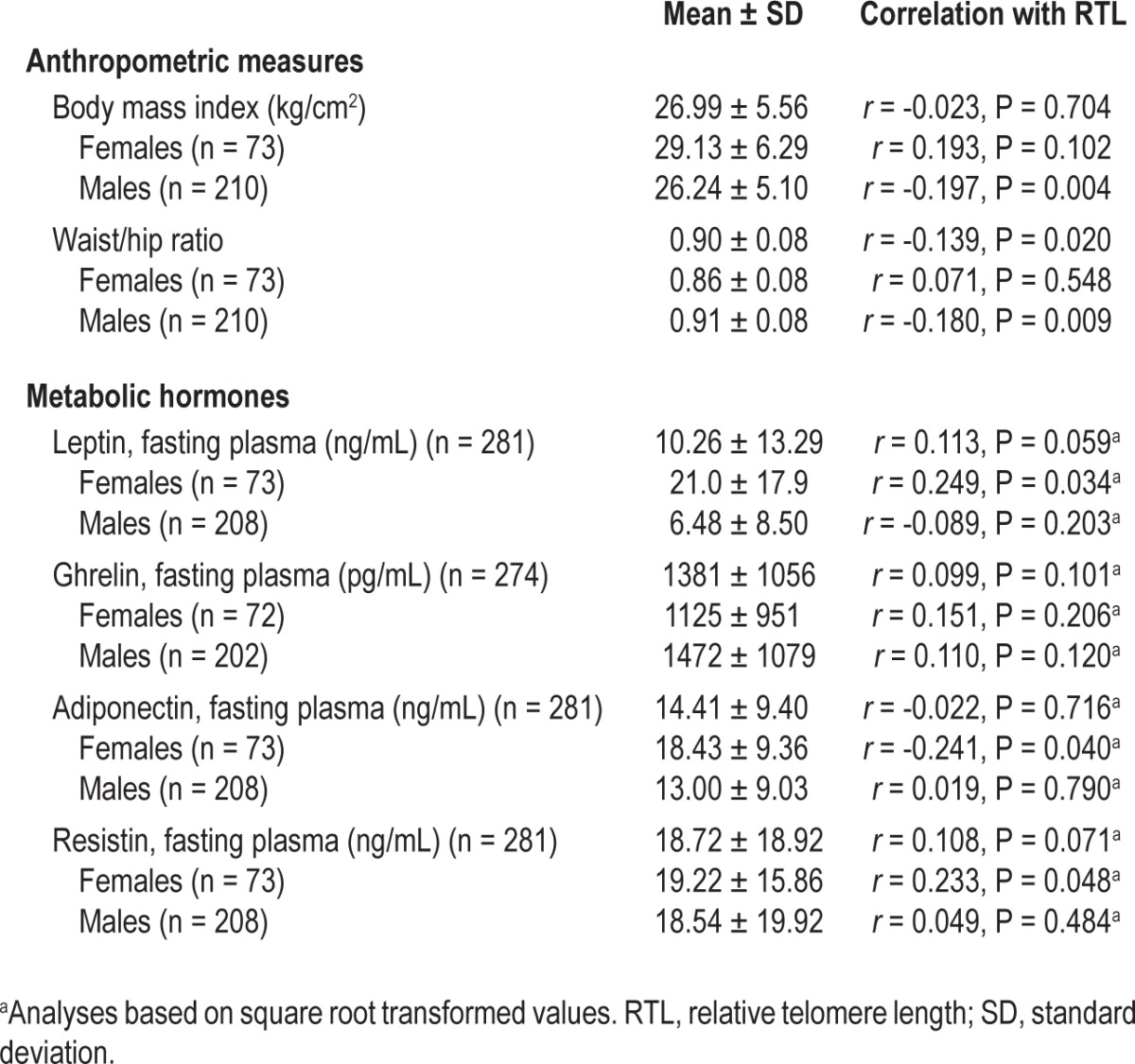
Relative telomere length by anthropometric and metabolic variables reported by sex (n = 283)
Sleep
Sample sleep characteristics are presented in Table 4.
Table 4.
Relative telomere length by sample sleep characteristics
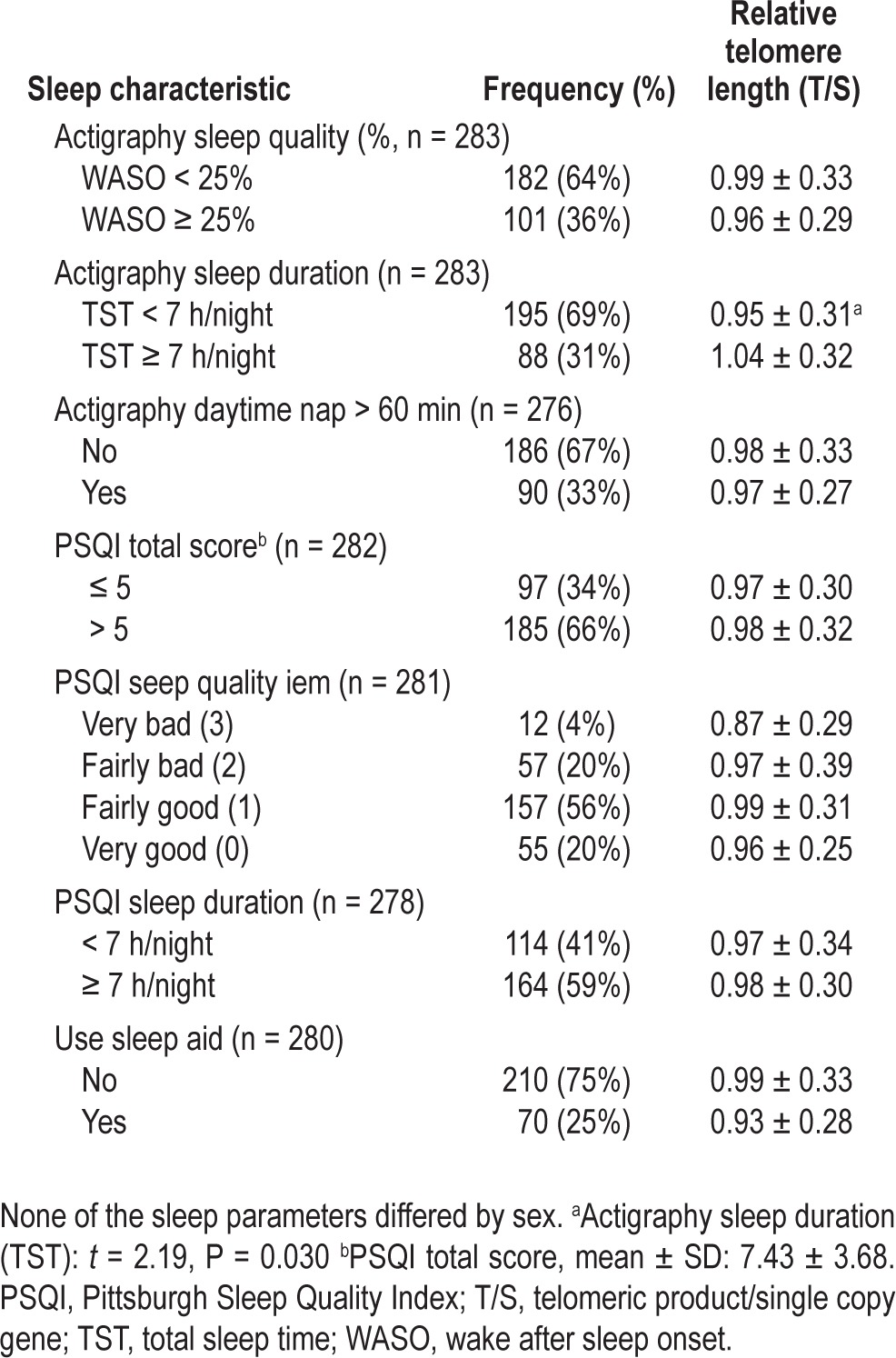
TST by actigraphy recording across the 3 nights averaged 6.2 ± 1.6 h. As previously reported,24 short sleep duration was associated with lower CD4+ T-cell count and higher viral load, and short sleepers were also more likely to be African American, have less education, and have lower income. TST was correlated with self-reported sleep duration on the PSQI (r = 0.30, P < 0.001), although self-reported sleep duration (7.1 ± 1.5 h) was generally higher than the actigraphy estimate (P < 0.001). TST was not related to PSQI total score, habitual sleep efficiency, or the sleep quality item. Three-night mean WASO was 20.9 ± 14.8%. WASO was correlated with PSQI total score (r = 0.12, P = 0.041) and PSQI habitual sleep efficiency score (r = 0.15, P = 0.015), but was not related to the PSQI sleep quality or duration items.
There were no significant differences in actigraphy sleep parameters between the women and men in this sample. However, women's PSQI responses indicated significantly worse sleep efficiency (79.5% ± 15.6%) compared to men (85.5% ± 14.7%) in this sample (t = 2.79, P = 0.006). There were no sex differences in PSQI total scores or the PSQI sleep duration or sleep quality items.
Leukocyte Relative Telomere Length
RTL had a mean T/S ratio of 0.98 ± 0.32 (median 0.96, range 0.24-2.03 T/S ratio). In bivariate analyses, RTL was inversely related to age (r = -0.179, P = 0.003) and education (t = 2.25, P = 0.025) such that older adults and more educated adults had shorter RTL. RTL was also significantly shorter for men compared to women (t = 2.04, P = 0.045). RTL was not associated with race, income, having a partner, or having children.
RTL was unrelated to any HIV clinical characteristics including CD4 cell count, viral load, time since diagnosis, or antiretroviral therapy. Symptoms of depression or anxiety were also unrelated to RTL. In bivariate analyses, RTL was not associated with smoking, intake of alcohol or caffeine, or hypertension. For the full sample of men and women, waist/ hip ratio was related to RTL, but BMI was not (Table 3). When examined separately, however, RTL was negatively correlated with BMI and waist/hip ratio among males, but not among females. Sex differences were also observed in associations between RTL and metabolic hormones; RTL was associated with leptin, adiponectin, and resistin among females, but not among males (Table 3).
RTL was not strongly correlated with TST as a continuous linear measure (r = 0.115, P = 0.052) or as a self-report measure from the PSQI item (r = 0.102, P = 0.089). However, as indicated in Table 4, participants who had at least 7 h of sleep by actigraphy had significantly longer RTL than participants who had less than 7 h of sleep (t = 2.19, P = 0.030). Similar patterns of longer telomeres for longer sleepers were observed among men and women, although these differences did not reach significance for the men (mean difference = 0.078, P = 0.072) or women (mean difference = 0.103, P = 0.276). Furthermore, when sleep duration was split into four categories (see Figure 1), sleeping at least 6 h per night was a more sensitive threshold for longer telomeres among women (mean difference = 0.164, P = 0.072), but not men (mean difference = 0.015, P = 0.705).
Figure 1.
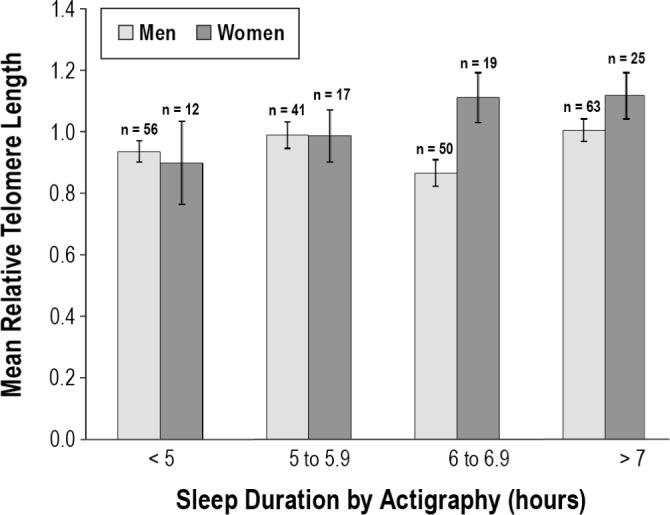
Objective sleep duration and relative telomere length in men and women (n = 283). Sleeping an average of 7 h or more per night was associated with longer telomeres. The differences were not significant when analyzed separately for men (mean difference = 0.078, P = 0.072) and women (mean difference = 0.103, P = 0.276). For this small sample of women, sleeping at least 6 hours may be a more sensitive threshold for longer telomeres (mean difference = 0.164, P = 0.072). Error bars indicate ± 1 standard error.
For the full sample, RTL was not significantly related to sleep quality as estimated by actigraphy WASO (r = -0.104, P = 0.079), PSQI total score (r = -0.070), or PSQI sleep quality item (rho = -0.065) in bivariate analyses. However, when examined separately for men and women, notable sex differences in the relationship between RTL and self-reported sleep quality became apparent. A higher PSQI total score (worse sleep quality) was associated with shorter RTL among men (r = -0.139, P = 0.043), but not among women (r = -0.050, P = 0.677). Furthermore, a higher score on the PSQI sleep quality item was associated with a shorter telomere among men (r = -0.137, P = 0.048) but slightly longer telomeres among women (r = 0.124, P = 0.303), although the association was not significant in the small sample of women. Associations between WASO and RTL were not significant for both men and women.
Multivariable Regression Model
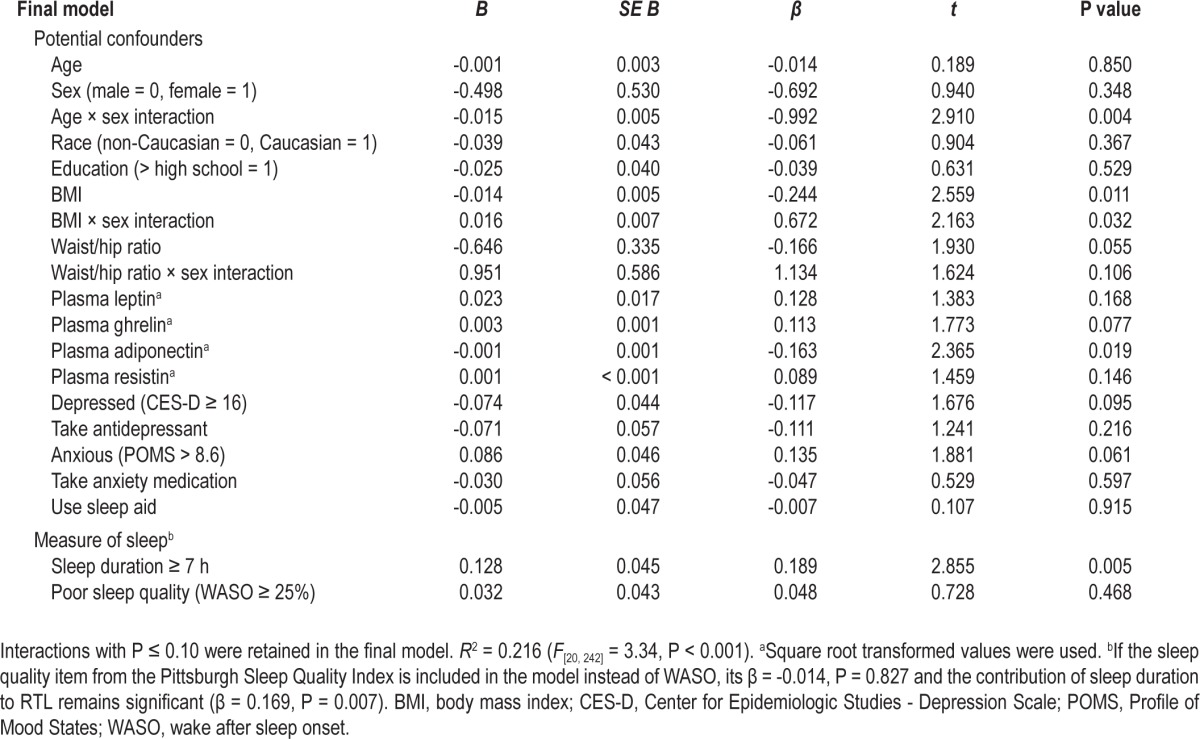
To determine whether the bivariate relationship between RTL and sleep duration ≥ 7 h (β = 0.129, t = 2.19, P = 0.030) was influenced by other factors associated with sleep and RTL, potentially confounding demographic variables, clinical factors, and metabolic indicators were entered into a two-step multiple regression analysis. WASO (< or ≥ 25%) was also included in the model to control for the confounding effect of poor sleep quality on sleep duration. In the final model, the interaction of age and sex was a significant predictor of RTL with age having a more negative influence on RTL for women than for men. Fasting plasma adiponectin levels, BMI, and the interaction of BMI by sex were also significant predictors of the variance in RTL. As shown in Table 5, the relationship between RTL and sleep duration ≥ 7 h was enhanced (β = 0.189, t = 2.86, P = 0.005) in the overall model, even when controlling for sleep quality. The overall model was significant and accounted for 21.6% of the variance in RTL (F[20,242] = 3.34, P < 0.001). Longer RTL was explained by five significant variables after controlling for other potential factors: younger age among women, lower BMI, the interaction of lower BMI and male sex, lower plasma adiponectin levels, and sleep duration of 7 h or more per night.
Table 5.
Multiple regression analysis of variables associated with relative telomere length (n = 263)
Alternative Sleep Parameters
Although dichotomized sleep duration (TST ≥ 7 h) was the only significant sleep parameter in bivariate analyses, after controlling for potentially confounding variables, the continuous measure of sleep duration (TST) was also a significant predictor of RTL. When the continuous measure of TST was in the model in place of the dichotomous 7-h variable, the β coefficient was = 0.175 (P = 0.022) and sleep quality remained unrelated to RTL.
Similarly, the results observed in the final model were unaffected by the selected measures of sleep quality. Sleep duration remained significant and sleep quality remained non-significant regardless of what value was used to dichotomize WASO or using the continuous measure of sleep quality (WASO%). If the PSQI sleep quality item was included in the multivariable model instead of WASO, its β coefficient was -0.014 (P = 0.827) and the contribution of sleep duration to RTL remained significant (β = 0.169, P = 0.007). Despite the sex differences in associations between PSQI sleep quality and RTL (Figure 2), the interaction was not significant (P = 0.127) and its inclusion in the model did not strengthen the association between sleep quality and RTL (β = -0.073, P = 0.339); nor did inclusion in the model attenuate the association between sleep duration and RTL (β = 0.165, P = 0.008).
Figure 2.
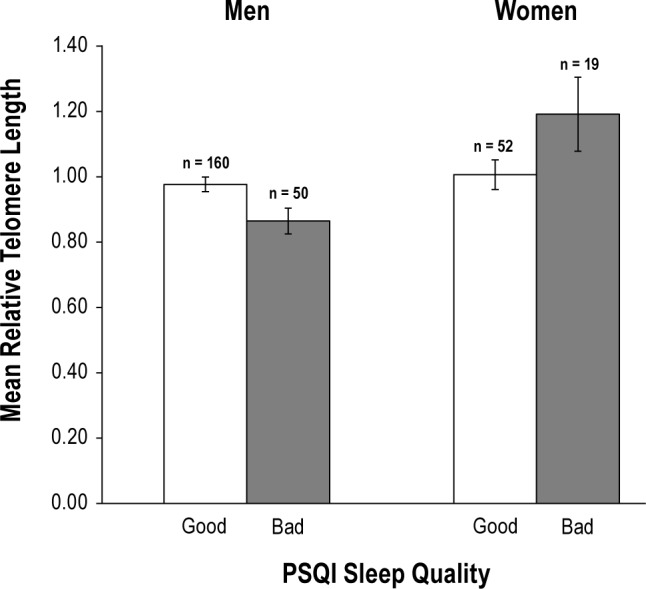
Subjective sleep quality and relative telomere length in men and women (n = 281). Good = very/fairly good; Bad = vary/fairly bad. Good sleep quality was associated with longer telomeres among men (mean difference = 0.112, P = 0.016), but slightly (not significantly) shorter telomeres among women (mean difference = 0.185, P = 0.072). Error bars indicate ± 1 standard error.
Sex and Race Differences
Given sex differences on a number of metabolic covariates, the regression model in Table 5 was repeated for men and women separately. The dichotomized sleep duration regression coefficient was significant for women (β = 0.266, P = 0.028), but not men (β = 0.148, P = 0.078), and a similar pattern was observed when continuous sleep duration was used (women: β = 0.320, P = 0.020; men: β = 0.112, P = 0.244). Sleep quality was not significant for men (β = 0.076, P = 0.364) or women (β = -0.024, P = 0.851) when using WASO dichotomized at ≥ 25%, nor when using the PSQI sleep quality item (men: β = -0.098, P = 0.241; women: β = 0.116, P = 0.324).
To determine whether the association between sleep duration and RTL was similar for Caucasian and non-Caucasian samples, the final regression model was repeated by race. The regression coefficients for dichotomized sleep duration ≥ 7 h were similar, but slightly stronger for Caucasian (β = 0.216, P = 0.038) than for non-Caucasian (β = 0.152, P = 0.081) adults in the sample. Sleep quality was not associated with RTL in racial subgroup analyses regardless of the selected measure of sleep quality.
DISCUSSION
This study used a cross-sectional convenience sample of men and women living with HIV/AIDS to describe the relationship between RTL and sleep duration and quality using both objective actigraphy and PSQI self-report measures. Even after controlling for demographic and clinical variables that included HIV-disease factors and BMI and metabolic hormones, longer sleep duration (TST) by objective actigraphy estimates was a significant factor in preserving RTL. In contrast, sleep quality measured by objective actigraphy estimates (WASO) or by subjective sleep quality was not significant in accounting for variance in RTL.
RTL and Demographic Factors
Findings in this sample of adults with a chronic illness support findings from prior studies that demonstrate RTL associations with age and sex.25,26 In fact, the interaction of age with sex was the strongest predictor of RTL, reinforcing the understanding that telomere length is a marker of cellular aging even in a sample of adults living with HIV disease and comorbidities,27 and highlighting that the influence of age on RTL likely differs for men and women. As expected, we found a significant sex difference in RTL, with women having longer RTL (1.05) than men (0.95) in bivariate analyses. Diez Roux et al.28 reported lower RTL values, but a similar sex difference in their sample of healthy men (0.75) and women (0.85) who were approximately 20 y older than our sample.
Race was unrelated to RTL. Diez Roux et al.28 reported that African Americans and Hispanics had shorter RTL than Caucasians, but differences were not seen until older age, and our sample of HIV-infected adults was an average of 20 y younger. In contrast, Needham et al.26 reported that African American adults had longer telomeres than Caucasians after controlling for age and sex. We also confirm prior research showing that RTL was unrelated to income5,26,28,29 and partner status3,26 in samples of healthy adults. We found a counterintuitive association between higher education and shorter RTL in bivariate analyses, and although this association contradicts prior findings,26 it disappeared after accounting for the confounding effects of sex and race.
RTL and Clinical Factors
Although prior studies have reported associations between RTL and depression30–33 and anxiety,34–36 no such associations were evident in our sample. Other studies have found no relationship between RTL and depression or anxiety,3,34,37 or only found associations among specific age groups.38,39 In addition, a recent study40 has suggested that the RTL-depression association needs to be evaluated within a psychosocial-behavioral context because factors such as sleep, among others, can moderate the relationship.
Clinical factors related to HIV status, number of comorbid conditions, and medication for sleep, depression, or anxiety also were not associated with RTL. Although prior studies have reported that adults with HIV have shorter RTL than healthy controls,41,42 studies of RTL and HIV clinical variables among adults with HIV have yielded inconsistent results. Malan-Muller et al.42 found no associations between RTL and HIV variables in their study of women with HIV, whereas Pathai et al.41 reported an association between RTL and CD4+ T-cell counts, but only among patients on ART with undetectable viral load. In a study of children with HIV, Cote et al.43 found that detectable viral load was associated with shorter RTL. Imam et al.44 found an association between RTL and duration of HIV infection, but no effect of ART on RTL and no difference in RTL between HIV-infected childbearing women and uninfected controls. Finally, Pommier et al.13 reported that HIV-infected adults had shorter telomeres than age-matched controls, but only for HIV patients with CD4+ counts below 200 cells/mm3. Such disparate findings may reflect differences in sampling or in how clinical indicators of HIV status were analyzed.
Although diet habits, fat intake, metabolic hormones, and BMI have been linked to shortened RTL in prior studies,6–8,45 to our knowledge, metabolic hormones such as leptin, ghrelin, adiponectin, and resistin have not been evaluated in studies of sleep duration and RTL. Our finding that adiponectin plasma levels predict RTL is consistent with a study of Arab men and women,46 although other studies did not observe an association.47 Although metabolic hormones could potentially explain the relationship between RTL and BMI as well as sleep duration, hormone values differed by sex in our sample, and some of the bivariate relationships with RTL differed in the opposite direction for men and women. However, our multivariable model demonstrates that the association between RTL and BMI persists even when accounting for sex and metabolic hormones. Furthermore, sleep duration remains a significant factor in explaining RTL, even with age, sex, BMI, and metabolic hormones in the overall model.
The relationship between BMI and RTL is complex, particularly when a study sample includes both men and women. BMI was not related to RTL in our sample until the effects of sex and the interaction of sex and BMI were controlled. Shorter RTL was associated with higher BMI in some samples of women.3,5,48 In a younger sample of women (mean age, 32 y), BMI was only associated with RTL among the women who reported intimate partner violence.17 Jackowska et al.4 found no relationship between BMI and RTL for either men or women in their healthy British sample. In our HIV sample, the interaction between BMI and sex was critical in the regression model for a better understanding of this relationship, and thus, further research related to this interaction is warranted.
RTL and Sleep Duration
Given established associations between sleep duration and demographic and clinical factors, as well as the complexity associated with sex differences in metabolic factors, multiple regression was used to determine the relative contribution of sleep duration to RTL while controlling for relevant covariates. The relationship between RTL and sleep duration of at least 7 h per night remained significant and unchanged after controlling for age, sex, BMI, waist/hip ratio, metabolic hormones, and other common correlates of sleep duration including symptoms of anxiety and depression, medication usage, and self-reported or objective sleep quality. This finding was also replicated when we used continuous total sleep time as the predictor in place of the dichotomous 7-h sleep duration.
In the bivariate analysis, the 7-h cutoff point for TST was significantly associated with RTL, whereas the continuous TST variable was less convincing. A weaker relationship between RTL and continuous measures of sleep duration (either self-report PSQI or actigraphy) may result because sleep duration effects are not linear and excessively long total sleep time can be as deleterious as excessively short sleep time. Employment and work hours may also be a factor in sleep duration for other study samples, and it is important to keep in mind that most of our sample was unemployed and not sleep restricted due to work schedules. Our finding of an association between RTL and sleep duration ≥ 7 h is consistent with recent findings of a trend for healthy older men, although no relationship was seen for women.4 In contrast, we observed a dose-response type of relationship between RTL and sleep duration for women but not men (Figure 1).
RTL and Sleep Quality
Sleep quality was assessed using both objective actigraphy WASO values and self-report measures. We found no association between RTL and WASO values. The association between short RTL and self-report measures of poor sleep quality was evident for men in our sample, but not for women. Sex differences in self-reported sleep quality have been well established49–51 and may help explain the differing results between men and women in this study. Others have also reported no association between RTL and sleep by self-report measures in healthy women3,5 and studies that use objective sleep measures should be encouraged in future research on this topic.
Study Limitations and Recommendations
This study of telomere length and sleep in a cross-sectional sample of HIV-positive adults has some limitations to consider when interpreting the findings. First, although sleep was objectively assessed with movement counts using wrist actigraphy, we did not assess sleep stages or sleep architecture with polysomnography, we only sampled from 3 consecutive weekdays at one time point, and we did not examine the potential for extra sleep some adults obtain on weekends. We assessed daytime sleep and total 24-h sleep, and found no relationship to RTL. However, most of our sample was on disability and weekdays were not as likely to vary from weekends or days off. Unlike sleep quality, which can fluctuate over time for various reasons, sleep duration is a fairly stable personal characteristic52 and thus may be more likely to correlate with RTL, a measure of cumulative life stress and cellular aging. Another limitation of this study was the one-time measure of RTL, which may fluctuate over time. We did not include a measure of telomerase activity,53 which is an even more dynamic measure of cellular aging, and future studies should consider the timing of these measures before and after sleep studies in controlled laboratory settings. Finally, these results from our sample of HIV-positive adults need to be replicated in other samples of healthy adults and adults living with other types of chronic illness.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by a grant from the National Institute of Mental Health (NIMH, 5 R01 MH074358). Data collection was supported by the General Clinical Research Center in the UCSF CTSA (1 UL RR024131). This study was conducted at the University of California, San Francisco. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge contributions to the study from Traci Coggins, Ryan Kelly, Yeonsu Song, Kristen Nelson, Matthew Shullick, Steve Bruce, and Harvey Davis.
ABBREVIATIONS
- ART
anti-retroviral therapy
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies - Depression Scale
- CRC
Clinical Research Center
- HBG
human beta-globin gene
- qPCR
quantitative polymerase chain reaction
- POMS
Profile of Mood States
- PSQI
Pittsburgh Sleep Quality Index
- RTL
relative telomere length
- T/S
telomeric product/single copy gene
- TST
total sleep time
- UCSF
University of California, San Francisco
- WASO
wake after sleep onset
REFERENCES
- 1.Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res. 2012;730:85–9. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108:E513–8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackowska M, Hamer M, Carvalho LA, Erusalimsky JD, Butcher L, Steptoe A. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study. PLoS One. 2012;7:e47292. doi: 10.1371/journal.pone.0047292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prather AA, Puterman E, Lin J, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge MP, O'Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson MW, Sverstiuk A, Hendel H, Cheung TW, Zagury JF, Rappaport J. Analysis of telomere length and thymic output in fast and slow/non-progressors with HIV infection. Biomed Pharmacother. 2000;54:21–31. doi: 10.1016/s0753-3322(00)88637-0. [DOI] [PubMed] [Google Scholar]
- 10.Tucker V, Jenkins J, Gilmour J, et al. T-cell telomere length maintained in HIV-infected long-term survivors. HIV Med. 2000;1:116–22. doi: 10.1046/j.1468-1293.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 11.van Baarle D, Nanlohy NM, Otto S, Plunkett FJ, Fletcher JM, Akbar AN. Progressive telomere shortening of Epstein-Barr virus-specific memory T cells during HIV infection: contributor to exhaustion? J Infect Dis. 2008;198:1353–7. doi: 10.1086/592170. [DOI] [PubMed] [Google Scholar]
- 12.Kaushal S, Landay AL, Lederman MM, et al. Increases in T cell telomere length in HIV infection after antiretroviral combination therapy for HIV-1 infection implicate distinct population dynamics in CD4+ and CD8+ T cells. Clin Immunol. 1999;92:14–24. doi: 10.1006/clim.1999.4726. [DOI] [PubMed] [Google Scholar]
- 13.Pommier JP, Gauthier L, Livartowski J, et al. Immunosenescence in HIV pathogenesis. Virology. 1997;231:148–54. doi: 10.1006/viro.1997.8512. [DOI] [PubMed] [Google Scholar]
- 14.Lee KA, Gay C, Portillo CJ, et al. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. J Pain Symptom Manage. 2009;38:882–93. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere shortening in formerly abused and never abused women. Biol Res Nurs. 2012;14:115–23. doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20:24–7. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 20.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.McNair DM, Lorr M., Droppleman LF. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 23.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Lee KA, Gay C, Portillo CJ, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med. 2012;8:67–75. doi: 10.5664/jcsm.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10:913–21. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 26.Needham BL, Adler N, Gregorich S, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 28.Diez Roux AV, Ranjit N, Jenny NS, et al. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8:251–7. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seifarth JE, McGowan CL, Milne KJ. Sex and life expectancy. Gend Med. 2012;9:390–401. doi: 10.1016/j.genm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27:1111–6. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 31.Wikgren M, Maripuu M, Karlsson T, et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Rizo C, Fernandez-Egea E, Miller BJ, et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–97. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- 35.Okereke OI, Prescott J, Wong JY, Han J, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS One. 2012;7:e40516. doi: 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladwig KH, Brockhaus AC, Baumert J, et al. Posttraumatic stress disorder and not depression Is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 Study. PLoS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaffer JA, Epel E, Kang MS, et al. Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One. 2012;7:e48318. doi: 10.1371/journal.pone.0048318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips AC, Robertson T, Carroll D, et al. Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study. Psychosom Med. 2013;75:288–96. doi: 10.1097/PSY.0b013e318289e6b5. [DOI] [PubMed] [Google Scholar]
- 40.Puterman E, Epel ES, Lin J, et al. Multisystem resiliency moderates the major depression-telomere length association: Findings from the Heart and Soul Study. Brain Behav Immun. 2013;33:65–73. doi: 10.1016/j.bbi.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pathai S, Lawn SD, Gilbert CE, et al. Accelerated biological aging in HIV-infected individuals in South Africa: a case-control study. AIDS. 2013;27:2375–84. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length - a potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected] PLoS One. 2013;8:e58351. doi: 10.1371/journal.pone.0058351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cote HC, Soudeyns H, Thorne A, et al. Leukocyte telomere length in HIV-infected and HIV-exposed uninfected children: shorter telomeres with uncontrolled HIV viremia. PLoS One. 2012;7:e39266. doi: 10.1371/journal.pone.0039266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imam T, Jitratkosol MH, Soudeyns H, et al. Leukocyte telomere length in HIV-infected pregnant women treated with antiretroviral drugs during pregnancy and their uninfected infants. J Acquir Immune Defic Syndr. 2012;60:495–502. doi: 10.1097/QAI.0b013e31825aa89c. [DOI] [PubMed] [Google Scholar]
- 45.Njajou OT, Cawthon RM, Blackburn EH, et al. Shorter telomeres are associated with obesity and weight gain in the elderly. Int J Obes (Lond) 2012;36:1176–9. doi: 10.1038/ijo.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Attas OS, Al-Daghri NM, Alokail MS, et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: the influence of circulating adiponectin. Eur J Endocrinol. 2010;163:601–7. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz VA, Mainous AG, Player MS, Everett CJ. Telomere length and adiposity in a racially diverse sample. Int J Obes (Lond) 2010;34:261–5. doi: 10.1038/ijo.2009.198. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–20. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–10. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- 50.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56:1218–27. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 51.van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–75. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 53.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]


